Navigating Diagnostic and Treatment Challenges of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia
Abstract
1. Introduction
2. Challenge #1: Screening and Confirmation
2.1. Screening for Pulmonary Hypertension
2.2. Echocardiography as a Screening and Diagnostic Tool
2.3. Confirmation of Diagnosis
Cardiac Catheterization Is Not Routinely Performed
2.4. Adjunct Testing May Be Helpful
3. Challenge #2: Multitiered Management of BPD-PH
3.1. Focusing on Optimization of Respiratory Disease as First-Line Therapy
3.2. Management of Chronic Respiratory Failure: Ventilator
3.3. Management of Chronic Respiratory Failure: Oxygen
3.4. Identifying Confounding Variables: Left-to-Right Intracardiac Shunts, Pulmonary Vein Stenosis
3.5. Treating Comorbidities to Minimize Respiratory Complications
Nutrition and Feeding
3.6. Fluid Management
3.7. Lack of Approved PH Therapies and Lack of Data in This Population Should Not Deter Use When Indicated
PH Pharmacotherapy
4. Challenge #3: Long-Term Follow-Up Care of BPD-PH Infants
5. Overcoming the Challenges Listed
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Vyas-Read, S.; Varghese, N.P.; Suthar, D.; Backes, C.; Lakshminrusimha, S.; Petit, C.J.; Levy, P.T. Prematurity and Pulmonary Vein Stenosis: The Role of Parenchymal Lung Disease and Pulmonary Vascular Disease. Children 2022, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Brugha, R.; Quyam, S.; Moledina, S. Diagnosis and management of pulmonary hypertension in infants with bronchopulmonary dysplasia: A guide for paediatric respiratory specialists. Breathe 2022, 18, 220209. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H.; Hansmann, G.; Archer, S.L.; Ivy, D.D.; Adatia, I.; Chung, W.K.; Hanna, B.D.; Rosenzweig, E.B.; Raj, J.U.; Cornfield, D.; et al. Pediatric Pulmonary Hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015, 132, 2037–2099. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, U.; Feinstein, J.A.; Adatia, I.; Austin, E.D.; Mullen, M.P.; Hopper, R.K.; Hanna, B.; Romer, L.; Keller, R.L.; Fineman, J.; et al. Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J. Pediatr. 2017, 188, 24–34.e1. [Google Scholar] [CrossRef] [PubMed]
- Lagatta, J.M.; Hysinger, E.B.; Zaniletti, I.; Wymore, E.M.; Vyas-Read, S.; Yallapragada, S.; Nelin, L.D.; Truog, W.E.; Padula, M.A.; Porta, N.F.; et al. The Impact of Pulmonary Hypertension in Preterm Infants with Severe Bronchopulmonary Dysplasia through 1 Year. J. Pediatr. 2018, 203, 218–224.e3. [Google Scholar] [CrossRef] [PubMed]
- Mourani, P.M.; Abman, S.H. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 839–855. [Google Scholar] [CrossRef]
- El-Saie, A.; Varghese, N.P.; Webb, M.K.; Villafranco, N.; Gandhi, B.; Guaman, M.C.; Shivanna, B. Bronchopulmonary dysplasia-associated pulmonary hypertension: An updated review. Semin. Perinatol. 2023, 47, 151817. [Google Scholar] [CrossRef]
- Altit, G.; Dancea, A.; Renaud, C.; Perreault, T.; Lands, L.C.; Sant’Anna, G. Pathophysiology, screening and diagnosis of pulmonary hypertension in infants with bronchopulmonary dysplasia—A review of the literature. Paediatr. Respir. Rev. 2017, 23, 16–26. [Google Scholar] [CrossRef]
- Kinsella, J.P.; Greenough, A.; Abman, S.H. Bronchopulmonary dysplasia. Lancet 2006, 367, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Nawaytou, H.; Hills, N.K.; Clyman, R.I. Patent ductus arteriosus and the risk of bronchopulmonary dysplasia-associated pulmonary hypertension. Pediatr. Res. 2023, 94, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Altit, G.; Bhombal, S.; Hopper, R.K.; Tacy, T.A.; Feinstein, J. Death or resolution: The “natural history” of pulmonary hypertension in bronchopulmonary dysplasia. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2019, 39, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.H.; Hutchison, A.A.; Lakshminrusimha, S.; Morin, F.C.; Wynn, R.J.; Ryan, R.M. Characteristics of pulmonary hypertension in preterm neonates. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2007, 27, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Altit, G.; Lee, H.C.; Hintz, S.; Tacy, T.A.; Feinstein, J.A.; Bhombal, S. Practices surrounding pulmonary hypertension and bronchopulmonary dysplasia amongst neonatologists caring for premature infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2018, 38, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.B.; Abman, S.H.; Adatia, I.; Beghetti, M.; Bonnet, D.; Haworth, S.; Ivy, D.D.; Berger, R.M. Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019, 53, 1801916. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Koestenberger, M.; Alastalo, T.-P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2019, 38, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Keszler, M. Persistent Pulmonary Hypertension of the Newborn. NeoReviews 2015, 16, e680–e692. [Google Scholar] [CrossRef] [PubMed]
- Arjaans, S.; Zwart, E.A.H.; Ploegstra, M.; Bos, A.F.; Kooi, E.M.W.; Hillege, H.L.; Berger, R.M.F. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2018, 32, 258–267. [Google Scholar] [CrossRef]
- Levy, P.T.; Levin, J.; Leeman, K.T.; Mullen, M.P.; Hansmann, G.; Kourembanas, S. Diagnosis and management of pulmonary hypertension in infants with bronchopulmonary dysplasia. Semin. Fetal Neonatal Med. 2022, 27, 101351. [Google Scholar] [CrossRef]
- Abman, S.H.; Ivy, D.D.; Archer, S.L.; Wilson, K.; AHA/ATS Joint Guidelines for Pediatric Pulmonary Hypertension Committee. Executive Summary of the American Heart Association and American Thoracic Society Joint Guidelines for Pediatric Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2016, 194, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Miller, J.I.; Kinsella, J.P.; Baker, C.D.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015, 191, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.N.; Ivy, D.D. Echocardiography in pediatric pulmonary hypertension. Front. Pediatr. 2014, 2, 124. [Google Scholar] [CrossRef]
- Condon, D.F.; Nickel, N.P.; Anderson, R.; Mirza, S.; de Jesus Perez, V.A. The 6th World Symposium on Pulmonary Hypertension: What’s old is new. F1000Research 2019, 8, 888. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Jain, A.; El-Khuffash, A.; Giesinger, R.; Weisz, D.; Freud, L.; Levy, P.T.; Bhombal, S.; de Boode, W.; Leone, T.; et al. Guidelines and Recommendations for Targeted Neonatal Echocardiography and Cardiac Point-of-Care Ultrasound in the Neonatal Intensive Care Unit: An Update from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2024, 37, 171–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Z.; Zhang, F.; Xu, H.; Wang, H.; Zhao, X. The value of pulmonary artery acceleration time in evaluating pulmonary vascular disease in preterm infants with bronchopulmonary dysplasia. Echocardiography 2023, 40, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Nagiub, M.; Lee, S.; Guglani, L. Echocardiographic assessment of pulmonary hypertension in infants with bronchopulmonary dysplasia: Systematic review of literature and a proposed algorithm for assessment. Echocardiography 2015, 32, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Schweintzger, S.; Kurath-Koller, S.; Burmas, A.; Grangl, G.; Fandl, A.; Noessler, N.; Avian, A.; Gamillscheg, A.; Chouvarine, P.; Hansmann, G.; et al. Normal Echocardiographic Reference Values of the Right Ventricular to Left Ventricular Endsystolic Diameter Ratio and the Left Ventricular Endsystolic Eccentricity Index in Healthy Children and in Children with Pulmonary Hypertension. Front. Cardiovasc. Med. 2022, 9, 950765. [Google Scholar] [CrossRef]
- Abraham, S.; Weismann, C.G. Left Ventricular End-Systolic Eccentricity Index for Assessment of Pulmonary Hypertension in Infants. Echocardiography 2016, 33, 910–915. [Google Scholar] [CrossRef]
- King, M.E.; Braun, H.; Goldblatt, A.; Liberthson, R.; Weyman, A.E. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: A cross-sectional echocardiographic study. Circulation 1983, 68, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Altit, G.; Bhombal, S.; Feinstein, J.; Hopper, R.K.; Tacy, T.A. Diminished right ventricular function at diagnosis of pulmonary hypertension is associated with mortality in bronchopulmonary dysplasia. Pulm. Circ. 2019, 9, 2045894019878598. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Nunes, G.; Wutthigate, P.; Simoneau, J.; Dancea, A.; Beltempo, M.; Renaud, C.; Altit, G. The biventricular contribution to chronic pulmonary hypertension of the extremely premature infant. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2023, 43, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Koestenberger, M.; Nagel, B.; Ravekes, W.; Urlesberger, B.; Raith, W.; Avian, A.; Halb, V.; Cvirn, G.; Fritsch, P.; Gamillscheg, A. Systolic right ventricular function in preterm and term neonates: Reference values of the tricuspid annular plane systolic excursion (TAPSE) in 258 patients and calculation of Z-score values. Neonatology 2011, 100, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Malikiwi, A.; Paul, E.; Tan, K.; Menahem, S. Right Ventricular Function in Infants with Bronchopulmonary Dysplasia: Association with Respiratory Sequelae. Neonatology 2016, 109, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Dioneda, B.; Holland, M.R.; Sekarski, T.J.; Lee, C.K.; Mathur, A.; Cade, W.T.; Cahill, A.G.; Hamvas, A.; Singh, G.K. Right ventricular function in preterm and term neonates: Reference values for right ventricle areas and fractional area of change. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Mah, K.; Mertens, L. Echocardiographic Assessment of Right Ventricular Function in Paediatric Heart Disease: A Practical Clinical Approach. CJC Pediatr. Congenit. Heart Dis. 2022, 1, 136–157. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.M.; Chiesa, G.; Durighel, G.; Goldring, S.T.; Fitzpatrick, J.A.; Uribe, S.; Razavi, R.; Hajnal, J.V.; Edwards, A.D. Functional cardiac MRI in preterm and term newborns. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F86–F91. [Google Scholar] [CrossRef]
- Breatnach, C.R.; El-Khuffash, A.; James, A.; McCallion, N.; Franklin, O. Serial measures of cardiac performance using tissue Doppler imaging velocity in preterm infants <29weeks gestations. Early Hum. Dev. 2017, 108, 33–39. [Google Scholar] [CrossRef]
- Koestenberger, M.; Nagel, B.; Ravekes, W.; Gamillscheg, A.; Pichler, G.; Avian, A.; Heinzl, B.; Binder, C.; Cvirn, G.; Urlesberger, B. Right ventricular performance in preterm and term neonates: Reference values of the tricuspid annular peak systolic velocity measured by tissue Doppler imaging. Neonatology 2013, 103, 281–286. [Google Scholar] [CrossRef]
- Moore, S.S.; De Carvalho Nunes, G.; Dancea, A.; Wutthigate, P.; Simoneau, J.; Beltempo, M.; Sant’anna, G.; Altit, G. Early cardiac function and death, severe bronchopulmonary dysplasia and pulmonary hypertension in extremely preterm infants. Pediatr. Res. 2024, 95, 293–301. [Google Scholar] [CrossRef]
- Levy, P.T.; El-Khuffash, A.; Patel, M.D.; Breatnach, C.R.; James, A.T.; Sanchez, A.A.; Abuchabe, C.; Rogal, S.R.; Holland, M.R.; McNamara, P.J.; et al. Maturational Patterns of Systolic Ventricular Deformation Mechanics by Two-Dimensional Speckle-Tracking Echocardiography in Preterm Infants over the First Year of Age. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2017, 30, 685–698.e1. [Google Scholar] [CrossRef] [PubMed]
- El-Khuffash, A.; Schubert, U.; Levy, P.T.; Nestaas, E.; de Boode, W.P. European Special Interest Group ‘Neonatologist Performed Echocardiography’ (NPE). Deformation imaging and rotational mechanics in neonates: A guide to image acquisition, measurement, interpretation, and reference values. Pediatr. Res. 2018, 84 (Suppl. S1), 30–45. [Google Scholar] [CrossRef]
- Tissot, C.; Singh, Y.; Sekarski, N. Echocardiographic Evaluation of Ventricular Function-for the Neonatologist and Pediatric Intensivist. Front. Pediatr. 2018, 6, 79. [Google Scholar] [CrossRef]
- Jain, A.; El-Khuffash, A.F.; Kuipers, B.C.; Mohamed, A.; Connelly, K.A.; McNamara, P.J.; Jankov, R.P.; Mertens, L. Left Ventricular Function in Healthy Term Neonates During the Transitional Period. J. Pediatr. 2017, 182, 197–203.e2. [Google Scholar] [CrossRef]
- Lu, J.C.; Ensing, G.J.; Yu, S.; Thorsson, T.; Donohue, J.E.; Dorfman, A.L. 5/6 Area length method for left-ventricular ejection-fraction measurement in adults with repaired tetralogy of Fallot: Comparison with cardiovascular magnetic resonance. Pediatr. Cardiol. 2013, 34, 231–239. [Google Scholar] [CrossRef]
- Abushaban, L.; Rathinasamy, J.; Sharma, P.N.; Vel, M.T. Normal reference ranges for the left ventricular mass and left ventricular mass index in preterm infants. Ann. Pediatr. Cardiol. 2020, 13, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, S.; Seguchi, M.; Ohzeki, T. Left ventricular diastolic performance in neonates. Circ. J. Off. J. Jpn. Circ. Soc. 2005, 69, 1094–1098. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torres, A.J. Hemodynamic assessment of atrial septal defects. J. Thorac. Dis. 2018, 10, S2882–S2889. [Google Scholar] [CrossRef]
- Lin, Y.; Amin, E.K.; Keller, R.L.; Teitel, D.F.; Nawaytou, H.M. Doppler Echocardiographic Features of Pulmonary Vein Stenosis in Ex-Preterm Children. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2022, 35, 435–442. [Google Scholar] [CrossRef]
- Mahgoub, L.; Kaddoura, T.; Kameny, A.R.; Lopez Ortego, P.; Vanderlaan, R.D.; Kakadekar, A.; Dicke, F.; Rebeyka, I.; Calderone, C.A.; Redington, A.; et al. Pulmonary vein stenosis of ex-premature infants with pulmonary hypertension and bronchopulmonary dysplasia, epidemiology, and survival from a multicenter cohort. Pediatr. Pulmonol. 2017, 52, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, Z.; Issa, Z.; Siblini, G.; Moiduddin, N.; Di Salvo, G. Normal Range of Left Ventricular Strain, Dimensions and Ejection Fraction Using Three-dimensional Speckle-Tracking Echocardiography in Neonates. J. Cardiovasc. Echogr. 2015, 25, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Truong, U.; Meinel, K.; Haddad, F.; Koestenberger, M.; Carlsen, J.; Ivy, D.; Jone, P.-N. Update on noninvasive imaging of right ventricle dysfunction in pulmonary hypertension. Cardiovasc. Diagn. Ther. 2020, 10, 1604–1624. [Google Scholar] [CrossRef] [PubMed]
- Mawad, W.; Fadnes, S.; Løvstakken, L.; Henry, M.; Mertens, L.; Nyrnes, S.A. Pulmonary Hypertension in Children is Associated With Abnormal Flow Patterns in the Main Pulmonary Artery as Demonstrated by Blood Speckle Tracking. CJC Pediatr. Congenit. Heart Dis. 2022, 1, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Marzlin, N.; Hays, A.G.; Peters, M.; Kaminski, A.; Roemer, S.; O’leary, P.; Kroboth, S.; Harland, D.R.; Khandheria, B.K.; Tajik, A.J.; et al. Myocardial Work in Echocardiography. Circ. Cardiovasc. Imaging 2023, 16, e014419. [Google Scholar] [CrossRef]
- Koestenberger, M.; Ravekes, W.; Everett, A.D.; Stueger, H.P.; Heinzl, B.; Gamillscheg, A.; Cvirn, G.; Boysen, A.; Fandl, A.; Nagel, B. Right ventricular function in infants, children and adolescents: Reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2009, 22, 715–719. [Google Scholar] [CrossRef]
- Clancy, D.J.; Mclean, A.; Slama, M.; Orde, S.R. Paradoxical septal motion: A diagnostic approach and clinical relevance. Australas J. Ultrasound Med. 2018, 21, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.S.; Sayed-Ahmed, E.Y.; Kroeker, C.A.G.; Sun, Y.-H.; Ter Keurs, H.E.D.J.; Shrive, N.G.; Tyberg, J.V. Compression of interventricular septum during right ventricular pressure loading. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H2639–H2648. [Google Scholar] [CrossRef]
- Revanna, G.K.; Kunjunju, A.; Sehgal, A. Bronchopulmonary dysplasia associated pulmonary hypertension: Making the best use of bedside echocardiography. Prog. Pediatr. Cardiol. 2017, 46, 39–43. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Murzi, B.; Assanta, N.; Spadoni, I.; De Lucia, V.; Crocetti, M.; Cresti, A.; Gallotta, M.; Marotta, M.; et al. Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2014, 27, 1279–1292.e2. [Google Scholar] [CrossRef]
- Hansmann, G.; Sallmon, H.; Roehr, C.C.; Kourembanas, S.; Austin, E.D.; Koestenberger, M.; European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr. Res. 2021, 89, 446–455. [Google Scholar] [CrossRef] [PubMed]
- El-Khuffash, A.; Molloy, E.J. Are B-type natriuretic peptide (BNP) and N-terminal-pro-BNP useful in neonates? Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F320–F324. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.S.; Keller, T.; Kribs, A.; Hünseler, C. Diseases associated with prematurity in correlation with N-terminal pro-brain natriuretic peptide levels during the early postnatal life. Eur. J. Pediatr. 2023, 182, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart Br. Card Soc. 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Balanos, G.M.; Talbot, N.P.; Dorrington, K.L.; Robbins, P.A. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J. Appl. Physiol. Bethesda Md. 1985 2003, 94, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Ambalavanan, N.; Mourani, P. Pulmonary hypertension in bronchopulmonary dysplasia. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rios, D.I.; Joye, S.; Baczynski, M.; Rios, D.; Giesinger, R.E.; McNamara, P.J.; Jain, A. Cardiopulmonary physiological effects of diuretic therapy in preterm infants with chronic pulmonary hypertension. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2023, 43, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Yung, D.; Jackson, E.O.; Blumenfeld, A.; Redding, G.; DiGeronimo, R.; McGuire, J.K.; Riker, M.; Tressel, W.; Berkelhamer, S.; Eldredge, L.C. A multidisciplinary approach to severe bronchopulmonary dysplasia is associated with resolution of pulmonary hypertension. Front. Pediatr. 2023, 11, 1077422. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Abman, S.H. Oxygen Targets in Neonatal Pulmonary Hypertension: Individualized, “Precision-Medicine” Approach. Clin. Perinatol. 2024, 51, 77–94. [Google Scholar] [CrossRef]
- Balfour-Lynn, I.M.; Field, D.J.; Gringras, P.; Hicks, B.; Jardine, E.; Jones, R.C.; Magee, A.G.; Primhak, R.A.; Samuels, M.P.; Shaw, N.J.; et al. BTS guidelines for home oxygen in children. Thorax 2009, 64 (Suppl. S2), ii1–ii26. [Google Scholar] [CrossRef]
- Balink, S.; Onland, W.; Vrijlandt, E.J.L.E.; Andrinopoulou, E.-R.; Bos, A.F.; Dijk, P.H.; Goossens, L.; Hulsmann, A.R.; Nuytemans, D.H.; Reiss, I.K.M.; et al. Supplemental oxygen strategies in infants with bronchopulmonary dysplasia after the neonatal intensive care unit period: Study protocol for a randomised controlled trial (SOS BPD study). BMJ Open 2022, 12, e060986. [Google Scholar] [CrossRef]
- Webb, M.K.; Guaman, M.C.; Tejtel, S.K.S.; Cambronero, N.; Coleman, R.D.; Chartan, C.A.; Furtun, B.Y.; Morris, S.A.; Varghese, N.P.; Villafranco, N.M. Atrial septal defect closure is associated with improved clinical status in patients ≤ 10 kg with bronchopulmonary dysplasia. Pulm. Circ. 2023, 13, e12299. [Google Scholar] [CrossRef]
- Kumar, K.R.; Clark, D.A.; Kim, E.M.; Perry, J.D.; Wright, K.; Thomas, S.A.; Thompson, E.J.; Greenberg, R.G.; Smith, P.B.; Benjamin, D.K.; et al. Association of Atrial Septal Defects and Bronchopulmonary Dysplasia in Premature Infants. J. Pediatr. 2018, 202, 56–62.e2. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Ruoss, J.L.; Stanford, A.H.; Lakshminrusimha, S.; McNamara, P.J. Hemodynamic consequences of respiratory interventions in preterm infants. J. Perinatol. 2022, 42, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H.; Collaco, J.M.; Shepherd, E.G.; Keszler, M.; Cuevas-Guaman, M.; Welty, S.E.; Truog, W.E.; McGrath-Morrow, S.A.; Moore, P.E.; Rhein, L.M.; et al. Interdisciplinary Care of Children with Severe Bronchopulmonary Dysplasia. J. Pediatr. 2017, 181, 12–28.e1. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.; Guimarães, H.; Pereira-da-Silva, L. The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach. Int. J. Environ. Res. Public Health 2021, 18, 6245. [Google Scholar] [CrossRef]
- Miller, A.N.; Curtiss, J.; Kielt, M.J. Nutritional Needs of the Infant with Bronchopulmonary Dysplasia. NeoReviews 2024, 25, e12–e24. [Google Scholar] [CrossRef] [PubMed]
- Bonadies, L.; Cavicchiolo, M.E.; Priante, E.; Moschino, L.; Baraldi, E. Prematurity and BPD: What general pediatricians should know. Eur. J. Pediatr. 2023, 182, 1505–1516. [Google Scholar] [CrossRef]
- Wang, L.J.; Hu, Y.; Wang, W.; Zhang, C.Y.; Bai, Y.Z.; Zhang, S.C. Gastroesophageal Reflux Poses a Potential Risk for Late Complications of Bronchopulmonary Dysplasia: A Prospective Cohort Study. Chest 2020, 158, 1596–1605. [Google Scholar] [CrossRef]
- Akinola, E.; Rosenkrantz, T.S.; Pappagallo, M.; McKay, K.; Hussain, N. Gastroesophageal reflux in infants < 32 weeks gestational age at birth: Lack of relationship to chronic lung disease. Am. J. Perinatol. 2004, 21, 57–62. [Google Scholar] [CrossRef]
- Mendes, T.B.; Mezzacappa, M.A.M.S.; Toro, A.A.D.C.; Ribeiro, J.D. Risk factors for gastroesophageal reflux disease in very low birth weight infants with bronchopulmonary dysplasia. J. Pediatr. 2008, 84, 154–159. [Google Scholar] [CrossRef]
- Nobile, S.; Noviello, C.; Cobellis, G.; Carnielli, V.P. Are Infants with Bronchopulmonary Dysplasia Prone to Gastroesophageal Reflux? A Prospective Observational Study with Esophageal pH-Impedance Monitoring. J. Pediatr. 2015, 167, 279–285.e1. [Google Scholar] [CrossRef]
- Radford, P.J.; Stillwell, P.C.; Blue, B.; Hertel, G. Aspiration complicating bronchopulmonary dysplasia. Chest 1995, 107, 185–188. [Google Scholar] [CrossRef]
- Varghese, N.; Rios, D. Pulmonary Hypertension Associated with Bronchopulmonary Dysplasia: A Review. Pediatr. Allergy Immunol. Pulmonol. 2019, 32, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Bamat, N.A.; Kirpalani, H.; Feudtner, C.; Jensen, E.A.; Laughon, M.M.; Zhang, H.; Monk, H.M.; Passarella, M.; Lorch, S.A. Medication use in infants with severe bronchopulmonary dysplasia admitted to United States children’s hospitals. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2019, 39, 1291–1299. [Google Scholar] [CrossRef]
- Lewis, T.; Truog, W.; Nelin, L.; Napolitano, N.; McKinney, R.L. Pharmacoepidemiology of Drug Exposure in Intubated and Non-Intubated Preterm Infants With Severe Bronchopulmonary Dysplasia. Front. Pharmacol. 2021, 12, 695270. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Levy, P.T.; El-Khuffash, A.; Gu, H.; Hamvas, A.; Singh, G.K. Left Ventricle Phenotyping Utilizing Tissue Doppler Imaging in Premature Infants with Varying Severity of Bronchopulmonary Dysplasia. J. Clin. Med. 2021, 10, 2211. [Google Scholar] [CrossRef]
- Fike, C.D.; Aschner, J.L. Pharmacotherapy for Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia: Past, Present, and Future. Pharmaceuticals 2023, 16, 503. [Google Scholar] [CrossRef] [PubMed]
- Duong-Quy, S.; Hua-Huy, T.; Pham, H.; Tang, X.; Mercier, J.C.; Baud, O.; Dinh-Xuan, A.T. Early inhaled nitric oxide at high dose enhances rat lung development after birth. Nitric Oxide Biol. Chem. 2014, 38, 8–16. [Google Scholar] [CrossRef]
- Jeremiasen, I.; Naumburg, E.; Westöö, C.; GWeismann, C.; Tran-Lundmark, K. Vasodilator therapy for pulmonary hypertension in children: A national study of patient characteristics and current treatment strategies. Pulm. Circ. 2021, 11, 20458940211057892. [Google Scholar] [CrossRef]
- Dillon, K.; Lamba, V.; Philip, R.R.; Weems, M.F.; Talati, A.J. Efficacy of Sildenafil in Infants with Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension. Children 2023, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Darland, L.K.; Dinh, K.L.; Kim, S.; Placencia, J.L.; Varghese, N.P.; Ruiz, F.; Mallory, G.B.; Fernandes, C.J. Evaluating the safety of intermittent intravenous sildenafil in infants with pulmonary hypertension. Pediatr. Pulmonol. 2017, 52, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Bailey, M.; Spears, T.; Esther, C.R.; Laughon, M.M.; Hornik, C.P.; Jackson, W. Safety of sildenafil in premature infants with severe bronchopulmonary dysplasia (SILDI-SAFE): A multicenter, randomized, placebo-controlled, sequential dose-escalating, double-masked, safety study. BMC Pediatr. 2020, 20, 559. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.E.; Hornik, C.D.; Martz, K.; Jacangelo, J.; Anand, R.; Greenberg, R.; Hornik, C.; Zimmerman, K.; Smith, P.B.; Benjamin, D.K.; et al. Safety of sildenafil in premature infants at risk of bronchopulmonary dysplasia: Rationale and methods of a phase II randomized trial. Contemp. Clin. Trials Commun. 2022, 30, 101025. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Yi, C.; Liu, T.; Lei, W.; Hung, J.; Liu, C.; Chen, C. Effects of phosphodiesterase-5 inhibitor sildenafil on esophageal secondary peristalsis: Studies with high-resolution manometry. J. Gastroenterol. Hepatol. 2021, 36, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Donda, K.; Zambrano, R.; Moon, Y.; Percival, J.; Vaidya, R.; Dapaah-Siakwan, F.; Luo, S.; Duncan, M.R.; Bao, Y.; Wang, L.; et al. Riociguat prevents hyperoxia-induced lung injury and pulmonary hypertension in neonatal rats without effects on long bone growth. PLoS ONE 2018, 13, e0199927. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, S.; Ishida, H.; Suginobe, H.; Tsuru, H.; Wang, R.; Yoshihara, C.; Ueyama, A.; Narita, J.; Ishii, R.; Kogaki, S.; et al. Riociguat can ameliorate bronchopulmonary dysplasia in the SU5416 induced rat experimental model. Exp. Lung Res. 2021, 47, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Torok, R.D.; Gardner, R.A.; Barker, P.C.; McCrary, A.W.; Li, J.S.; Hornik, C.P.; Laughon, M.M.; Jackson, W.M. Correlating Severity of Pulmonary Hypertension by Echocardiogram with Mortality in Premature Infants with Bronchopulmonary Dysplasia. Am. J. Perinatol. 2024. [Google Scholar] [CrossRef]
- Avitabile, C.M.; Zhang, X.; Ampah, S.B.; Wang, Y.; Ash, D.; Nilan, K.; Mercer-Rosa, L.; Fierro, J.L.; Frank, D.B.; Gibbs, K.A. Factors associated with discontinuation of pulmonary vasodilator therapy in children with bronchopulmonary dysplasia-associated pulmonary hypertension. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2022, 42, 1246–1254. [Google Scholar] [CrossRef]
- Tracy, M.K.; Berkelhamer, S.K. Bronchopulmonary Dysplasia and Pulmonary Outcomes of Prematurity. Pediatr. Ann. 2019, 48, e148–e153. [Google Scholar] [CrossRef]
- Goss, K.N.; Beshish, A.G.; Barton, G.P.; Haraldsdottir, K.; Levin, T.S.; Tetri, L.H.; Battiola, T.J.; Mulchrone, A.M.; Pegelow, D.F.; Palta, M.; et al. Early Pulmonary Vascular Disease in Young Adults Born Preterm. Am. J. Respir. Crit. Care Med. 2018, 198, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.B.; Bates, A.; Mullen, M.P.; Abman, S.H.; Austin, E.D.; Everett, A.; Fineman, J.; Feinstein, J.; Hopper, R.K.; Kinsella, J.P.; et al. Cardiac Catheterization and Hemodynamics in a Multicenter Cohort of Children with Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2022, 19, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
| Estimation of Pulmonary Pressures | |
|---|---|
| RVSP estimation by tricuspid regurgitant jet velocity | Concern of PH if the RVSP > 40 mmHg by the TR jet (TR jet with an RV–RA gradient of >35 mmHg, assuming an RA pressure of 5 mmHg). RVSP > 1/2 of systemic pressure is concerning for abnormal pulmonary pressure. About 60% of echocardiograms may have a quantifiable TR jet with a full envelope. Some use the cutoff of the TR jet > 33.6 mmHg) [12,22]. |
| Mean and diastolic PAP by pulmonary insufficiency jet | Often not available in BPD-PH scans. However, when available, it provides estimates of PAP during the diastolic phase of the cardiac cycle [24,25]. (mPAP = 4 × [early diastolic PI velocity]2 + estimated RA pressure) |
| Gradient and directionality through restrictive PDA or VSD | May inform on systemic-to-pulmonary relationship based on directionality. Velocity gradient may inform on sPAP and dPAP when compared to systemic blood pressure at the time of the echocardiogram. Equalization of pressures occurs with unrestrictive shunts—limiting the interpretation of underlying PVR [12,24]. |
| Pulmonary artery acceleration time/right ventricular ejection time (PAAT/RVET) | This ratio provides some insight on the RV afterload. In a situation where the RV afterload is increased (either due to high pulmonary vascular resistance or other contributors—flow/pressure transmission), this ratio decreases. Ratio is measured from the pulsed wave (PW) Doppler envelope of the right ventricular outflow tract. A low ratio suggests an increased pulmonary afterload (abnormal < 0.31; some use a cutoff of <0.25) [26,27,28]. |
| LV eccentricity index at peak systole | The left ventricular (LV) end-systolic eccentricity index provides a quantifiable metric of septal deformation. The index is computed as the ratio of the diameter parallel to the septum to the diameter perpendicular to the septum at peak of systole. In a situation where there is a flat septal configuration or a bowing septum, this ratio will decrease. This provides a continuous quantifiable metric of the “septal motion.” In the absence of a congenital cardiac anomaly, ventricles will equalize pressure with their corresponding outflow tract at the peak of systole. As such, the RV–LV relationship may inform on the systemic-to-pulmonary systolic pressure relationship. In the expected setting, the LV systolic pressure should be above the RV systolic pressure, and the LV should form a near-perfect circular configuration at the peak of systole. The left ventricular (LV) end-systolic eccentricity index (EI) ≥ 1.3 has been associated with PH in BPD infants [29,30]. |
| LV septal motion | Septal flattening (or bowing toward LV) at peak of systole indicates an increased RV–LV systolic pressure relationship. Flattening concerning for systolic PA pressure is greater than 50% systemic pressure [31]. |
| Evaluation of RV Function/Dimensions | |
| TAPSE | Tricuspid annular plane systolic excursion (TAPSE) is a marker evaluating RV systolic function using the M-Mode tracking motion of the tricuspid valve (line of interrogation crossing the apex and attachment of the tricuspid valve to the RV-free wall). It estimates the longitudinal displacement of the tricuspid valve from peak diastole to peak systole. Low values (by age) indicate RV dysfunction [32,33,34]. |
| RV-FAC | FAC is calculated after obtaining the end diastolic (EDA) and the end systolic area (ESA) of the RV (FAC = [EDA − ESA]/EDA), and also provides an important marker of RV function. Normative values have been published (although normal FAC values quoted to be most common when >35%) [35,36,37]. |
| RV-MPI by TDI | Evaluates the RV myocardial performance index using tissue Doppler imaging. Combined marker of RV systolic and diastolic performance [35]. |
| RV output estimation | Assesses RV stroke volume and cardiac output. Values < 150 mL/kg/min are of concern [26,38]. |
| RV E/A ratio | Assesses RV diastolic function [37]. |
| RV S’ by TDI | Measures RV systolic velocity using tissue Doppler imaging (peak longitudinal contraction velocity). May be decreased in the context of systolic dysfunction [37,39,40]. |
| RV E/E’ by TDI | Estimates RV filling/diastolic function [37,39,40]. |
| RV EDA | Evaluates RV end-diastolic area [37]. |
| RV/LV | RV/LV ratio > 1 at peak of systole in parasternal short axis is concerning for RV dilation [24,41]. |
| RV longitudinal strain | Speckle-tracking echocardiography allows for assessment of RV longitudinal deformation during contraction. Associated with later mortality in those with BPD-PH diagnosis. Normative values have been published by age and vendor [32,33,42,43]. |
| Evaluation of LV Function/Dimensions | |
| Shortening fraction | May be computed from the 2D or Motion-Modes. Ratio between the end diastolic and peak systolic diameters of the internal cavity of the LV at the tip of the mitral valve. Concern with angle of image acquisition and assessment of partial/segmental LV function. Normal: 28–46% [44]. |
| EF by biplane | Standard measure of LV ejection fraction. Assumes a mathematical bullet-shaped LV, which may not be true in the context of adverse septal motion. Normal > 55% [44,45]. |
| EF by 5/6 area length | Alternative measure of LV ejection fraction. Normal > 55% [46]. |
| LV-EDV | LV end-diastolic volume assessment [44,46]. |
| LV mass | Assesses LV hypertrophy, potentially leading to a decreased LV compliance and an increased end-diastolic pressure [47]. |
| LV output estimate | Evaluates LV stroke volume and cardiac output. Values < 150 mL/kg/min are of concern [26]. |
| LV S’ by TDI | Measures LV systolic velocity using tissue Doppler imaging (peak longitudinal contraction velocity). May be decreased in the context of systolic dysfunction [39]. |
| LV E/E’ (free wall and septal) | Estimates LV filling pressures [39]. |
| LV E/A | Assesses LV diastolic function [48]. |
| LV longitudinal or circumferential strain | Speckle-tracking echocardiography allows for assessment of LV longitudinal and circumferential deformation during contraction. In healthy children, the mean LV global longitudinal strain is −20% (95% CI, −19.5% to −21%) and the mean global circumferential strain is −22% (95% CI, −20% to −25%) [33,42,43]. |
| Evaluation of Shunts | |
| Atrial shunt evaluation | A bidirectional or right-to-left shunt suggests higher right-sided atrial pressure (often secondary to RV diastolic dysfunction). These patients may have concomitant hepatomegaly and dilated inferior vena cava and subhepatic veins (with occasionally >50% retrograde flow by pulse-wave Doppler) [26,28,49]. |
| Post-tricuspid shunt evaluation | A bidirectional or right-to-left shunt provides information on the pressure relationship between the pulmonary and systemic sides; unrestrictive shunts lead to equalized systolic pressure [26,28,49]. |
| Assessment for Concomitant Anomalies | |
| Pulmonary veins | Assessment of pulmonary venous flow at each ostium to rule out signs of pulmonary veins stenosis (mean gradient < 4 mmHg, biphasic/triphasic flow) [50,51]. |
| Pulmonary valve, main pulmonary artery and right/left pulmonary arteries | Evaluation for signs of RV outflow tract obstruction, or stenosis/obstruction in the pulmonary arteries [10]. |
| Future Parameters in Investigation | |
| LV-EF by 3D echocardiography | 3D-volume capture of the LV in order to estimate LV dimensions and function by ejection fraction (or strain). Limited data are available in the neonatal population [52]. |
| RV-EF by 3D echocardiography | Detailed RV ejection fraction assessment using 3D-echocardiography and modeling. Advanced techniques are often challenging in preterm infants and require specific expertise, equipment, and assessment tools [53]. |
| Blood speckle-tracking | Assessment of vortex formation in the LV and RV. Estimation of transcavitary pressure gradients [54]. |
| Myocardial work assessment | Incorporates markers of left or right ventricular afterload into strain analysis [55]. |
| Metric | Echocardiography Image Example |
|---|---|
| Pulmonary insufficiency jet | 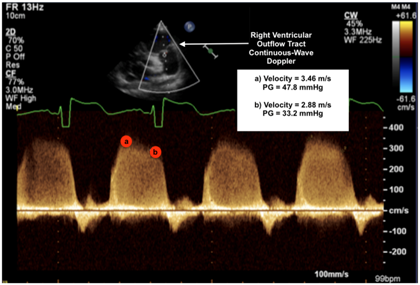 |
| Legend: Mean pulmonary arterial pressure (mPAP): Peak/early diastolic velocity estimates the PA–RV (pulmonary artery to right ventricle) gradient in early diastole to be 32.59 mmHg using the modified Bernoulli equation (4 × velocity2). As such, mPAP is estimated as 47.8 mmHg (4 × 3.462) + expected right atrial (RA) pressure (~5 mmHg) = 52.8 mmHg [24]. | |
| Diastolic pulmonary arterial pressure (dPAP): End-diastolic velocity estimates the PA–RV gradient in late diastole to be 33.2 mmHg using the modified Bernoulli equation. As such, dPAP is estimated as 33.2 mmHg + expected RA pressure = 38.2 mmHg [24]. | |
| TAPSE—tricuspid annular plane systolic execution | 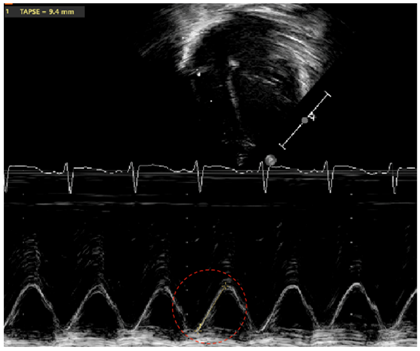 |
| Legend: Marker of longitudinal systolic function of the right ventricle (RV) (which primarily contracts longitudinally). The M(motion)-Mode is used with the line of interrogation passing through the attachment of the tricuspid valve at the level of the free wall of the RV, and through the RV apex. The distance travelled from end-diastole to peak of systole is measured by following the line of the attachment of the tricuspid valve on the M-Mode tracing through time (red circle). Occasionally, superimposed tissue Doppler allows to follow the period of systole with increased precision. Z-scores have been published by gestational age/postmenstrual age, as well as chronological age for term infants [34,56]. | |
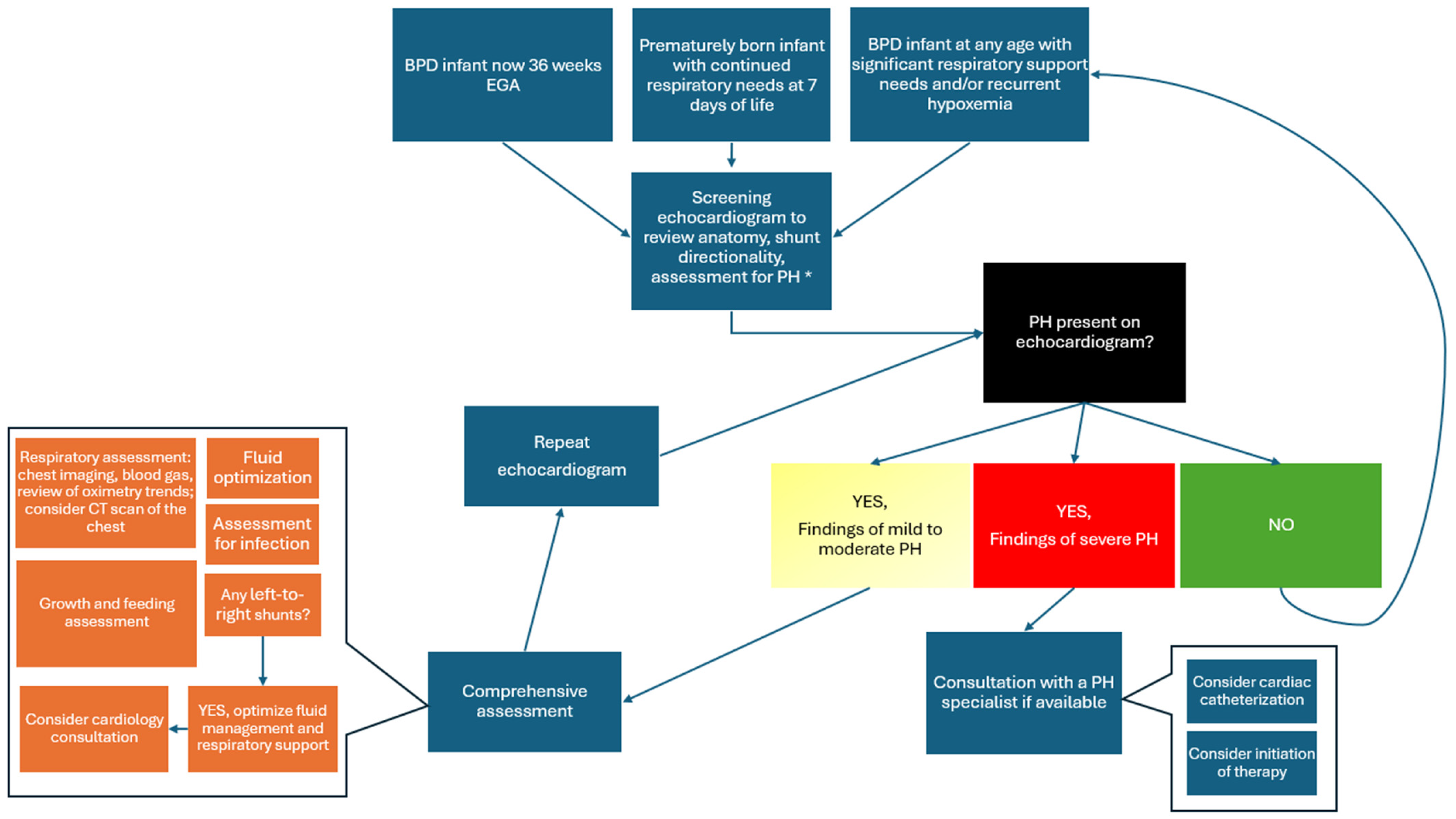
| Tricuspid regurgitation jet | 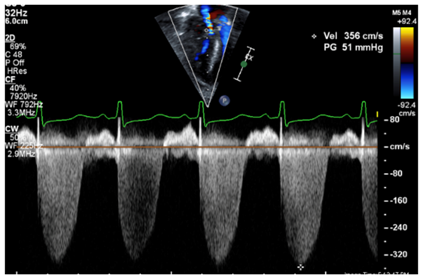 |
| Legend: The tricuspid regurgitant jet velocity provides information on the velocity of flow during systole from the RV to the RA. This allows to estimate, using the Bernoulli equation, the pressure gradient between the RV and the RA. A full envelope is generated when the line of interrogation is parallel to the tricuspid regurgitant jet. With an estimated RA pressure (typically 0–5 mmHg in a normal setting, but likely further increased in RV diastolic impairment), one is able to estimate the peak systolic RV pressure. Assuming that the RV and pulmonary arterial pressure are equalized at the peak of systole, one may infer the peak systolic pulmonary arterial pressure. The velocity measurement should be performed along the contour of the spectral Doppler envelope, avoiding overestimation of the measurement. In this case, the peak systolic RV–RA gradient has a velocity of 3.56 m/s, providing an estimated RV–RA pressure gradient of 51 mmHg (4 × 3.562). With an RA pressure of 5 mmHg, this provides a peak systolic RV pressure estimated at 56 mmHg (abnormal > 40 mmHg). | |
| Fractional area of change by RV | 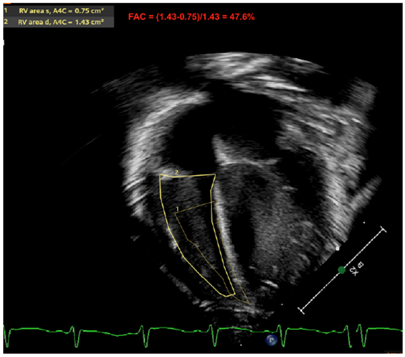 |
| Legend: The fractional area change of the RV is another marker used to estimate RV systolic function. The contour at the endocardial border is traced at the peak of systole and at the peak of diastole. The fraction ([RV End Diastolic Area] − [RV End Systolic Area])/[RV End Diastolic Area] is expressed in % of area shortening. An apical RV focused view is used. This marker may be calculated in the apical four-chamber view. Some reports have also used the RV inflow–outflow view (or RV three-chamber view, or RV “tet” view—which outlines the RV anterior and inferior walls) to compute this metric, although the American Society of Echocardiography officially recommends its evaluation in apical four-chamber view—which outlines the RV free wall and RV septum. Here, the RV-FAC is 47.6%, which is considered normal (>35%) | |
| RV E/A ratio |  |
| Legend: E (early filling velocity), A (late/atrial contraction filling velocity). This metric is used in the pediatric literature to evaluate diastolic performance. Data are lacking regarding the newborn. However, under normal circumstances at a few weeks of life, it is expected that the RV compliance is now increased (compared to the early postnatal period) and that the filling in the early phase (passive) occurs at a higher velocity than during the atrial contraction. Here, the ratio is >1.0, indicating that the E>A (0.62 m/s > 0.50 m/s)—which is considered normal. | |
| PAAT/RVET | 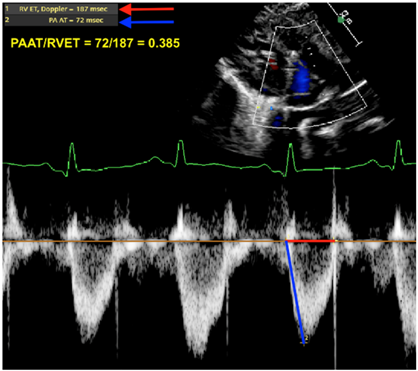 |
| Legend: The pulmonary artery acceleration time to the right ventricular ejection time is a ratio providing some insight on the RV afterload. In situations where the RV afterload is increased (either due to high pulmonary vascular resistance or other contributors—flow/pressure transmission), this ratio decreases. The pulsewave Doppler envelope of the RV outflow tract shifts from a parabolic shape to a more triangular shape with a steeper diastolic upstroke. Here, the ratio is 0.385 (abnormal < 0.31; some use a cutoff of <0.25). | |
| Eccentricity index | 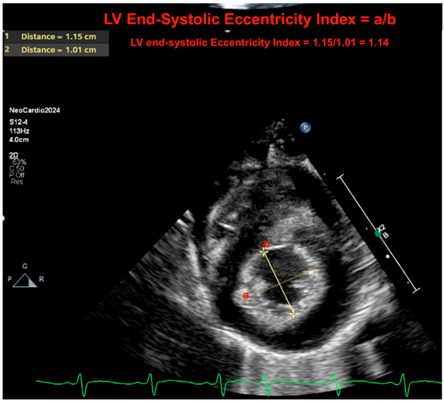 |
| Legend: The LV end-systolic eccentricity index provides a quantifiable metric of septal deformation. The index is computed as the ratio of the diameter parallel to the septum to the diameter perpendicular to the septum at peak of systole. In situations where there is a flat septal configuration or a bowing septum, this ratio will decrease. This provides a continuous quantifiable metric of the “septal motion.” In the absence of a congenital cardiac anomaly, ventricles will equalize pressure with their corresponding outflow tract at the peak of systole. As such, the RV–LV relationship may inform on the systemic to pulmonary systolic pressure relationship. In the expected setting, the LV systolic pressure should be below the RV systolic pressure, and the LV should form a near-perfect circular configuration at the peak of systole. Here, the ratio is 1.14 (normal if <1.3). Letters a = 1; b = 2. The equation is a/b. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varghese, N.P.; Altit, G.; Gubichuk, M.M.; Siddaiah, R. Navigating Diagnostic and Treatment Challenges of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia. J. Clin. Med. 2024, 13, 3417. https://doi.org/10.3390/jcm13123417
Varghese NP, Altit G, Gubichuk MM, Siddaiah R. Navigating Diagnostic and Treatment Challenges of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia. Journal of Clinical Medicine. 2024; 13(12):3417. https://doi.org/10.3390/jcm13123417
Chicago/Turabian StyleVarghese, Nidhy P., Gabriel Altit, Megan M. Gubichuk, and Roopa Siddaiah. 2024. "Navigating Diagnostic and Treatment Challenges of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia" Journal of Clinical Medicine 13, no. 12: 3417. https://doi.org/10.3390/jcm13123417
APA StyleVarghese, N. P., Altit, G., Gubichuk, M. M., & Siddaiah, R. (2024). Navigating Diagnostic and Treatment Challenges of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia. Journal of Clinical Medicine, 13(12), 3417. https://doi.org/10.3390/jcm13123417






