Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios
Abstract
1. Introduction
2. Materials and Methods
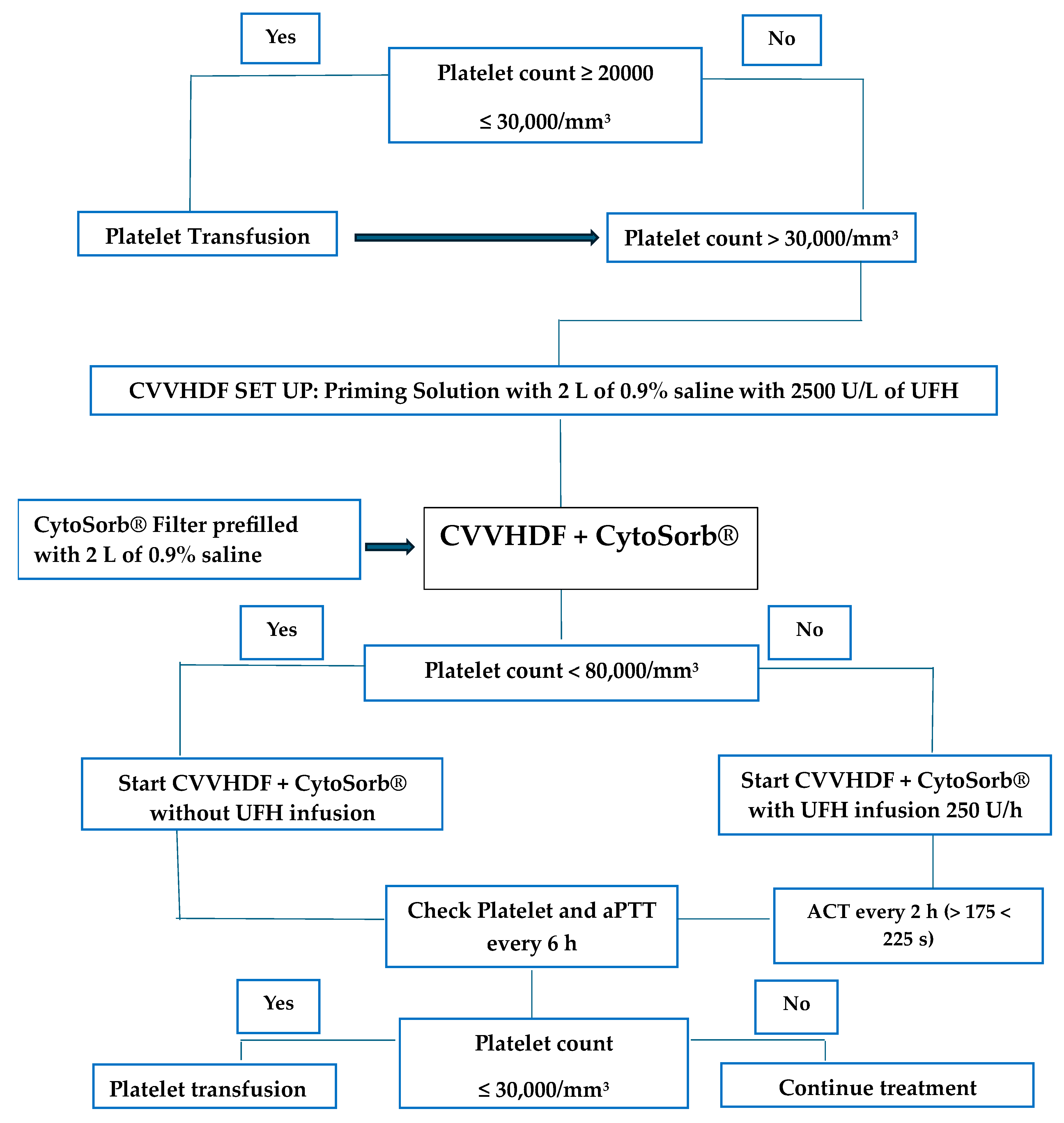
2.1. Extracorporeal Treatment Procedure
2.2. Data Collection
2.3. Statistical Analysis
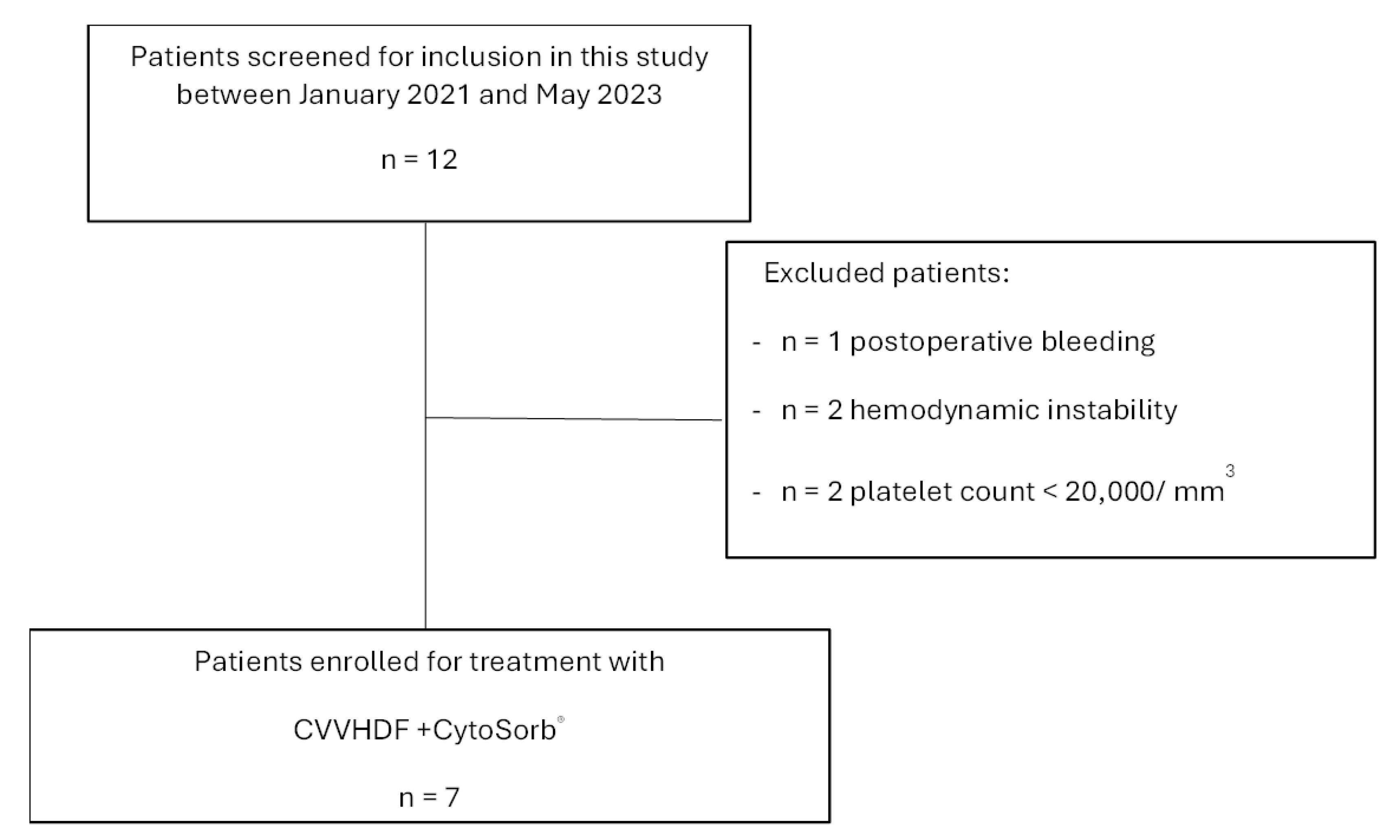
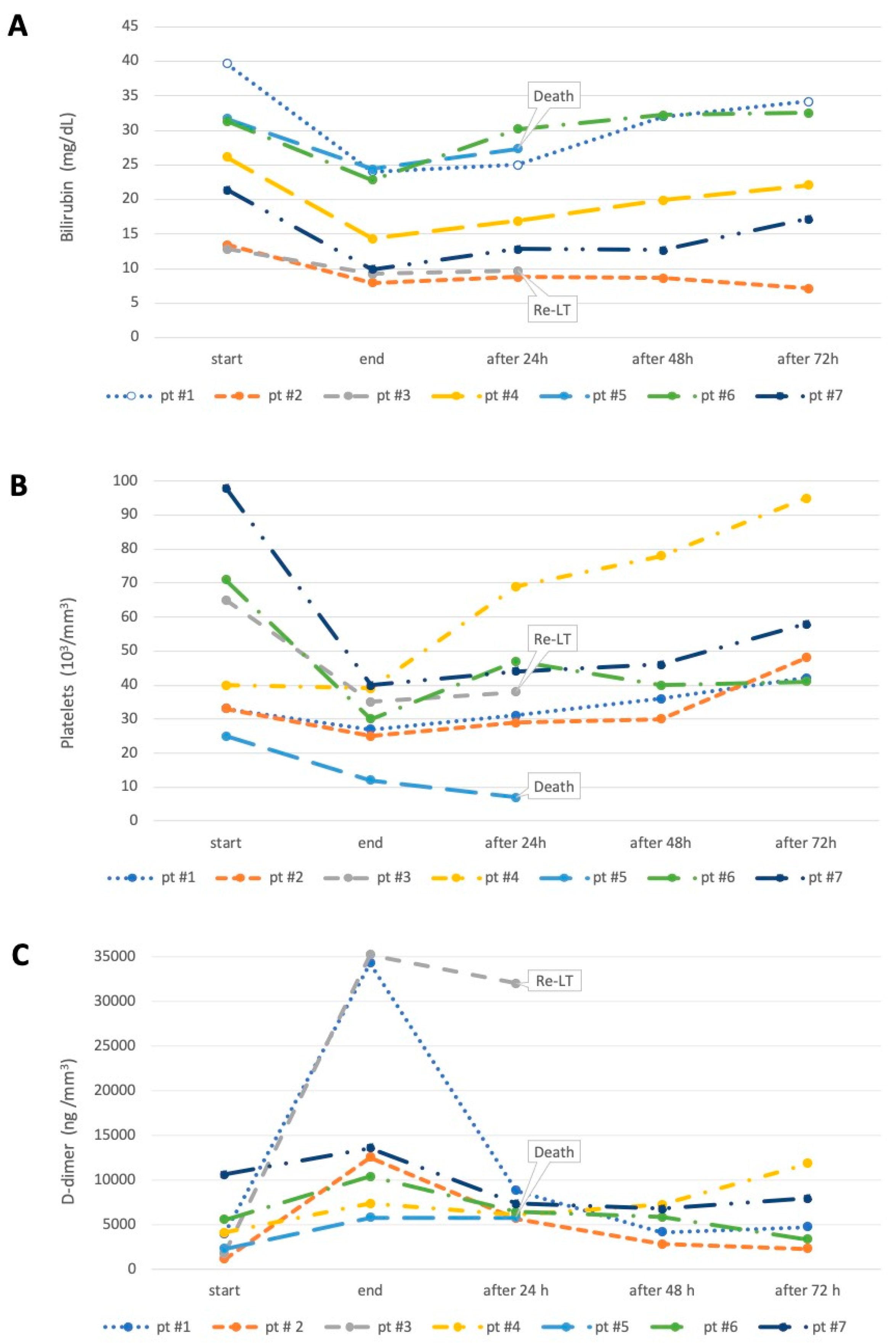
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moosburner, S.; Wiering, L.; Roschke, N.N.; Winter, A.; Demir, M.; Gaßner, J.M.G.V.; Zimmer, M.; Ritschl, P.; Globke, B.; Lurje, G.; et al. Validation of risk scores for allograft failure after liver transplantation in Germany: A retrospective cohort analysis. Hepatol. Commun. 2023, 7, e0012. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, H.E.; Kunz, D.; Yagmur, E.; Timmer-Stranghöner, A.; Vidacek, D.; Siewert, E.; Bach, J.; Geier, A.; Purucker, E.A.; Gressner, A.M.; et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J. Hepatol. 2005, 42, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Mirza, S.; Javed, N.; Hanif, H.; Ryu, M.; Mirza, R.T.; Sheikh, A.B. Extracorporeal Liver Support: An Updated Review of Mechanisms and Current Literature. J. Community Hosp. Intern. Med. Perspect. 2022, 12, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, R.; Cavaliere, F.; Sollazzi, L.; Perilli, V.; Melchionda, I.; Agnes, S.; Gasbarrini, A.; Avolio, A.W. Molecular adsorbent recirculating system (Mars) in patients with primary nonfunction and other causes of graft dysfunction after liver transplantation in the era of extended criteria donor organs. Transplant. Proc. 2009, 41, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bañares, R.; Nevens, F.; Larsen, F.S.; Jalan, R.; Albillos, A.; Dollinger, M.; Saliba, F.; Sauerbruch, T.; Klammt, S.; Ockenga, J.; et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: The RELIEF trial. J. Hepatol. 2013, 57, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Bonavia, A.; Groff, A.; Karamchandani, K.; Singbartl, K. Clinical utility of extracorporeal cytokine hemadsorption therapy: A review of the literature. Blood. Purif. 2018, 46, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Dominik, A.; Stange, J. Similarities; Differences; and Potential Synergies in the Mechanism of Action of Albumin Dialysis Using the MARS Albumin Dialysis Device and the CytoSorb Hemoperfusion Device in the Treatment of Liver Failure. Blood. Purif. 2021, 50, 119–128. [Google Scholar] [CrossRef]
- Ocskay, K.; Tomescu, D.; Faltlhauser, A.; Jacob, D.; Friesecke, S.; Malbrain, M.; Kogelmann, K.; Bogdanski, R.; Bach, F.; Fritz, H.; et al. Hemoadsorption in ‘Liver Indication’-Analysis of 109 Patients’ Data from the CytoSorb International Registry. J. Clin. Med. 2021, 10, 5182. [Google Scholar] [CrossRef]
- Tomescu, D.; Popescu, M.; David, C.; Sima, R.; Dima, S. Haemoadsorption by CytoSorb® in patients with acute liver failure: A case series. Int. J. Artif. Organs 2021, 44, 560–564. [Google Scholar] [CrossRef]
- Avolio, A.W.; Lai, Q.; Cillo, U.; Romagnoli, R.; De Simone, P. L-GrAFT and EASE scores in liver transplantation: Need for reciprocal external validation and comparison with other scores. J. Hepatol. 2021, 75, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care. Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. J. Hepatol. 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy definition; nomenclature; diagnosis; and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. J. Hepatol. 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Lang, H.; Vollmer Barbosa, C.; Tian, Z.; Melk, A.; Schmidt, B.M.W. Efficacy of CytoSorb®: A systematic review and meta-analysis. Crit. Care 2023, 27, 215. [Google Scholar] [CrossRef] [PubMed]
- Poli, E.C.; Rimmelé, T.; Schneider, A.G. Hemoadsorption with CytoSorb®. Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; David, C.; Marcu, A.; Olita, M.R.; Mihaila, M.; Tomescu, D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. J. Clin. Med. 2023, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Greimel, A.; Habler, K.; Gräfe, C.; Maciuga, N.; Brozat, C.I.; Vogeser, M.; Zoller, M.; Happich, F.L.; Liebchen, U.; Frank, S.; et al. Extracorporeal adsorption of protective and toxic bile acids and bilirubin in patients with cholestatic liver dysfunction: A prospective study. Ann. Intensive Care 2023, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Turan, C.; Szigetváry, C.E.; Kói, T.; Engh, M.A.; Atakan, I.; Zubek, L.; Terebessy, T.; Hegyi, P.; Molnár, Z. Hemoad-sorption Therapy for Critically Ill Patients with Acute Liver Dysfunction: A Meta-Analysis and Systematic Review. Biomedicines 2023, 12, 67. [Google Scholar] [CrossRef]
- Dhokia, V.D.; Madhavan, D.; Austin, A.; Morris, C.G. Novel use of Cytosorb™ haemadsorption to provide biochemical control in liver impairment. J. Intensive Care Soc. 2019, 20, 174–181. [Google Scholar] [CrossRef]
- de Boer, M.T.; Christensen, M.C.; Asmussen, M.; van der Hilst, C.S.; Hendriks, H.G.; Slooff, M.J.; Porte, R.J. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth. Analg. 2008, 106, 32–44. [Google Scholar] [CrossRef]
- Pereboom, I.T.A.; De Boer, M.T.; Haagsma, E.B.; Hendriks, H.G.D.; Lisman, T.; Porte, R.J. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth. Analg. 2009, 108, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Teofili, L.; Valentini, C.G.; Aceto, P.; Bartolo, M.; Sollazzi, L.; Agnes, S.; Gaspari, R.; Avolio, A.W. High intraoperative blood product requirements in liver transplantation: Risk factors and impact on the outcome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 64–75. [Google Scholar] [PubMed]
- Shiba, H.; Ishida, Y.; Fujiwara, Y.; Wakiyama, S.; Gocho, T.; Ito, R.; Sakamoto, T.; Tsutsui, N.; Lida, T.; Matsumoto, M.; et al. Practice to minimize the use of blood products improve outcome after hepatic resection for hepatocellular carcinoma. Hepatogastroenterology 2013, 60, 1681–1683. [Google Scholar]
- Fiorelli, S.; Biancofiore, G.; Feltracco, P.; Lavezzo, B.; De Gasperi, A.; Pompei, L.; Masiero, L.; Testa, S.; Ricci, A.; Della Rocca, G.; et al. Acute kidney injury after liver transplantation, perioperative risk factors, and outcome: Prospective observational study of 1681 patients (OLTx Study). Minerva. Anestesiol. 2022, 88, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wei, X.; Yue, J.; Yang, Y.; Zhang, W.; Zhu, T. Impact of Perioperative Massive Transfusion on Long Term Outcomes of Liver Transplantation: A Retrospective Cohort Study. Int. J. Med. Sci. 2021, 18, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, R.; Spinazzola, G.; Aceto, P.; Avolio, A.W.; Delli Compagni, M.; Postorino, S.; Michi, T.; Fachechi, D.C.; Modoni, A.; Antonelli, M. Intensive Care Unit-Acquired Weakness after Liver Transplantation: Analysis of Seven Cases and a Literature Review. J. Clin. Med. 2023, 12, 7529. [Google Scholar] [CrossRef] [PubMed]
- Monet, C.; De Jong, A.; Aarab, Y.; Piron, L.; Prades, A.; Carr, J.; Belafia, F.; Chanques, G.; Guiu, B.; Pageaux, G.P.; et al. Adverse events, short- and long-term outcomes of extra corporeal liver therapy in the intensive care unit: 16 years experience with MARS® in a single center. Crit. Care 2022, 26, 282. [Google Scholar] [CrossRef]
- Toyoda, Y.; Tateno, K.; Takeda, Y.; Kobayashi, Y. Significance of mild thrombocytopenia in maintenance hemodialysis patients; a retrospective cohort study. Platelets 2022, 33, 735–742. [Google Scholar] [CrossRef]
- Daugirdas, J.T.; Bernardo, A.A. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney. Int. 2012, 82, 147–157. [Google Scholar] [CrossRef]
- Griffin, J.M.; Tariq, A.; Menez, S.; Kyeso, Y.; Chedid, A.; Ramakrishnan, V.; Schulman, S.P.; Sperati, C.J.; Choi, M.J.; McEvoy, J.W.; et al. Higher Prevalence of Concurrent Thrombocytopenia in Patients Receiving Continuous Renal Replacement Therapy in the Cardiac Intensive Care Unit. Blood. Purif. 2021, 50, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Johnson, D.W. The incidence of thrombocytopenia associated with continuous renal replacement therapy in critically ill patients. Ren. Fail. 2015, 37, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Guru, P.K.; Singh, T.D.; Akhoundi, A.; Kashani, K.B. Association of Thrombocytopenia and Mortality in Critically Ill Patients on Continuous Renal Replacement Therapy. Nephron 2016, 133, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Klingel, R.; Schaefer, M.; Schwarting, A.; Himmelsbach, F.; Altes, U.; Uhlenbusch-Körwer, I.; Hafner, G. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol. Dial. Transplant. 2004, 19, 164–170. [Google Scholar] [CrossRef]
- Dornia, C.; Philipp, A.; Bauer, S.; Stroszczynski, C.; Schreyer, A.G.; Müller, T.; Koehl, G.E.; Lehle, K. D-dimers Are a Predictor of Clot Volume Inside Membrane Oxygenators During Extracorporeal Membrane Oxygenation. Artif. Organs. 2015, 39, 782–787. [Google Scholar] [CrossRef]
| Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Age, years | 58 | 49 | 56 | 53 | 63 | 75 | 69 |
| Sex, male/female | Male | Male | Male | Male | Male | Male | Male |
| Body mass index, Kg/m2 | 26.9 | 23.2 | 21.9 | 22.5 | 21.5 | 27.7 | 21.5 |
| Etiology of liver disease | GD | GD | Severe GD | GD | GD | PHLF | PHLF |
| CytoSorb® session, N | 3 | 2 | 2 | 2 | 2 | 2 | 3 |
| RRT | No | Yes | Yes | Yes | Yes | No | No |
| Mechanical ventilation | No | No | Yes | Yes | Yes | No | No |
| Time from surgery, days | 7 | 5 | 2 | 10 | 26 | 17 | 23 |
| ° Parameter at T0/T1 | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
| HE grade | II/II | I/I | IV/IV | II/II | II/II | I/I | I/I |
| SOFA score | 9/10 | 8/7 | 18/17 | 10/10 | 14/14 | 7/9 | 6/6 |
| MELD score | 25/24 | 32/31 | 40/40 | 35/32 | 37/37 | 24/24 | 18/18 |
| Vasopressor therapy | no/no | no/no | yes/yes | no/no | yes/yes + | no/yes | no/no |
| Total bilirubin, mg/mL | 39.7/24.0 | 13.4/7.9 | 12.8/9.2 | 26.1/14.3 | 31.7/24.4 | 31.3/22.8 | 21.4/9.9 |
| Creatinine, mg/mL | 0.7/0.5 | 1.7/1.2 | 2.2/1.6 | 1.8/0.8 | 0.6/0.4 | 1.1/1.0 | 0.6/0.5 |
| Ammonia, µg/dL | 79/36 | 142/74 | 398/454 | 45/32 | 67/29 | 93/90 | 52/47 |
| Bile acid, μg/mL | - | 41.2/8.5 | - | - | - | 97/93 | - |
| Arterial lactate, mmol/L | 1.0/1.0 | 0.6/1.2 | 5.0/2.9 | 1.4/1.4 | 0.9/1.1 | 1.8/2.0 | 0.9/0.9 |
| AST, U/L | 40/25 | 29/37 | 1117/352 | 50/47 | 40/47 | 185/202 | 192/160 |
| ALT, U/L | 42/17 | 168/90 | 2296/793 | 52/13 | 13/13 | 107/103 | 238/150 |
| ALP, U/L | 94/78 | 163/93 | 97/87 | 179/148 | 500/435 | 110/112 | 286/224 |
| GGT, U/L | 117/66 | 183/53 | 59/41 | 158/127 | 71/64 | 108/100 | 161/135 |
| Hemoglobin, g/dL | 9.2/8.0 ^ | 9.1/9.0 | 9.3/8.2 | 8.4/8.1 | 11.0/11.2 | 9.5/9.1 | 8.5/8.3 ^ |
| Platelets count, ×103/mm3 | 33/27 * | 33/25 * | 65/35 * | 40/39 * | 25/12 * | 71/30 * | 98/65 |
| Leucocytes, ×103/mm3 | 12.7/19.5 | 2.3/3.4 | 9.3/11.8 | 5.8/6.2 | 6.2/3.5 | 15.0/9.3 | 10.3/5.9 |
| INR | 1.5/1.7 | 1.3/1.4 | 3.1/3.5 | 1.2/1.3 | 1.5/1.6 | 1.4/1.6 | 1.0/1.3 |
| aPTT, s | 40.7/83.7 | 33.7/52.2 | 60.2/91.0 | 48.0/49.2 | 70.5/76.6 | 67.0/93.0 | 47.9/58.2 |
| D-dimer, ng/mm3 | 3926/ 34,308 | 1076/ 12,508 | 1760/ 35,200 | 4084/ 7336 | 2263/ 5732 | 5523/ 10,354 | 10,605/ 13,572 |
| Fibrinogen, mg/mm3 | 201/180 | 190/195 | 179/166 | 379/326 | 403/354 | 261/226 | 597/518 |
| Interleukine-6, pg/mL | - | - | - | - | - | 15.9/29.3 | 94.2/59.1 |
| 28 days outcome | Alive | Alive | Alive re-LT | Alive | Dead | Dead | Alive |
| Variable | Pre-Treatment | Post-Treatment | p |
|---|---|---|---|
| Creatinine, mg/mL | 1.24 (0.6–1.8) | 0.86 (0.5–1.2) | 0.006 |
| Urea nitrogen, mg/dL | 45 (22.6–66.1) | 30 (14.3–45.3) | 0.01 |
| Arterial lactate, mmol/L | 1.6 (0.6–2.3) | 1.37 (0.7–2.2) | 0.89 |
| AST, U/L | 233.4 (26.1–325.9) | 133.14 (12.3–253.9) | 0.52 |
| ALT, U/L | 404.0 (24.4–480.5) | 175 (21.3–273.7) | 0.11 |
| Total bilirubin, mg/mL | 25.2 (15.5–34.9) | 16.1 (9.1–22.9) | 0.001 |
| Conjugated bilirubin, mg/mL | 18.9 (11.5–26.5) | 12.3 (7.3–17.2) | 0.005 |
| GGT, U/L | 118.7 (75.8–162.2) | 87.43 (46.9–127.8) | 0.10 |
| ALP, U/L | 204.1 (96.2–302.0) | 168.2 (49.7–286.4) | 0.02 |
| Ammonia, µg/dL | 123.4 (45.3–184.5) | 122.17 (26.5–205.8) | 0.10 |
| Hemoglobin, g/dL | 9.3 (8.5–10.0) | 9.5 (8.3–10.6) | 0.68 |
| Platelets count, ×103/mm3 | 49.4 (28.6–69.2) | 30.86 (20.8–40.8) | 0.02 |
| Leucocytes, ×103/mm3 | 8.8 (4.2–13.6) | 8.5 (3.2–13.8) | 0.70 |
| INR | 1.6 (1.0–2.0) | 1.8 (1.0–2.4) | 0.02 |
| aPTT, s | 52.57 (39.7–65.4) | 73.94 (54.1–93.7) | 0.01 |
| Fibrinogen, mg/mm3 | 315.7 (188.3–439.2) | 286.86 (174.1–399.5) | 0.16 |
| D-dimer, ng/mm3 | 4176 (1615–6631) | 17001 (5504–28,498) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspari, R.; Aceto, P.; Spinazzola, G.; Piervincenzi, E.; Chioffi, M.; Giuliante, F.; Antonelli, M.; Avolio, A.W. Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios. J. Clin. Med. 2024, 13, 2853. https://doi.org/10.3390/jcm13102853
Gaspari R, Aceto P, Spinazzola G, Piervincenzi E, Chioffi M, Giuliante F, Antonelli M, Avolio AW. Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios. Journal of Clinical Medicine. 2024; 13(10):2853. https://doi.org/10.3390/jcm13102853
Chicago/Turabian StyleGaspari, Rita, Paola Aceto, Giorgia Spinazzola, Edoardo Piervincenzi, Maurizio Chioffi, Felice Giuliante, Massimo Antonelli, and Alfonso Wolfango Avolio. 2024. "Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios" Journal of Clinical Medicine 13, no. 10: 2853. https://doi.org/10.3390/jcm13102853
APA StyleGaspari, R., Aceto, P., Spinazzola, G., Piervincenzi, E., Chioffi, M., Giuliante, F., Antonelli, M., & Avolio, A. W. (2024). Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios. Journal of Clinical Medicine, 13(10), 2853. https://doi.org/10.3390/jcm13102853







