Temporomandibular Joint Injections and Lavage: An Overview of Reviews
Abstract
1. Introduction
1.1. Background
1.2. Rationale
1.3. Objectives
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias Assessment
2.8. Synthesis Methods
3. Results
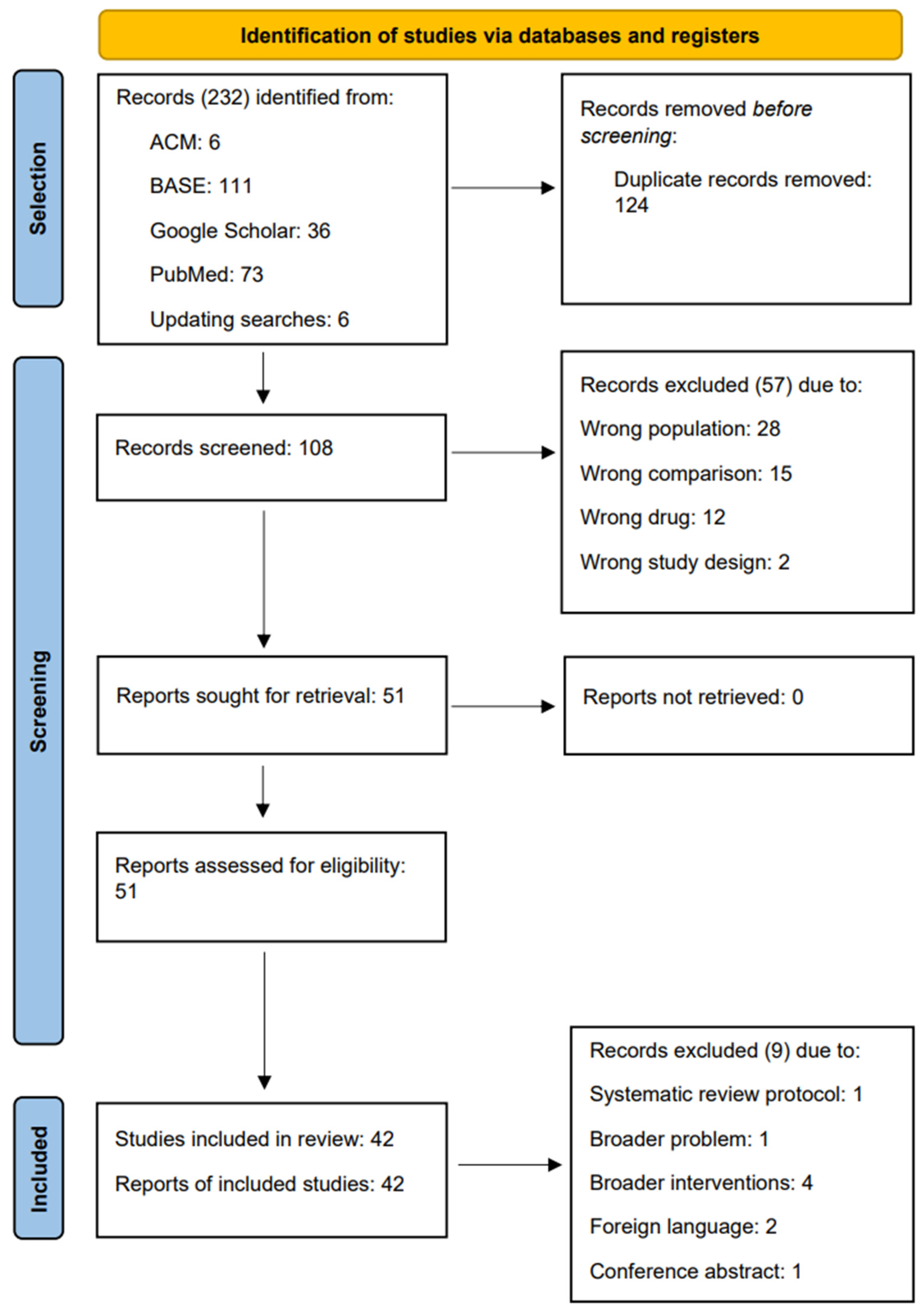
3.1. Systematic Review Selection
3.2. Characteristics of Systematic Reviews
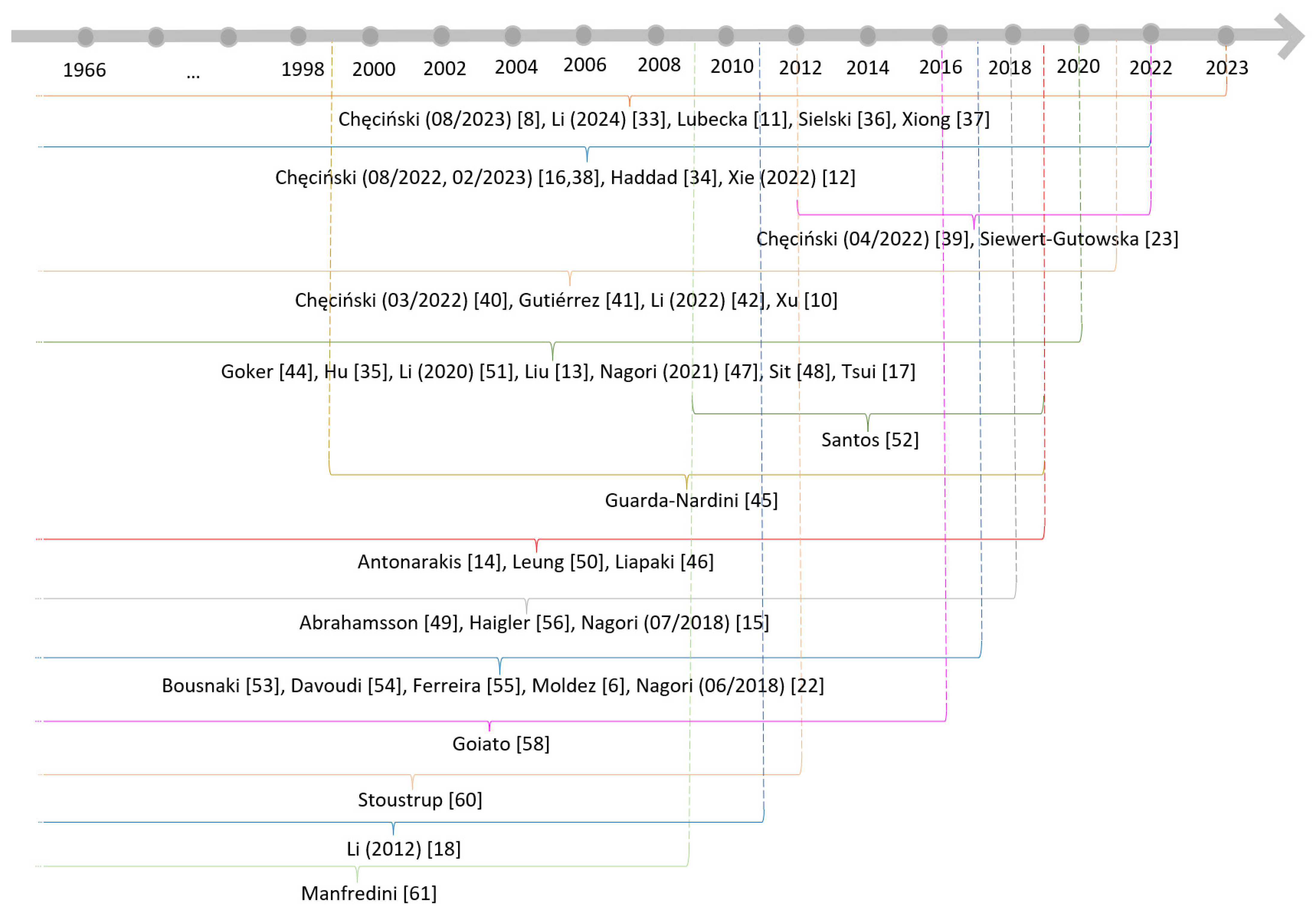
3.3. Primary Study Overlap
3.4. Risk of Bias in Systematic Reviews
3.5. Synthesis of Results
3.5.1. Generalized Osteoarthritis
3.5.2. TMJ Hypomobility
3.5.3. Recurrent TMJ Dislocation
4. Discussion
4.1. Main Findings
4.2. General Interpretation of the Results
4.2.1. AC in TMJ Hypomobility
4.2.2. CS in TMJ Hypomobility
4.2.3. HA in TMJ Hypomobility
4.2.4. PRP in TMJ Hypomobility
4.2.5. Local Anesthetics in TMJ Hypomobility
4.2.6. Different Compartment Injections in TMJ Hypomobility
4.3. Limitations of the Evidence
4.4. Limitations of the Overview Methods
4.5. Strengths of the Overview
4.6. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Search Engine | Search Query |
|---|---|
| Association for Computing Machinery: Guide to Computing Literature | [All: temporomandibular] AND [[All: injection] OR [All: injections] OR [All: intraarticular] OR [All: intra-articular] OR [All: intracavitary] OR [All: intra-cavitary] OR [All: periarticular] OR [All: peri-articular] OR [All: arthrocentesis] OR [All: lavage] OR [All: rinse] OR [All: rinsing]] AND [All: review] AND [Title: systematic] |
| Bielefeld Academic Search Engine | temporomandibular AND (injection OR injections OR intraarticular OR intra-articular OR intracavitary OR intra-cavitary OR periarticular OR peri-articular OR arthrocentesis OR lavage OR rinse OR rinsing) AND review tit:systematic |
| Google Scholar | allintitle: temporomandibular AND (injection OR injections OR intraarticular OR intra-articular OR intracavitary OR intra-cavitary OR periarticular OR peri-articular OR arthrocentesis OR lavage OR rinse OR rinsing) AND systematic AND review |
| National Library of Medicine: PubMed | temporomandibular AND (injection OR injections OR intraarticular OR intra-articular OR intracavitary OR intra-cavitary OR periarticular OR peri-articular OR arthrocentesis OR lavage OR rinse OR rinsing) AND systematic [Title] AND review |
| Search Engine | Identified Records | Filtered Records |
|---|---|---|
| Association for Computing Machinery: Guide to Computing Literature | 37 | 6 |
| Bielefeld Academic Search Engine | 180 | 111 |
| Google Scholar | about 29100 | 36 |
| National Library of Medicine: PubMed | 117 | 73 |
References
- Iturriaga, V.; Bornhardt, T.; Velasquez, N. Temporomandibular Joint: Review of Anatomy and Clinical Implications. Dent. Clin. N. Am. 2023, 67, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Chęciński, M.; Chęcińska, K.; Bliźniak, F.; Lubecka, K.; Turosz, N.; Rąpalska, I.; Michcik, A.; Chlubek, D.; Sikora, M. Temporomandibular Joint (TMJ) Replacement Affects Quality of Life: A Systematic Review and Synthesis of Clinical Trials. Appl. Sci. 2024, 14, 2912. [Google Scholar] [CrossRef]
- Albilia, J.; Herrera-Vizcaíno, C.; Weisleder, H.; Choukroun, J.; Ghanaati, S. Liquid Platelet-Rich Fibrin Injections as a Treatment Adjunct for Painful Temporomandibular Joints: Preliminary Results. CRANIO® 2020, 38, 292–304. [Google Scholar] [CrossRef]
- Schmidt, C.; Reich, R.; Koos, B.; Ertel, T.; Ahlers, M.O.; Arbogast, M.; Feurer, I.; Habermann-Krebs, M.; Hilgenfeld, T.; Hirsch, C.; et al. Controversial Aspects of Diagnostics and Therapy of Arthritis of the Temporomandibular Joint in Rheumatoid and Juvenile Idiopathic Arthritis-An Analysis of Evidence- and Consensus-Based Recommendations Based on an Interdisciplinary Guideline Project. J. Clin. Med. 2022, 11, 1761. [Google Scholar] [CrossRef]
- Moldez, M.; Camones, V.; Ramos, G.; Padilla, M.; Enciso, R. Effectiveness of Intra-Articular Injections of Sodium Hyaluronate or Corticosteroids for Intracapsular Temporomandibular Disorders: A Systematic Review and Meta-Analysis. J. Oral Facial Pain Headache 2018, 32, 53–66. [Google Scholar] [CrossRef]
- De Nordenflycht, D.; Ayala, A.; Orellana, L.; Tesch, R.D.S. Intra-Articular Injections in the TMJ Inferior Joint Space: A Scoping Review. J. Oral Rehabil. 2023, 50, 1316–1329. [Google Scholar] [CrossRef]
- Chęciński, M.; Chęcińska, K.; Rąpalska, I.; Turosz, N.; Chlubek, D.; Sikora, M. Autologous Blood Injections in Temporomandibular Hypermobility: A Systematic Review. J. Clin. Med. 2023, 12, 5590. [Google Scholar] [CrossRef]
- Monje-Gil, F.; Nitzan, D.; González-Garcia, R. Temporomandibular Joint Arthrocentesis. Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e575–e581. [Google Scholar] [CrossRef]
- Xu, J.; Ren, H.; Zhao, S.; Li, Q.; Li, C.; Bao, G.; Kang, H. Comparative Effectiveness of Hyaluronic Acid, Platelet-Rich Plasma, and Platelet-Rich Fibrin in Treating Temporomandibular Disorders: A Systematic Review and Network Meta-Analysis. Head Face Med. 2023, 19, 39. [Google Scholar] [CrossRef]
- Lubecka, K.; Chęcińska, K.; Bliźniak, F.; Chęciński, M.; Turosz, N.; Michcik, A.; Chlubek, D.; Sikora, M. Intra-Articular Local Anesthetics in Temporomandibular Disorders: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 13, 106. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, K.; Ye, G.; Yao, X.; Yu, M.; Ouyang, H. Effectiveness of Intra-Articular Injections of Sodium Hyaluronate, Corticosteroids, Platelet-Rich Plasma on Temporomandibular Joint Osteoarthritis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Evid.-Based Dent. Pract. 2022, 22, 101720. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Zhang, X. Do Intra-articular Injections of Analgesics Improve Outcomes after Temporomandibular Joint Arthrocentesis?: A Systematic Review and Meta-analysis. J. Oral Rehabil. 2021, 48, 95–105. [Google Scholar] [CrossRef]
- Antonarakis, G.S.; Blanc, A.; Courvoisier, D.S.; Scolozzi, P. Effect of Intra-Articular Corticosteroid Injections on Pain and Mouth Opening in Juvenile Idiopathic Arthritis with Temporomandibular Involvement: A Systematic Review and Meta-Analysis. J. Cranio-Maxillofac. Surg. 2020, 48, 772–778. [Google Scholar] [CrossRef]
- Nagori, S.A.; Jose, A.; Gopalakrishnan, V.; Roy, I.D.; Chattopadhyay, P.K.; Roychoudhury, A. The Efficacy of Dextrose Prolotherapy over Placebo for Temporomandibular Joint Hypermobility: A Systematic Review and Meta-Analysis. J. Oral Rehabil. 2018, 45, 998–1006. [Google Scholar] [CrossRef]
- Chęciński, M.; Chęcińska, K.; Turosz, N.; Sikora, M.; Chlubek, D. Intra-Articular Injections into the Inferior versus Superior Compartment of the Temporomandibular Joint: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1664. [Google Scholar] [CrossRef]
- Tsui, H.C.; Lam, C.M.; Leung, Y.Y.; Li, K.Y.; Wong, N.S.M.; Li, D.T.S. Lavage Volume of Arthrocentesis in the Management of Temporomandibular Disorders: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2622. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Lv, J.; Shi, Z. Inferior or Double Joint Spaces Injection Versus Superior Joint Space Injection for Temporomandibular Disorders: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2012, 70, 37–44. [Google Scholar] [CrossRef]
- Soni, A. Arthrocentesis of Temporomandibular Joint- Bridging the Gap Between Non-Surgical and Surgical Treatment. Ann. Maxillofac. Surg. 2019, 9, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Guarda-Nardini, L.; Ferronato, G.; Manfredini, D. Two-Needle vs. Single-Needle Technique for TMJ Arthrocentesis plus Hyaluronic Acid Injections: A Comparative Trial over a Six-Month Follow Up. Int. J. Oral Maxillofac. Surg. 2012, 41, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, A.; Ghosh, R.; Pandey, R.; Kumar, S. A Comparative Study between Concentric Single-Needle Puncture Technique and Conventional 2-Needle Technique for Temporomandibular Joint Arthrocentesis Plus Corticosteroid Injections. Craniomaxillofacial Trauma Reconstr. 2020, 13, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Nagori, S.A.; Roy Chowdhury, S.K.; Thukral, H.; Jose, A.; Roychoudhury, A. Single Puncture versus Standard Double Needle Arthrocentesis for the Management of Temporomandibular Joint Disorders: A Systematic Review. J. Oral Rehabil. 2018, 45, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Siewert-Gutowska, M.; Pokrowiecki, R.; Kamiński, A.; Zawadzki, P.; Stopa, Z. State of the Art in Temporomandibular Joint Arthrocentesis-A Systematic Review. J. Clin. Med. 2023, 12, 4439. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, E.; Ferreira, L.A.; Poluha, R.L.; Setogutti, E.; Iwaki, L.C.V.; Iwaki Filho, L. Comparison of Two Needles Arthrocentesis versus Double Needle Cannula Arthrocentesis in the Treatment of Temporomandibular Disc Displacement. CRANIO® 2022, 40, 358–364. [Google Scholar] [CrossRef]
- Kütük, N.; Baş, B.; Kazan, D.; Yüceer, E. Is Repeated Arthrocentesis Beneficial in the Treatment of Temporomandibular Disorders: A Retrospective Study. J. Oral Maxillofac. Surg. 2019, 77, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Pollock, M.; Fernandes, R.M.; Pieper, D.; Tricco, A.C.; Gates, M.; Gates, A.; Hartling, L. Preferred Reporting Items for Overviews of Reviews (PRIOR): A Protocol for Development of a Reporting Guideline for Overviews of Reviews of Healthcare Interventions. Syst. Rev. 2019, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- The ACM Guide to Computing Literature. Available online: https://libraries.acm.org/digital-library/acm-guide-to-computing-literature (accessed on 4 May 2023).
- BASE—Bielefeld Academic Search Engine|What Is BASE? Available online: https://www.base-search.net/about/en/index.php (accessed on 4 May 2023).
- Google Scholar. Available online: https://scholar.google.com/ (accessed on 18 May 2023).
- PubMed Overview. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 14 November 2023).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- University of Bristol. ROBIS Tool. Available online: https://www.bristol.ac.uk/population-health-sciences/projects/robis/robis-tool/ (accessed on 18 May 2023).
- Li, J.; Chen, H. Intra-Articular Injection of Platelet-Rich Plasma vs Hyaluronic Acid as an Adjunct to TMJ Arthrocentesis: A Systematic Review and Meta-Analysis. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101676. [Google Scholar] [CrossRef] [PubMed]
- Haddad, C.; Zoghbi, A.; El Skaff, E.; Touma, J. Platelet-rich Plasma Injections for the Treatment of Temporomandibular Joint Disorders: A Systematic Review. J. Oral Rehabil. 2023, 50, 1330–1339. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, X.; Liu, S.; Xu, F. Ultrasound-Guided vs Conventional Arthrocentesis for Management of Temporomandibular Joint Disorders: A Systematic Review and Meta-Analysis. Cranio J. Craniomandib. Pract. 2023, 41, 264–273. [Google Scholar] [CrossRef]
- Sielski, M.; Chęcińska, K.; Chęciński, M.; Sikora, M. Injectable Platelet-Rich Fibrin (I-PRF) Administered to Temporomandibular Joint Cavities: A Scoping Review. J. Clin. Med. 2023, 12, 3326. [Google Scholar] [CrossRef]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2023, 10, 1204144. [Google Scholar] [CrossRef] [PubMed]
- Chęciński, M.; Chęcińska, K.; Turosz, N.; Kamińska, M.; Nowak, Z.; Sikora, M.; Chlubek, D. Autologous Stem Cells Transplants in the Treatment of Temporomandibular Joints Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Cells 2022, 11, 2709. [Google Scholar] [CrossRef]
- Chęciński, M.; Chęcińska, K.; Nowak, Z.; Sikora, M.; Chlubek, D. Treatment of Mandibular Hypomobility by Injections into the Temporomandibular Joints: A Systematic Review of the Substances Used. J. Clin. Med. 2022, 11, 2305. [Google Scholar] [CrossRef]
- Chęciński, M.; Sikora, M.; Chęcińska, K.; Nowak, Z.; Chlubek, D. The Administration of Hyaluronic Acid into the Temporomandibular Joints’ Cavities Increases the Mandible’s Mobility: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1901. [Google Scholar] [CrossRef]
- Gutiérrez, I.Q.; Sábado-Bundó, H.; Gay-Escoda, C. Intraarticular Injections of Platelet Rich Plasma and Plasma Rich in Growth Factors with Arthrocenthesis or Arthroscopy in the Treatment of Temporomandibular Joint Disorders: A Systematic Review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e327–e335. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Han, N. Diverse Therapies for Disc Displacement of Temporomandibular Joint: A Systematic Review and Network Meta-Analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1012–1022. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef] [PubMed]
- Goker, F. Evaluation of Arthrocentesis with Hyaluronic Acid Injections for Management of Temporomandibular Disorders: A Systematic Review and Case Series. J. Biol. Regul. Homeost. Agents 2021, 35, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Guarda-Nardini, L.; De Almeida, A.; Manfredini, D. Arthrocentesis of the Temporomandibular Joint: Systematic Review and Clinical Implications of Research Findings. J. Oral Facial Pain Headache 2021, 35, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Liapaki, A.; Thamm, J.R.; Ha, S.; Monteiro, J.L.G.C.; McCain, J.P.; Troulis, M.J.; Guastaldi, F.P.S. Is There a Difference in Treatment Effect of Different Intra-Articular Drugs for Temporomandibular Joint Osteoarthritis? A Systematic Review of Randomized Controlled Trials. Int. J. Oral Maxillofac. Surg. 2021, 50, 1233–1243. [Google Scholar] [CrossRef]
- Nagori, S.A.; Bansal, A.; Jose, A.; Roychoudhury, A. Comparison of Outcomes with the Single-Puncture and Double-Puncture Techniques of Arthrocentesis of the Temporomandibular Joint: An Updated Systematic Review and Meta-Analysis. J. Oral Rehabil. 2021, 48, 1056–1065. [Google Scholar] [CrossRef]
- Sit, R.W.-S.; Reeves, K.D.; Zhong, C.C.; Wong, C.H.L.; Wang, B.; Chung, V.C.; Wong, S.Y.; Rabago, D. Efficacy of Hypertonic Dextrose Injection (Prolotherapy) in Temporomandibular Joint Dysfunction: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 14638. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, H.; Eriksson, L.; Abrahamsson, P.; Häggman-Henrikson, B. Treatment of Temporomandibular Joint Luxation: A Systematic Literature Review. Clin. Oral Investig. 2020, 24, 61–70. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Wu, F.H.W.; Chan, H.H. Ultrasonography-Guided Arthrocentesis versus Conventional Arthrocentesis in Treating Internal Derangement of Temporomandibular Joint: A Systematic Review. Clin. Oral Investig. 2020, 24, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Li, D.T.S.; Wong, N.S.M.; Li, S.K.Y.; McGrath, C.P.; Leung, Y.Y. Timing of Arthrocentesis in the Management of Temporomandibular Disorders: An Integrative Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2021, 50, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.C.A.V.; Maia, I.H.T.; Soares, P.A.; dos Santos, N.E.B.; de Souza Cavalcante, J.G.; Leitão, A.K.A.; Feitosa, V.P. Viscosupplementation in the Treatment of Articular Temporomandibular Disorders—Systematic Review/Viscossuplementação No Tratamento Das Desordens Temporomandibulares Articulares—Revisão Sistemática. Braz. J. Health Rev. 2020, 3, 18616–18627. [Google Scholar] [CrossRef]
- Bousnaki, M.; Bakopoulou, A.; Koidis, P. Platelet-Rich Plasma for the Therapeutic Management of Temporomandibular Joint Disorders: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2018, 47, 188–198. [Google Scholar] [CrossRef]
- Davoudi, A.; Khaki, H.; Mohammadi, I.; Daneshmand, M.; Tamizifar, A.; Bigdelou, M.; Ansaripoor, F. Is Arthrocentesis of Temporomandibular Joint with Corticosteroids Beneficial? A Systematic Review. Med. Oral Patol. Oral Cirugia Bucal 2018, 23, e367–e375. [Google Scholar] [CrossRef]
- Ferreira, N.; Masterson, D.; Lopes de Lima, R.; de Souza Moura, B.; Oliveira, A.T.; Kelly da Silva Fidalgo, T.; Carvalho, A.C.P.; DosSantos, M.F.; Grossmann, E. Efficacy of Viscosupplementation with Hyaluronic Acid in Temporomandibular Disorders: A Systematic Review. J. Cranio-Maxillofac. Surg. 2018, 46, 1943–1952. [Google Scholar] [CrossRef]
- Haigler, M.C.; Abdulrehman, E.; Siddappa, S.; Kishore, R.; Padilla, M.; Enciso, R. Use of Platelet-Rich Plasma, Platelet-Rich Growth Factor with Arthrocentesis or Arthroscopy to Treat Temporomandibular Joint Osteoarthritis. J. Am. Dent. Assoc. 2018, 149, 940–952.e2. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, V.; Bornhardt, T.; Manterola, C.; Brebi, P. Effect of Hyaluronic Acid on the Regulation of Inflammatory Mediators in Osteoarthritis of the Temporomandibular Joint: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2017, 46, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; da Silva, E.V.F.; de Medeiros, R.A.; Túrcio, K.H.L.; Dos Santos, D.M. Are Intra-Articular Injections of Hyaluronic Acid Effective for the Treatment of Temporomandibular Disorders? A Systematic Review. Int. J. Oral Maxillofac. Surg. 2016, 45, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Varedi, P.; Bohluli, B. Autologous Blood Injection for Treatment of Chronic Recurrent TMJ Dislocation: Is It Successful? Is It Safe Enough? A Systematic Review. Oral Maxillofac. Surg. 2015, 19, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Stoustrup, P.; Kristensen, K.D.; Verna, C.; Küseler, A.; Pedersen, T.K.; Herlin, T. Intra-Articular Steroid Injection for Temporomandibular Joint Arthritis in Juvenile Idiopathic Arthritis: A Systematic Review on Efficacy and Safety. Semin. Arthritis Rheum. 2013, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Piccotti, F.; Guarda-Nardini, L. Hyaluronic Acid in the Treatment of TMJ Disorders: A Systematic Review of the Literature. Cranio 2010, 28, 166–176. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Yu, L.; He, S.; Li, F.; Lin, Y.; Xu, J.; Chen, S. Disc–Condyle Relationship Alterations Following Stabilization Splint Therapy or Arthrocentesis plus Hyaluronic Acid Injection in Patients with Anterior Disc Displacement: A Retrospective Cohort Study. Oral Radiol. 2023, 39, 198–206. [Google Scholar] [CrossRef]
- Li, F.; Wu, C.; Sun, H.; Zhou, Q. Effect of Platelet-Rich Plasma Injections on Pain Reduction in Patients with Temporomandibular Joint Osteoarthrosis: A Meta-Analysis of Randomized Controlled Trials. J. Oral Facial Pain Headache 2020, 34, 149–156. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Problem | Temporomandibular disorders | Cadaver or animal studies |
| Intervention | Arthrocentesis and/or intra- or peri-articular injection | More invasive cointervention, e.g., arthroscopy (non-surgical treatment, e.g., physiotherapy, pharmacotherapy, and/or splint therapy was allowed) |
| Control (applies to the synthesis of systematic reviews on randomized controlled trials) | Other eligible interventions or placebo administration | No randomization |
| Outcomes (applies to the synthesis of systematic reviews on randomized controlled trials) | Articular pain, mandibular abduction, or quality of life index | No quantitative assessment |
| Timeframe | Unlimited | Unaccepted paper, e.g., in the preprint stage |
| Settings | Systematic reviews published in English | No eligibility criteria, information sources, or search strategy |
| First Author, Publication Year | Coverage | Source Studies | Total Number of Patients | Diagnosis | Unified Diagnosis | Intervention or Administered Substance | Control in Randomized Controlled Trials |
|---|---|---|---|---|---|---|---|
| Li, 2024 [33] | Until 2023 | 7 randomized controlled trials | 243 | TMJ internal derangement | Generalized osteoarthritis, TMJ hypomobility | PRP | HA |
| Chęciński, 08/2023 [8] | Until 2023 | 7 randomized controlled trials | 390 | TMJ hypermobility | TMJ hypermobility | UB | HD or placebo |
| Chęciński, 02/2023 [16] | Until 2022 | 4 studies with varying evidence | 337 | Various | TMJ hypomobility | Inferior TMJ space injection | N/A |
| Haddad, 2023 [34] | Until 2022 | 7 randomized controlled trials | 359 | Various | Generalized osteoarthritis | AC + PRP | AC + saline or AC + HA |
| Hu, 2023 [35] | Until 2020 | 4 randomized controlled trials | 144 | TMDs | TMJ hypomobility | Ultrasound-guided AC | Conventional AC |
| Lubecka, 2023 [11] | Until 2023 | 8 randomized controlled trials | 252 | TMJ arthralgia and hypomobility | TMJ hypomobility | Articaine, bupivacaine, lidocaine, mepivacaine | SS/morphine/HA/HD |
| Sielski, 2023 [36] | Until 2023 | 8 randomized controlled trials | 213 | TMDs | Generalized osteoarthritis | I-PRF | HA/SS injection or AC |
| Siewert-Gutowska, 2023 [23] | 2012–2022 | 25 studies with varying evidence | N/S (1099 interventions) | Disc displacement with or without reduction | TMJ hypomobility | AC | N/A |
| Xiong, 2023 [37] | Until 2023 | 24 randomized controlled trials | 1344 | Knee/hip/ankle/TMJ OA | Generalized osteoarthritis | PRP | HA/SS |
| Xu, 2023 [10] | Until 2021 | 12 randomized controlled trials | 421 | TMDs | Generalized osteoarthritis | HA, PRP, PRF | RL/SS |
| Chęciński, 08/2022 [38] | Until 2022 | 5 studies with varying evidence | 51 | TMDs | TMJ hypomobility | AC + MSCs or MSCs | N/A |
| Chęciński, 04/2022 [39] | 2012–2022 | 52 studies with varying evidence | N/S | TMDs | TMJ hypomobility or recurrent TMJ dislocation | Various | N/A |
| Chęciński, 03/2022 [40] | Until 2021 | 16 studies with varying evidence | 1007 | TMJ arthralgia | TMJ hypomobility | HA | N/A |
| Gutiérrez, 2022 [41] | Until 2021 | 8 randomized controlled trials | 404 | Various | Generalized osteoarthritis | PRP or PGRF + AC | Saline or RL + AC or AC without injection |
| Li, 2022 [42] | Until 2021 | 26 studies with varying evidence | N/S | Disc displacement with or without reduction | TMJ hypomobility | Various | N/A |
| Tsui, 2022 [17] | Until 2020 | 16 randomized controlled trials | 677 | TMDs | TMJ hypomobility | AC | AC |
| Xie, 2022 [12] | Until 2022 | 9 randomized controlled trials | 316 | TMJ osteoarthritis | TMJ hypomobility | CS, HA, PRP | Placebo |
| Derwich, 2021 [43] | N/S | 16 randomized controlled trials | N/S | TMJ OA | TMJ hypomobility | Various | Various |
| Goker, 2021 [44] | Until 2020 | 29 studies with varying evidence | N/S | TMDs | TMJ hypomobility | HA or AC + HA | N/A |
| Guarda-Nardini, 2021 [45] | 1999–2019 | 30 randomized controlled trials | N/S | TMDs (OA, ADDwoR, ADDwR, TMJ arthralgia) | TMJ hypomobility | AC | Various |
| Liapaki, 2021 [46] | Until 2019 | 9 randomized controlled trials | 434 | TMJ osteoarthritis | TMJ hypomobility | HA, CS, or blood products | Various |
| Liu, 2021 [13] | Until 2020 | 9 randomized controlled trials | 251 | TMDs treated with arthrocenthesis | TMJ hypomobility | AC + NSAIDs or AC + opioids | None, SS, RL or HA |
| Nagori, 2021 [47] | Until 2020 | 13 studies with varying evidence | 715 | TMDs (OA, ADDwR, ADDwoR, TMJ inflammatory and generative diseases, TMJ pain/clicking, restricted MMO) | TMJ hypomobility | Single-puncture AC | N/A |
| Sit, 2021 [48] | Until 2020 | 10 randomized controlled trials | 336 | TMDs diagnosed by any pre-defined or specified diagnostic criteria | Recurrent TMJ dislocation, TMJ hypomobility | HD | Various |
| Abrahamsson, 2020 [49] | Until 2018 | 8 randomized controlled trials | 338 | TMJ luxation | Recurrent TMJ dislocation | ABI or HD | Placebo or IMF |
| Antonarakis, 2020 [14] | Until 2019 | 11 studies with varying evidence | 334 | Juvenile idiopathic arthritis with TMJ involvement | Generalized osteoarthritis | CS | N/A |
| Leung, 2020 [50] | Until 2019 | 4 randomized controlled trials | 144 | Internal derangement of TMJ | TMJ hypomobility | Ultrasound-guided AC | Conventional AC |
| Li, 2020 [51] | Until 2020 | 11 studies with varying evidence | 442 | TMDs; Pain due to arthrogenic, with or without myogenic TMDs | TMJ hypomobility | AC | N/A |
| Santos, 2020 [52] | 2009–2019 | 7 studies with varying evidence | N/S (over 313) | TMDs (including OA of the TMJ) | TMJ hypomobility | HA | N/A |
| Bousnaki, 2018 [53] | Until 2017 | 6 randomized controlled trials | 323 | degenerative TMDs (including TMJ-OA, disc displacement with osteoarthritic lesions) | TMJ hypomobility | PRP | HA, SS, or RL |
| Davoudi, 2018 [54] | Until 2017 | 7 randomized controlled trials | 397 | Any kind of TMDs (arthralgia, osteoartheria, osteoarthritis, juvenile idiopathic arthritis, internal derangement) | Generalized osteoarthritis and TMJ hypomobility | AC + CS | HA, SS, or RL |
| Ferreira, 2018 [55] | Until 2017 | 21 randomized controlled trials | 882 | Osteoarthritis, anterior displacement of the TMJ disc with or without reduction, internal derangement of the TMJ | TMJ hypomobility | HA | Various |
| Haigler, 2018 [56] | Until 2018 | 5 studies with varying evidence | 285 | TMJ OA | TMJ hypomobility | PRP or PRGF | N/A |
| Moldez, 2018 [6] | Until 2017 | 7 randomized controlled trials | 425 | OA and/or internal derange- ment of the TMJ | Generalized osteoarthritis and TMJ hypomobility | CS or NaH | Placebo |
| Nagori, 07/2018 [15] | Until 2018 | 3 randomized controlled trials | 75 | Painful TMJ hypermobility (subluxation or dislocation) | Recurrent TMJ dislocation | HD | SS |
| Nagori, 06/2018 [22] | Until 2017 | 5 randomized controlled trials | 210 | TMDs (OA/ADDwoR/ADDwR) | TMJ hypomobility | Single-puncture AC | Double needle AC |
| Iturriaga, 2017 [57] | N/S | 2 randomized controlled trials | 87 | TMJ OA | TMJ hypomobility | HA | SS or RL |
| Goiato, 2016 [58] | Until 2016 | 8 studies with varying evidence | 350 | TMDs (OA/inflammatory joint disorder/rheumathoid arthritis) | Generalized osteoarthritis | AC + HA | N/A |
| Varedi, 2015 [59] | N/S | 7 studies with varying evidence | 122 | Chronic recurrent TMJ dislocation | Recurrent TMJ dislocation | UB | N/A |
| Stoustrup, 2013 [60] | Until 2012 | 7 studies with varying evidence | 268 | TMJ arthritis in juvenile idiopathic arthritis (JIA) | Generalized osteoarthritis | CS | N/A |
| Li, 2012 [18] | Until 2011 | 4 randomized controlled trials | 349 | TMDs diagnosed by clinical and/or radiological assessment | Generalized osteoarthritis | Inferior or double TMJ spaces injection | Superior TMJ space injection |
| Manfredini, 2010 [61] | Until 2009 | 19 studies with varying evidence | 604 | TMDs (TMJ disk displacement, inflammatory degenerative disorders) | TMJ hypomobility | HA | N/A |
| First Author, Publication Year | Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisal | Synthesis and Findings | Risk of Bias in the Review |
|---|---|---|---|---|---|
| Li, 2024 [33] | Low | Low | Low | Low | Low |
| Chęciński, 08/2023 [8] | Low | Low | Low | Low | Low |
| Haddad, 2023 [34] | Low | Low | High | Low | High |
| Hu, 2023 [35] | Low | Low | High | Low | High |
| Lubecka, 2023 [11] | Low | Low | Low | Low | Low |
| Sielski, 2023 [36] | Low | Low | Low | Low | Low |
| Xiong, 2023 [37] | Low | Low | Low | Low | Low |
| Xu, 2023 | Low | Low | High | Low | Low |
| Gutiérrez, 2022 [41] | Low | Low | High | High | High |
| Tsui, 2022 [17] | Low | Low | Low | Low | Low |
| Xie, 2022 [12] | Low | Low | Low | Low | Low |
| Derwich, 2021 [43] | Low | Low | Low | Unclear | Unclear |
| Guarda-Nardini, 2021 [45] | Low | Low | Unclear | Unclear | Unclear |
| Liapaki, 2021 [46] | Low | Low | Unclear | High | High |
| Liu, 2021 [13] | Low | Low | Unclear | Unclear | Unclear |
| Sit, 2021 [48] | Low | Low | Low | Low | Low |
| Abrahamsson, 2020 [49] | Low | Low | High | Low | High |
| Leung, 2020 [50] | Low | Low | Low | Low | Low |
| Bousnaki, 2018 [53] | Low | Low | High | Low | High |
| Davoudi, 2018 [54] | Low | Low | Low | Low | Low |
| Ferreira, 2018 [55] | Low | Low | High | Low | High |
| Moldez, 2018 [6] | Low | Low | Low | Low | Low |
| Nagori, 07/2018 [15] | Low | Low | Low | Low | Low |
| Nagori, 06/2018 [22] | Low | Low | High | Low | High |
| Iturriaga, 2017 [57] | Low | Low | High | Low | High |
| Stoustrup, 2013 [60] | Low | High | High | Unclear | High |
| Li, 2012 [18] | Low | Low | Low | Low | Low |
| Placebo | AC | AC + CS | CS | AC + HA | HA | AC + I-PRF | PRP | Bupivacaine | |
|---|---|---|---|---|---|---|---|---|---|
| Compared to placebo | X | No better effect (Xie 2022 [12]) | Better (Moldez 2018 [6]); No better effect (Xie 2022 [12]) | No better effect (Xie 2022 [12]) | Better up to 24 h (Lubecka 2023 [11]) | ||||
| Compared to AC | X | No significant difference (Davoudi 2018 [54]) | Better effect (Sielski 2023 [36]) | ||||||
| Compared to AC + CS | No significant diffference (Davoudi 2018 [54]) | X | |||||||
| Compared to CS | Same effect (Xie 2022 [12]) | X | No statistically significant difference (Moldez 2018 [6]) | ||||||
| Compared to AC + HA | X | May lead to comparable clinical outcomes (Li 2024 [33]) | |||||||
| Compared to HA | Worse (Moldez 2018 [6]); Same effect (Xie 2022 [12]) | No statistically significant difference (Moldez 2018 [6]) | X | ||||||
| Compared to AC + I-PRF | Worse effect (Sielski 2023 [36]) | May lead to comparable clinical outcomes (Li 2024 [33]) | X | ||||||
| Compared to PRP | Same effect (Xie 2022 [12]) | X | |||||||
| Compared to Bupicavaine | No better effect (Lubecka 2023 [11]) | X |
| AC | Small- Volume (<150 mL) AC | Ultrasound Guided AC | Inferior- or Double-Compartment Injection | Superior-Compartment Injection | |
|---|---|---|---|---|---|
| Compared to AC | X | At least as effective, if not more (Tsui 2022 [17]) | No better clinical outcomes (Leung 2020 [50]) | ||
| Compared to small- volume (<150mL) AC | As effective or less (Tsui 2022 [17]) | X | |||
| Compared to ultrasound guided AC | No better clinical outcomes (Leung 2020 [50]) | X | |||
| Compared to inferior- or double-compartment injection | X | Worse effect (Li 2012 [18]) | |||
| Compared to superior-compartment injection | Better effect (Li 2012 [18]) | X |
| Placebo | HD | UB | Intracavitary + Pericapsular UB | Pericapsular UB | |
|---|---|---|---|---|---|
| Compared to placebo | X | Significant reduction in mouth-opening and associated pain (Nagori 07/2018 [15]); Conferred a large positive effect that met the criteria for clinical relevance in the treatment of temporomandibular joint pain (Sit 2021 [48]) | |||
| Compared to HD | Did not lead to a reduction in mouth opening and associated pain (Nagori 07/2018 [15]); Worse effect (clinically relevant) in the treatment of temporomandibular joint pain (Sit 2021 [48]) | X | Was more efficient in limiting temporomandibular dislocations in a 3-month observation (Chęciński 08/2023 [8]) | ||
| Compared to UB | Was less efficient in limiting temporomandibular dislocations in a 3-month observation (Chęciński 08/2023 [8]) | X | |||
| Compared to intracavitary + pericapsular UB | X | No difference was observed (Chęciński 08/2023 [8]) | |||
| Compared to pericapsular UB | No difference was observed (Chęciński 08/2023 [8]) | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turosz, N.; Chęcińska, K.; Chęciński, M.; Lubecka, K.; Bliźniak, F.; Chlubek, D.; Olszowski, T.; Sikora, M. Temporomandibular Joint Injections and Lavage: An Overview of Reviews. J. Clin. Med. 2024, 13, 2855. https://doi.org/10.3390/jcm13102855
Turosz N, Chęcińska K, Chęciński M, Lubecka K, Bliźniak F, Chlubek D, Olszowski T, Sikora M. Temporomandibular Joint Injections and Lavage: An Overview of Reviews. Journal of Clinical Medicine. 2024; 13(10):2855. https://doi.org/10.3390/jcm13102855
Chicago/Turabian StyleTurosz, Natalia, Kamila Chęcińska, Maciej Chęciński, Karolina Lubecka, Filip Bliźniak, Dariusz Chlubek, Tomasz Olszowski, and Maciej Sikora. 2024. "Temporomandibular Joint Injections and Lavage: An Overview of Reviews" Journal of Clinical Medicine 13, no. 10: 2855. https://doi.org/10.3390/jcm13102855
APA StyleTurosz, N., Chęcińska, K., Chęciński, M., Lubecka, K., Bliźniak, F., Chlubek, D., Olszowski, T., & Sikora, M. (2024). Temporomandibular Joint Injections and Lavage: An Overview of Reviews. Journal of Clinical Medicine, 13(10), 2855. https://doi.org/10.3390/jcm13102855








