Utilization of Immunotherapy as a Neoadjuvant Therapy for Liver Transplant Recipients with Hepatocellular Carcinoma
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology 2004, 127 (Suppl. S1), S27–S34. [Google Scholar] [CrossRef]
- Anwar, W.A.; Khaled, H.M.; Amra, H.A.; El-Nezami, H.; Loffredo, C.A. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: Possibilities for prevention. Mutat. Res./Rev. Mutat. Res. 2008, 659, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Herszenyi, L.; Tulassay, Z. Epidemiology of gastrointestinal and liver tumors. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 249–258. [Google Scholar] [PubMed]
- Malagari, K.; Pomoni, M.; Moschouris, H.; Bouma, E.; Koskinas, J.; Stefaniotou, A.; Marinis, A.; Kelekis, A.; Alexopoulou, E.; Chatziioannou, A.J.C.; et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: Five-year survival analysis. Cardiovasc. Interv. Radiol. 2012, 35, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Saharia, A.; McMillan, R.; Victor, D.; Kodali, S.; Shetty, A.; Nolte Fong, J.V.; et al. Transplant Oncology: An Evolving Field in Cancer Care. Cancers 2021, 13, 4911. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Victor, D.; Kodali, S.; Cheah, Y.L.; Simon, C.J.; Noureddin, M.; Connor, A.; et al. Transplant Oncology: An Emerging Discipline of Cancer Treatment. Cancers 2023, 15, 5337. [Google Scholar] [CrossRef] [PubMed]
- Constantinidou, A.; Alifieris, C.; Trafalis, D.T. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol. Ther. 2019, 194, 84–106. [Google Scholar] [CrossRef] [PubMed]
- Finkelmeier, F.; Waidmann, O.; Trojan, J. Nivolumab for the treatment of hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 1169–1175. [Google Scholar] [CrossRef]
- Graziani, G.; Tentori, L.; Navarra, P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol. Res. 2012, 65, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Saung, M.T.; Pelosof, L.; Casak, S.; Donoghue, M.; Lemery, S.; Yuan, M.; Rodriguez, L.; Schotland, P.; Chuk, M.; Davis, G.; et al. FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist 2021, 26, 797–806. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Umoru, G.; Westhart, K.; Abudayyeh, A.; Saharia, A.; Ghobrial, R.M. Immunotherapy as a Neoadjuvant Therapy for a Patient with Hepatocellular Carcinoma in the Pretransplant Setting: A Case Report. Curr. Oncol. 2022, 29, 4267–4273. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Florman, S.S.; Schwartz, M.E. PD-1 inhibitor as bridge therapy to liver transplantation? Am. J. Transplant. 2021, 21, 1979–1980. [Google Scholar] [CrossRef] [PubMed]
- Schwacha-Eipper, B.; Minciuna, I.; Banz, V.; Dufour, J.F. Immunotherapy as a Downstaging Therapy for Liver Transplantation. Hepatology 2020, 72, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Chouik, Y.; Erard, D.; Demian, H.; Schulz, T.; Mazard, T.; Hartig-Lavie, K.; Antonini, T.; Mabrut, J.-Y.; Mohkam, K.; Rode, A. Case Report: Successful liver transplantation after achieving complete clinical remission of advanced HCC with Atezolizumab plus Bevacizumab combination therapy. Front. Immunol. 2023, 14, 1205997. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.C.; Lilly, L.B.; Hogg, D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: A case report and literature review. Melanoma Res. 2018, 28, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.E.; Shoushtari, A.N.; Walsh, M.M.; Grewal, P.; Lipson, E.J.; Carvajal, R.D. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J. Immunother. Cancer 2015, 3, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gok Yavuz, B.; Hasanov, E.; Lee, S.S.; Mohamed, Y.I.; Curran, M.A.; Koay, E.J.; Cristini, V.; Kaseb, A.O. Current Landscape and Future Directions of Biomarkers for Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 1195–1207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheiner, B.; Pomej, K.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Waidmann, O.; Himmelsbach, V.; Schulze, K.; von Felden, J.; Fründt, T.W.; et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—Development and validation of the CRAFITY score. J. Hepatol. 2022, 76, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Kawabe, N.; Hashimoto, S.; Miyahara, R.; Sawaki, A.; Nakano, T.; Nakaoka, K.; Tanaka, H.; Miyachi, Y.; Mii, A.; et al. Early Changes in Alpha-Fetoprotein Are a Useful Predictor of Efficacy of Atezolizumab plus Bevacizumab Treatment in Patients with Advanced Hepatocellular Carcinoma. Oncology 2022, 100, 12–21. [Google Scholar] [CrossRef] [PubMed]

| Total: N = 6 | |
| Age: 61.00 (59.00–64.00) | |
| Sex | |
| Male | 6 (100.00) |
| Ethnicity | |
| Hispanic or Latino | 1 (16.67) |
| Not Hispanic or Latino | 5 (83.33) |
| Race | |
| Asian | 2 (33.33) |
| Caucasian | 4 (66.67) |
| Stage of HCC at the Dx | |
| Stage IA | 1 (16.67) |
| Stage II | 2 (33.33) |
| Stage III | 1 (16.67) |
| Stage IIIB | 1 (16.67) |
| Stage IVA | 1 (16.67) |
| Grade of HCC at the Dx | |
| Grade 1 | 1 (16.67) |
| Grade 2 | 2 (33.33) |
| Grade 2–3 | 2 (33.33) |
| Grade 3 | 1 (16.67) |
| Size of Largest HCC lesion (cm) at the Dx 3.30 (3.00–4.80) | |
| ECOG = 1:6 (100.00) | |
| Child–Pugh (CP) class | |
| A5 | 1 (16.67) |
| A6 | 1 (16.67) |
| C10 | 3 (50.00) |
| C11 | 1 (16.67) |
| MELD from the Dx to OLT | |
| 10 to 31 | 1 (16.67) |
| 13 to 43 | 1 (16.67) |
| 19 to 30 | 1 (16.67) |
| 6 to 28 | 1 (16.67) |
| 6 to 8 | 1 (16.67) |
| 8 to 43 | 1 (16.67) |
| Comorbidities | |
| NASH and Cirrhosis | 1 (16.67) |
| HBV and Cirrhosis | 1 (16.67) |
| HTN, BPH, HCV, and Cirrhosis | 1 (16.67) |
| HTN, DM, HBV, and Cirrhosis | 1 (16.67) |
| HTN, HCV, and Cirrhosis | 1 (16.67) |
| Hemochromatosis and Cirrhosis | 1 (16.67) |
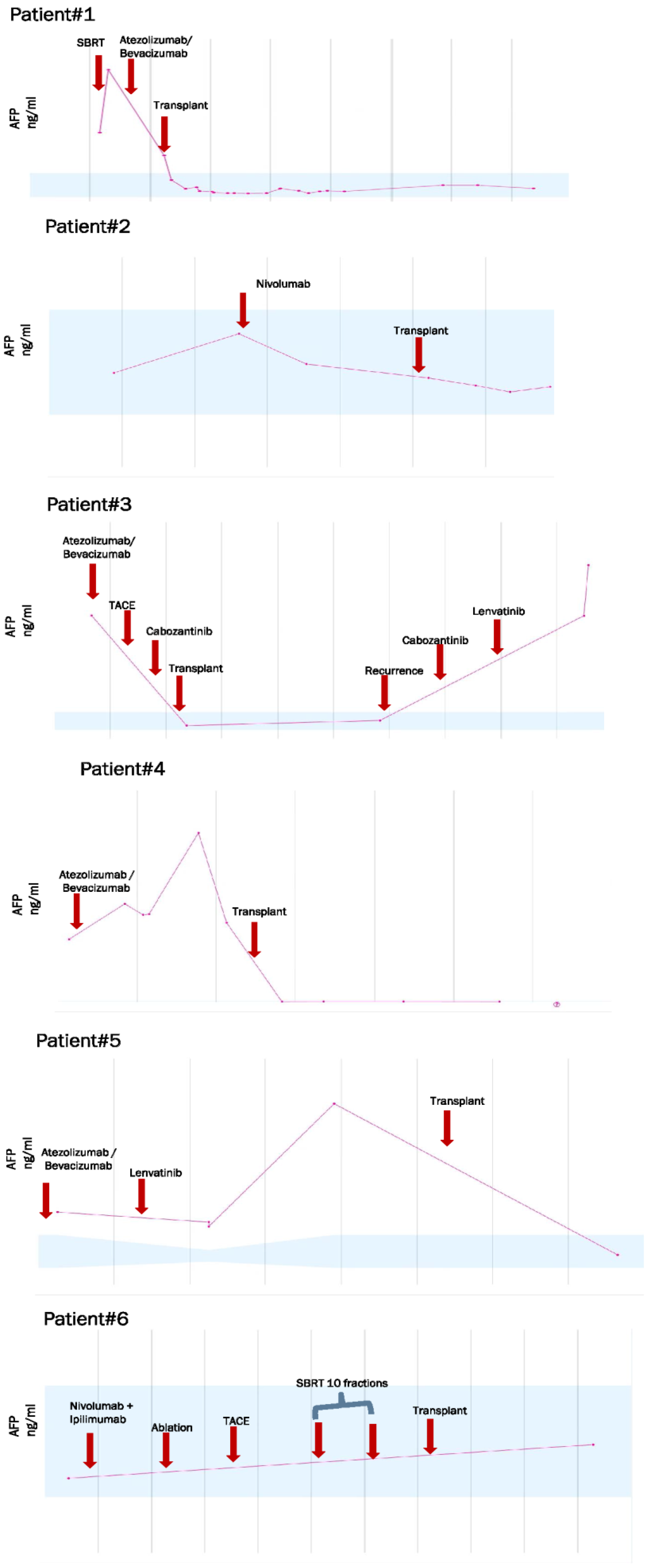
| AFP (ng/mL) prior OLT: 21.95 (2.00–53.80) | |
| Any treatment prior to ICPI | |
| No | 4 (66.67) |
| Yes | 2 (33.33) |
| Time for neoadjuvant: 635.50 (518.00–699.00) | |
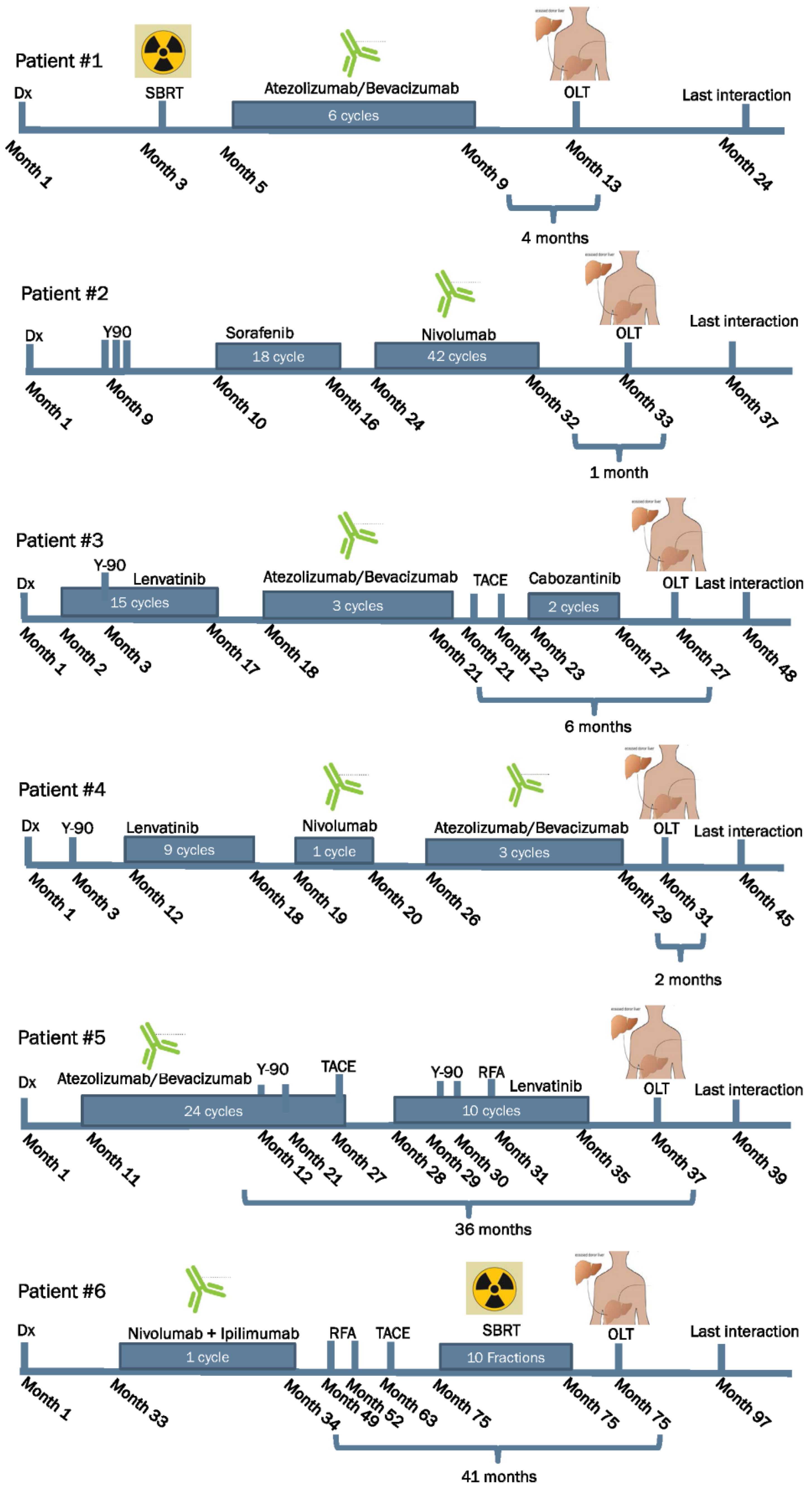
| Case ID | Age Years | Gender | Dx | Type of ICPI | LRT | WOP in Months | AFP before OLT | Type of Surgery | Rejection Yes or No | IS Agents after OLT |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | Male | HCC | PD-L1 | None | 4 | 2.0 ng/mL | OLT | No | Mycophenolate Tacrolimus |
| 2 | 61 | Male | HCC | PD-1 | Y-90/TACE | 1 | 1.8 ng/mL | OLT | No | Mycophenolate Tacrolimus |
| 3 | 58 | Male | HCC | PD-L1 | TACE/Y-90 | 6 | 4.4 ng/mL | OLT | No | Mycophenolate Tacrolimus |
| 4 | 61 | Male | HCC | PD-1 PD-L1 | Y-90 | 2 | 792.0 ng/mL | OLT | No | Mycophenolate Tacrolimus Everolimus |
| 5 | 68 | Male | HCC | PD-L1 | Y-90/ RFA/TACE | 36 | 41.2 ng/mL | OLT | No | Mycophenolate Tacrolimus |
| 6 | 59 | Male | HCC | PD-1 CTLA-4 | TACE/RFA | 41 | 2.7 ng/mL | OLT | No | Prednisone Tacrolimus Mycophenolate |
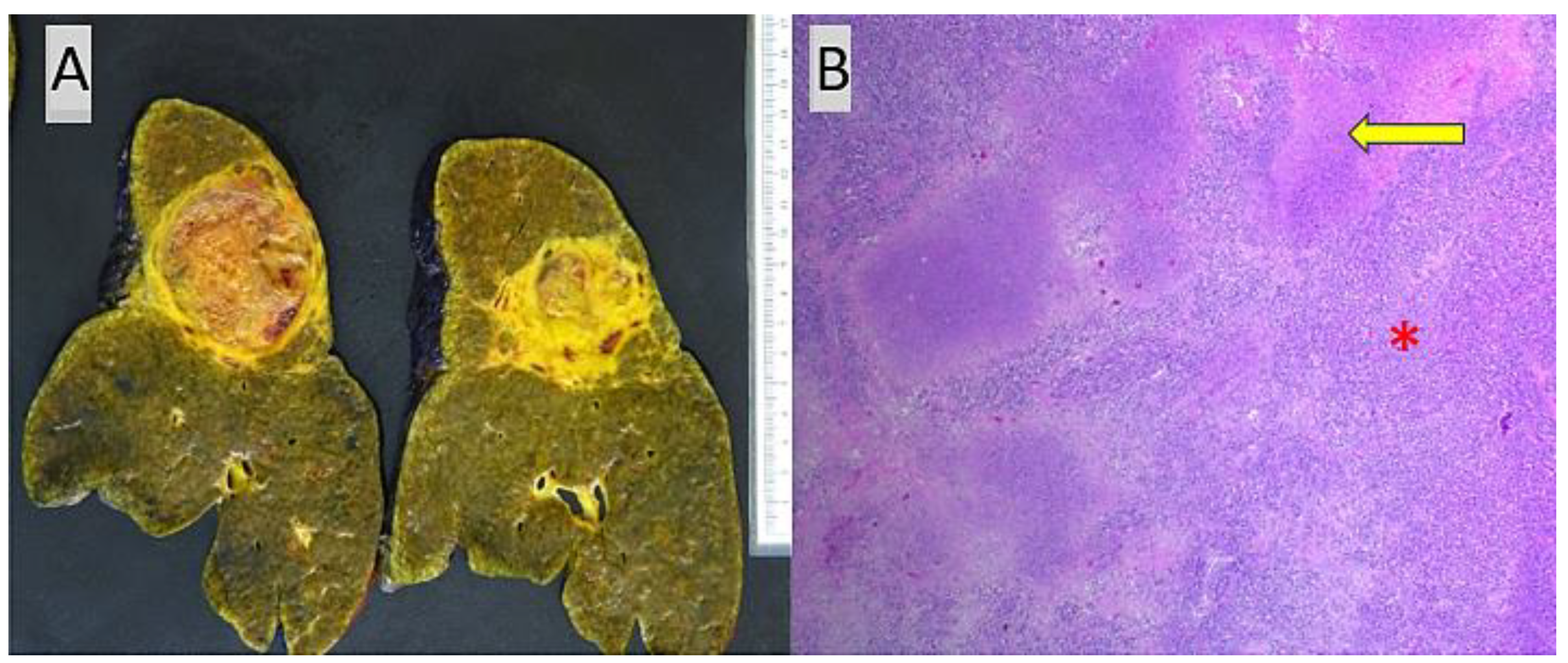
| Case ID | Tumor Size (cm) | Tumor Focality | Hepatic Segment(s) Involved by HCC | Grade | Tumor Necrosis | Lymphovascular Invasion | Perineural Invasion | Primary Tumor (ypT) | Lymph Nodes | Tumor Stage | Etiology Associated with HCC | Fibrosis Staging |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.3, 2.9, 1.2 | Multifocal, 3 tumor nodules | 2, 2 and 7 | Poorly Differentiated | 25% | Not identified | Not identified | T2(m) | One (ypN0) | II | Hepatitis B and C | Cirrhosis |
| 2 | 0.4 | Unifocal | 4 | Well-Differentiated | 99% | Not identified | Not identified | T1a | None | IA | Hepatitis C, alpha-1 antitrypsin deficiency | Cirrhosis |
| 3 | >20 | Multifocal (cirrhotomimetic) >30 tumor nodules | All segments | Moderately Differentiated | 20% | Present, small-caliber blood vessels | Not identified | T2(m) | None | II | Hepatitis B | Bridging (stage 3) fibrosis |
| 4 | 1.2–3 | Multifocal, 8 tumor nodules | 2, 3, 4, 7 and 8 | Moderately to Poorly Differentiated | 4 entirely viable tumor nodules, remainder 4 nodules with 10–95% necrosis | Present, small-caliber blood vessels | Present, large- caliber nerve fibers | T2(m) | None | II | NASH, hemochromatosis | Cirrhosis |
| 5 | 8, 5.2, 2.5 | Multifocal, 3 tumor nodules | 5/6, 2/4 and 7/8 | Moderately to Poorly Differentiated | 98% (largest tumor), 70% (second largest tumor), 100% (smallest tumor) | Present, small-and medium-caliber blood vessels | Not identified | T3(m) | Two (ypN0) | IIIA | NASH | Cirrhosis |
| 6 | 3.4 | Unifocal | Perihilar | Well to Moderately Differentiated | Not identified | Present, small-caliber blood vessels | Not identified | T2 | None | II | Hepatitis B | Cirrhosis with regression and portal vein thrombosis in Segment 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahim, M.; Esmail, A.; Divatia, M.K.; Xu, J.; Kodali, S.; Victor, D.W.; Brombosz, E.; Connor, A.A.; Saharia, A.; Elaileh, A.; et al. Utilization of Immunotherapy as a Neoadjuvant Therapy for Liver Transplant Recipients with Hepatocellular Carcinoma. J. Clin. Med. 2024, 13, 3068. https://doi.org/10.3390/jcm13113068
Abdelrahim M, Esmail A, Divatia MK, Xu J, Kodali S, Victor DW, Brombosz E, Connor AA, Saharia A, Elaileh A, et al. Utilization of Immunotherapy as a Neoadjuvant Therapy for Liver Transplant Recipients with Hepatocellular Carcinoma. Journal of Clinical Medicine. 2024; 13(11):3068. https://doi.org/10.3390/jcm13113068
Chicago/Turabian StyleAbdelrahim, Maen, Abdullah Esmail, Mukul K. Divatia, Jiaqiong Xu, Sudha Kodali, David W. Victor, Elizabeth Brombosz, Ashton A. Connor, Ashish Saharia, Ahmed Elaileh, and et al. 2024. "Utilization of Immunotherapy as a Neoadjuvant Therapy for Liver Transplant Recipients with Hepatocellular Carcinoma" Journal of Clinical Medicine 13, no. 11: 3068. https://doi.org/10.3390/jcm13113068
APA StyleAbdelrahim, M., Esmail, A., Divatia, M. K., Xu, J., Kodali, S., Victor, D. W., Brombosz, E., Connor, A. A., Saharia, A., Elaileh, A., Kaseb, A. O., & Ghobrial, R. M. (2024). Utilization of Immunotherapy as a Neoadjuvant Therapy for Liver Transplant Recipients with Hepatocellular Carcinoma. Journal of Clinical Medicine, 13(11), 3068. https://doi.org/10.3390/jcm13113068







