Current Evidence and Future Perspectives to Implement Continuous and End-Ischemic Use of Normothermic and Oxygenated Hypothermic Machine Perfusion in Clinical Practice
Abstract
1. Introduction
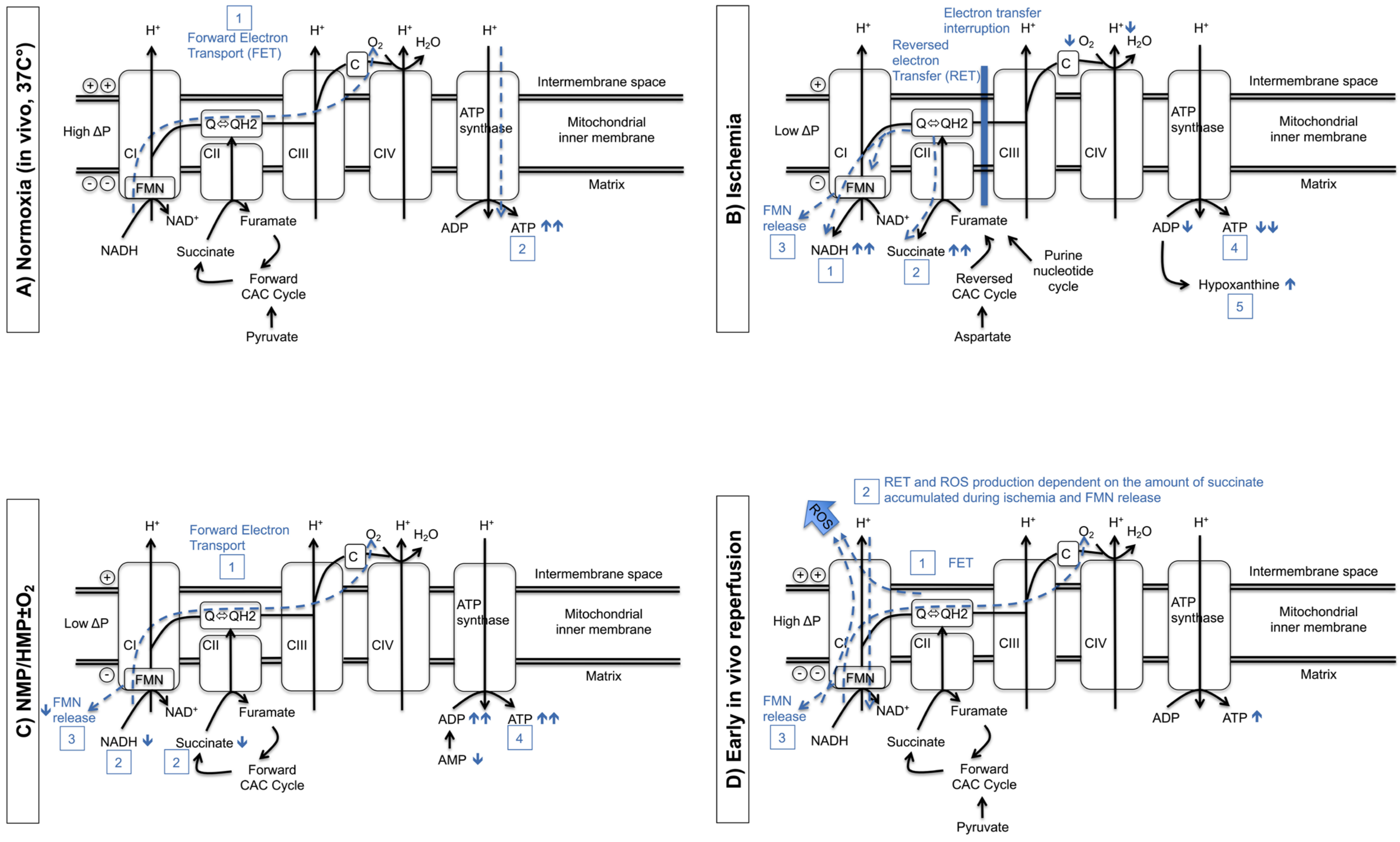
2. Mitochondria Play a Central Role in Dynamic Preservation Strategies
3. Hypothermic Machine Perfusion
3.1. Establishment of Hypothermic Machine Perfusion on the Kidney
3.2. HMP as an Alternative to Static Cold Storage
3.3. Kidney O2 Delivery during HMP?
3.3.1. Membrane Oxygenation
3.3.2. Bubble and Surface Oxygenation
3.4. Duration of Active Oxygenation during HMP
3.5. HMP as a Tool for Kidney Assessment Prior to Transplantation
3.6. Clinical Evidence for HMPO2: End-Ischemic versus Continuous Perfusion
| Meister FA et al., 2019 [78] | Ravaioli M et al., 2020 [63] | Jochmans I et al., 2020 [65] | Husen P et al., 2021 [66] | Houtzager J et al., 2021 [79] | Pravisani R et al., 2022 [80] | |
|---|---|---|---|---|---|---|
| Donor type | ECD DBD | ECD DBD | DCD > 50 y | ECD DBD | DBD DCD | NA |
| Perfusion device | Kidney Assist Transporter (Organ Assist) | Unique device developed by Medica S.P.A and Centro Iperbarico S.R.L. | Kidney Assist Transporter (Organ Assist) | Kidney Assist Transporter (Organ Assist) | Oxygenated Airdrive HMP system | Waves Machine |
| Type of study | Matched-case analysis | Matched-case analysis | RCT (COPE-COMPARE trial) | RCT (COPE-POMP trial) | Phase I | Retrospective study |
| Study groups (n = included patients) | 1. SCS + HMPO2 (n = 15) 2. SCS (n = 30) (historical cohort group) | 1. SCS + HMPO2 (n = 10) 2. SCS (n = 30) (historical cohort group) | 1. HMPO2 (n = 106) 2. HMP (n = 106) | 1. SCS + HMPO2 (n = 127) 2. SCS (n = 135) | 1. SCS(n = 4)/ HMP (n = 1) + HMPO2 | 1. SCS + HMPO2 (21%02) (n = 51) 2. SCS + HMP (n = 52) |
| Donor age, years | ||||||
| Study group 1 | 66.0 (±12) * | 71.5 (60–78) | 58.0 (54–63) | 64.0 (50–82) | 44.2 (19–64) | 62.0 (44–73) |
| Study group 2 | 66.0 (±8) * | 69.5 (59–79) | 58.0 (54–63) | 65.0 (51–84) | - | 60.0 (48–70) |
| Donor WIT, min | ||||||
| Study group 1 | - | - | 28.8 (22–36) | 34.0 (17–92) | NA | 50 (37–59) |
| Study group 2 | - | - | 28.8 (22–36) | 32.0 (10–80) | - | 44 (35–52) |
| Duration on MP, hour | ||||||
| Study group 1 | 2.5 ± 1.5 * | Mean 3.3 (1–6 h) | 11.0 (8.7–13.7) | 4.7 (0.8–17.1) | 8.5 (3–15) | 20.3 (18.1–22.7) |
| Study group 2 | - | - | 10.3 (8.9–14.0) | - | - | 20.3 (18.1–22.3) |
| p-value | NA | NA | 0.410 | NA | - | 0.678 |
| CIT, hour | ||||||
| Study group 1 | 10.8 ± 3.8 * | 14.5 (10.8–22) | 6.85 (4.5–9.1) | 13.2 (5.1–28.7) | 20.2 (11–29.5) | 29 (26.6–31) |
| Study group 2 | 11.2 ± 3.6 * | 14 (8–21) | 7.40 (4.8–9.9) | 12.9 (4–29.2) | - | 29.8 (27.6–31.5) |
| p-value | 0.563 | 0.896 | 0.210 | NA | - | 0.438 |
| DGF rate, n (%) | ||||||
| Study group 1 | 8 (53%) | 2 (20%) | 38 (36%) | 30 (23.6%) | 3 (60%) | 11 (21.5%) |
| Study group 2 | 10 (33%) | 12 (40%) | 38 (36%) | 38 (28.1%) | - | 13 (25%) |
| p-value | 0.197 | 0.607 | 0.990 | 0.400 | - | 0.648 |
| PNF rate, n (%) | ||||||
| Study group 1 | 1 (7%) | 0 (0%) | 3 (3%) | 8 (6.3%) | 1 (20%) | NA |
| Study group 2 | 0 (0%) | 1 (3.3%) | 5 (5%) | 8 (5.9%) | - | NA |
| p-value | 0.333 | 0.948 | 0.480 | 0.900 | - | NA |
| eGFR (mL/Min/1.73 m2) | (At 6 mo) | (At 1 year) | (At 1 year) | (At 1 year, serum creatinine level) | ||
| Study group 1 | 32 ± 14 * | NA | 50.5 ± 19.3 * | 39.9 ± 14.4 * | NA | 1.27 mg/dL |
| Study group 2 | 38 ± 17 * | NA | 46.7 ± 17.1 * | 41.2 ± 17.1 * | - | 1.40 mg/dL |
| p-value | 0.276 | NA | 0.120 | 0.530 | - | 0.319 |
| Graft survival, % | (At 6 months) | (At 1 year) | (At 1 year) | (At 1 year) | (At 1 year) | |
| Study group 1 | 93% | 100% | 10% | 92.1% | NA | 96.1% |
| Study group 2 | 100% | 93.3% | 90% | 93.3% | - | 100% |
| p-value | 0.333 | 0.894 | 0.028 | 0.630 | - | 0.495 |
| Postoperative complications, % (Clavien-Dindo grade 3 or more) | ||||||
| Study group 1 | NA | NA | 11% | NA | NA | 15.7% |
| Study group 2 | NA | NA | 13% | NA | - | 11.5% |
| p-value | NA | NA | 0.032 | NA | - | 0.775 |
| BPAR | ||||||
| Study group 1 | NA | NA | 15 (14%) | 23 (18.1%) | NA | 6 (11.7%) |
| Study group 2 | NA | NA | 27 (26%) | 18 (13.3%) | - | 4 (7.7%) |
| p-value | NA | NA | 0.040 | 0.290 | - | 0.741 |
4. Normothermic Machine Perfusion
4.1. Establishment of Normothermic Machine Perfusion of the Kidney
4.2. NMP as an Alternative to Cold Preservation
4.3. NMP as a Tool for Kidney Assessment Prior to Transplantation
4.4. Clinical Evidence for NMP: End-Ischemic versus Continuous Perfusion
| Nicholson M. et al., 2013 [113] | Chandhak P. et al., 2019 [95] | Rijkse E. et al., 2021 [114] | Mazilescu L. et al., 2022 [115] | |
|---|---|---|---|---|
| Donor type | ECD (1 DCD + 17 DBD) | DCD | ECD (7 DCD + 4 DBD) | 7 DCD + 6 DBD |
| Perfusion device | Designed on pediatric cardiopulmonary bypass technology (Medtronic) | Designed on pediatric cardiopulmonary bypass technology (Medtronic) | Kidney Assist | Designed on pediatric cardiopulmonary bypass technology (Medtronic) |
| Type of study | Cohort study | Clinical series | Clinical series | Clinical series |
| Study groups (n = included patients) |
|
|
|
|
| Donor age, years | ||||
| Study group 1 | 61 (60–62) | 48 (29–61) | 71 (66–72) | 61 (52–66) |
| Study group 2 | 62 (56–68) | 57 (31–61) | 69 (68–73) | 60 (51–64) |
| Donor WIT, min | ||||
| Study group 1 | 26 (20–32) | NA | 13 (0–17) + 18 (17–21) | 29 (26–35) |
| Study group 2 | 31 (27–35) | NA | 13 (2–15) + 22 (17.5–28.5) | 32 (30–36) |
| Duration on EVNP, min (Temp, °C—02 conc, % —MP pressure, mmHg) | 63 (47–79) | 60 | 120 | 171 |
| Study group 1 | (36 °C—100% O2—52/70 mmHg) | (36 °C—100% O2—75 mmHg) | (37 °C—100% O2—60 mmHg) | (37 °C—100% O2—65 mmHg) |
| CIT, min | ||||
| Study group 1 | 774 | 838 | 593 | 537 |
| Study group 2 | 738 | 608 | 600 | 616 |
| p-value | 0.614 | 0.010 | 0.893 | 0.450 |
| DGF rate, n (%) | ||||
| Study group 1 | 1 (5.6%) | 1 (14%) | 4 (36%) | 4 (30.8%) |
| Study group 2 | 17 (36.2%) | 3 (43%) | 28 (53%) | 10 (38.5%) |
| p-value | 0.014 | 0.560 | 0.320 | 0.733 |
| PNF rate, n (%) | ||||
| Study group 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Study group 2 | 1 (2%) | 1 (14%) | 4 (8%) | 0 (0%) |
| p-value | 1.000 | 1.000 | 0.347 | 1.000 |
| eGFR (ml/min/1.73 m2) | At 1 year | At 1 year | At 1 year | |
| Study group 1 | NA | 59 | >30: 6 (55%) | 75.3 |
| Study group 2 | NA | 53 | >30: 31 (58%) | 76.5 |
| p-value | NA | 0.640 | 0.809 | 0.595 |
| Graft survival, % | At 1 year | At 1 year | At 1 year | At 1 year |
| Study group 1 | 100% | 100% | 91% | 100% |
| Study group 2 | 96% | 86% | 91% | 96% |
| p-value | 1.000 | 0.310 | 0.537 | 0.480 |
| Postoperative complications, % (Clavien-Dindo grade 3 or more) | ||||
| Study group 1 | NA | NA | NA | 23.1 |
| Study group 2 | NA | NA | NA | 11.5 |
| p-value | NA | NA | NA | 0.380 |
| BPAR | ||||
| Study group 1 | 5 (27.7%) | NA | 4 (36%) | NA |
| Study group 2 | 11 (23.4%) | NA | 14 (26%) | NA |
| p-value | 0.753 | NA | 0.504 | NA |
5. Future Perspectives of HMPO2 and NMP in Clinical Practice
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| AMP | adenosine monophosphate |
| ATP | adenosine triphosphate |
| CI | (mitochondrial) complex I |
| CAC | citric acid cycle |
| CIT | cold ischemia time |
| DAMP | damage-associated molecular pattern |
| DBD | donation after brain death |
| DCD | donation after circulatory death |
| DGF | delayed graft function |
| ECD | expanded criteria donor |
| EVNP | ex vivo normothermic perfusion |
| FET | forward electron transport |
| FMN | flavin mononucleotide |
| FMNH2 | flavin mononucleotide release |
| eGFR | estimated glomerular filtration rate |
| HMGB1 | high-mobility group box 1 |
| HMP | hypothermic machine perfusion |
| HMPO2 IRI | Oyxgenated hypothermic machine perfusion ischemia-reperfusion injury |
| KT | kidney transplantation |
| NAD+ | oxidized nicotinamide adenine dinucleotide |
| NADH | reduced nicotinamide adenine dinucleotide |
| NMP | normothermic machine perfusion |
| PNF | primary nonfunction |
| pO2 | partial pressure of oxygen |
| QAS | quality assessment score |
| QH2 | Ubiquinol |
| ROS | reactive oxygen species |
| RCT | randomized clinical trial |
| RET | reversed electron transport |
| SCS | static cold storage |
| WIT | warm ischemia time |
References
- Matas, A.J.; Smith, J.M.; Skeans, M.A.; Thompson, B.; Gustafson, S.K.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Boyle, G.; Snyder, J.J.; et al. OPTN/SRTR 2013 Annual Data Report: Kidney. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2015, 15 (Suppl. S2), 1–34. [Google Scholar] [CrossRef]
- Heylen, L.; Jochmans, I.; Samuel, U.; Tieken, I.; Naesens, M.; Pirenne, J.; Sprangers, B. The duration of asystolic ischemia determines the risk of graft failure after circulatory-dead donor kidney transplantation: A Eurotransplant cohort study. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2018, 18, 881–889. [Google Scholar] [CrossRef]
- Hamed, M.O.; Chen, Y.; Pasea, L.; Watson, C.J.; Torpey, N.; Bradley, J.A.; Pettigrew, G.; Saeb-Parsy, K. Early graft loss after kidney transplantation: Risk factors and consequences. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2015, 15, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Saidi, R.F.; Elias, N.; Kawai, T.; Hertl, M.; Farrell, M.L.; Goes, N.; Wong, W.; Hartono, C.; Fishman, J.A.; Kotton, C.N.; et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: Realities and costs. Am. J. Transplant. 2007, 7, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Srinivasa, S.; Callaghan, C.J.; Watson, C.J.E.; Dark, J.H.; Fisher, A.J.; Wilson, C.H.; Friend, P.J.; Johnson, R.; Forsythe, J.L.; et al. Novel Organ Perfusion and Preservation Strategies in Transplantation—Where Are We Going in the United Kingdom? Transplantation 2020, 104, 1813–1824. [Google Scholar] [CrossRef]
- Mittal, S.; Adamusiak, A.; Horsfield, C.; Loukopoulos, I.; Karydis, N.; Kessaris, N.; Drage, M.; Olsburgh, J.; Watson, C.J.; Callaghan, C.J. A Re-evaluation of Discarded Deceased Donor Kidneys in the UK: Are Usable Organs Still Being Discarded? Transplantation 2017, 101, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Reese, P.P.; Harhay, M.N.; Abt, P.L.; Levine, M.H.; Halpern, S.D. New Solutions to Reduce Discard of Kidneys Donated for Transplantation. J. Am. Soc. Nephrol. 2016, 27, 973–980. [Google Scholar] [CrossRef]
- Pérez-Sáez, M.J.; Montero, N.; Redondo-Pachón, D.; Crespo, M.; Pascual, J. Strategies for an Expanded Use of Kidneys From Elderly Donors. Transplantation 2017, 101, 727–745. [Google Scholar] [CrossRef]
- Moeckli, B.; Sun, P.; Lazeyras, F.; Morel, P.; Moll, S.; Pascual, M.; Bühler, L.H. Evaluation of donor kidneys prior to transplantation: An update of current and emerging methods. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2019, 32, 459–469. [Google Scholar] [CrossRef]
- De Beule, J.; Jochmans, I. Kidney Perfusion as an Organ Quality Assessment Tool-Are We Counting Our Chickens Before They Have Hatched? J. Clin. Med. 2020, 9, 879. [Google Scholar] [CrossRef]
- Dare, A.J.; Pettigrew, G.J.; Saeb-Parsy, K. Preoperative assessment of the deceased-donor kidney: From macroscopic appearance to molecular biomarkers. Transplantation 2014, 97, 797–807. [Google Scholar] [CrossRef] [PubMed]
- De Beule, J.; Vandendriessche, K.; Pengel, L.H.M.; Bellini, M.I.; Dark, J.H.; Hessheimer, A.J.; Kimenai, H.; Knight, S.R.; Neyrinck, A.P.; Paredes, D.; et al. A systematic review and meta-analyses of regional perfusion in donation after circulatory death solid organ transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 34, 2046–2060. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Hessheimer, A.J.; Neyrinck, A.P.; Paredes, D.; Bellini, M.I.; Dark, J.H.; Kimenai, H.; Pengel, L.H.M.; Watson, C.J.E. Consensus statement on normothermic regional perfusion in donation after circulatory death: Report from the European Society for Organ Transplantation’s Transplant Learning Journey. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 34, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Hamelink, T.L.; Ogurlu, B.; De Beule, J.; Lantinga, V.A.; Pool, M.B.F.; Venema, L.H.; Leuvenink, H.G.D.; Jochmans, I.; Moers, C. Renal Normothermic Machine Perfusion: The Road Toward Clinical Implementation of a Promising Pretransplant Organ Assessment Tool. Transplantation 2021, 106, 268–279. [Google Scholar] [CrossRef]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 1382–1390. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.D. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 265. [Google Scholar] [CrossRef]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemother. Off. Organ Der Dtsch. Ges. Fur Transfus. Und Immunhamatol. 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Fuller, B.J.; Lee, C.Y. Hypothermic perfusion preservation: The future of organ preservation revisited? Cryobiology 2007, 54, 129–145. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Summers, D.M.; Johnson, R.J.; Hudson, A.; Collett, D.; Watson, C.J.; Bradley, J.A. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: A cohort study. Lancet 2013, 381, 727–734. [Google Scholar] [CrossRef]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018, 293, 9869–9879. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Guarrera, J.V.; de Jonge, J.; Martins, P.N.; Porte, R.J.; Clavien, P.A. Evolving Trends in Machine Perfusion for Liver Transplantation. Gastroenterology 2019, 156, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Vergauwen, M.; Smith, T.B.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 2030–2043. [Google Scholar] [CrossRef]
- Wilson, D.F.; Erecinska, M.; Drown, C.; Silver, I.A. The oxygen dependence of cellular energy metabolism. Arch. Biochem. Biophys. 1979, 195, 485–493. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. Oxygen supplementation supports energy production during hypothermic machine perfusion in a model of donation after circulatory death donors. Transplantation 2019, 103, 1980–1981. [Google Scholar] [CrossRef]
- Ten, V.; Galkin, A. Mechanism of mitochondrial complex I damage in brain ischemia/reperfusion injury. A hypothesis. Mol. Cell. Neurosci. 2019, 100, 103408. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.; Sosunov, S.; Niatsetskaya, Z.; Konrad, C.; Starkov, A.A.; Manfredi, G.; Wittig, I.; Ten, V.; Galkin, A. Redox-Dependent Loss of Flavin by Mitochondrial Complex I in Brain Ischemia/Reperfusion Injury. Antioxid. Redox Signal. 2019, 31, 608–622. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Mueller, M.; Aydin, S.; Dutkowski, P.; Gianello, P.; Mourad, M. Brief Bubble and Intermittent Surface Oxygenation Is a Simple and Effective Alternative for Membrane Oxygenation During Hypothermic Machine Perfusion in Kidneys. Transplant. Direct 2020, 6, e571. [Google Scholar] [CrossRef]
- Pell, V.R.; Chouchani, E.T.; Murphy, M.P.; Brookes, P.S.; Krieg, T. Moving Forwards by Blocking Back-Flow: The Yin and Yang of MI Therapy. Circ. Res. 2016, 118, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Nath, J.; Mourad, M. Simply Adding Oxygen during Hypothermic Machine Perfusion to Combat the Negative Effects of Ischemia-Reperfusion Injury: Fundamentals and Current Evidence for Kidneys. Biomedicines 2021, 9, 993. [Google Scholar] [CrossRef]
- Mouzas, G.L. The present status of organ preservation: A review. Postgrad. Med. J. 1967, 43, 712–715. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. The future of kidney preservation. Transplantation 1980, 30, 161–165. [Google Scholar] [CrossRef]
- Belzer, F.O.; Ashby, B.S.; Gulyassy, P.F.; Powell, M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N. Engl. J. Med. 1968, 278, 608–610. [Google Scholar] [CrossRef] [PubMed]
- t Hart, N.A.; der van Plaats, A.; Leuvenink, H.G.; van Goor, H.; Wiersema-Buist, J.; Verkerke, G.J.; Rakhorst, G.; Ploeg, R.J. Determination of an adequate perfusion pressure for continuous dual vessel hypothermic machine perfusion of the rat liver. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2007, 20, 343–352. [Google Scholar] [CrossRef]
- Maathuis, M.H.; Manekeller, S.; van der Plaats, A.; Leuvenink, H.G.; t Hart, N.A.; Lier, A.B.; Rakhorst, G.; Ploeg, R.J.; Minor, T. Improved kidney graft function after preservation using a novel hypothermic machine perfusion device. Ann. Surg. 2007, 246, 982–988; discussion 989–991. [Google Scholar] [CrossRef]
- Gallinat, A.; Fox, M.; Luer, B.; Efferz, P.; Paul, A.; Minor, T. Role of pulsatility in hypothermic reconditioning of porcine kidney grafts by machine perfusion after cold storage. Transplantation 2013, 96, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Smits, J.M.; Maathuis, M.H.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Watson, C.J.; Wells, A.C.; Roberts, R.J.; Akoh, J.A.; Friend, P.J.; Akyol, M.; Calder, F.R.; Allen, J.E.; Jones, M.N.; Collett, D.; et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: A UK multicenter randomized controlled trial. Am.J.Transplant. 2010, 10, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Chatauret, N.; Coudroy, R.; Delpech, P.O.; Vandebrouck, C.; Hosni, S.; Scepi, M.; Hauet, T. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2014, 14, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Jani, A.; Zimmerman, M.; Martin, J.; Lu, L.; Turkmen, K.; Ravichandran, K.; Pacic, A.; Ljubanovic, D.; Edelstein, C.L. Perfusion storage reduces apoptosis in a porcine kidney model of donation after cardiac death. Transplantation 2011, 91, 169–175. [Google Scholar] [CrossRef]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschlaeger, J.; Rauen, U.; Paul, A.; Minor, T. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation 2014, 98, 944–950. [Google Scholar] [CrossRef]
- Vaziri, N.; Thuillier, R.; Favreau, F.D.; Eugene, M.; Milin, S.; Chatauret, N.P.; Hauet, T.; Barrou, B. Analysis of machine perfusion benefits in kidney grafts: A preclinical study. J. Transl. Med. 2011, 9, 15. [Google Scholar] [CrossRef]
- Giraud, S.; Steichen, C.; Couturier, P.; Tillet, S.; Mallet, V.; Coudroy, R.; Goujon, J.M.; Hannaert, P.; Hauet, T. Influence of Hypoxic Preservation Temperature on Endothelial Cells and Kidney Integrity. BioMed. Res. Int. 2019, 2019, 8572138. [Google Scholar] [CrossRef] [PubMed]
- Nordling, S.; Brannstrom, J.; Carlsson, F.; Lu, B.; Salvaris, E.; Wanders, A.; Buijs, J.; Estrada, S.; Tolmachev, V.; Cowan, P.J.; et al. Enhanced protection of the renal vascular endothelium improves early outcome in kidney transplantation: Preclinical investigations in pig and mouse. Sci. Rep. 2018, 8, 5220. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.A.J.; Ginther, N.; Luo, Y.; Beck, G.; Ginther, R.; Ewen, M.; Matsche-Neufeld, R.; Shoker, A.; Sawicki, G. Early experience with hypothermic machine perfusion of living donor kidneys—A retrospective study. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2017, 30, 706–712. [Google Scholar] [CrossRef]
- Tozzi, M.; Franchin, M.; Soldini, G.; Ietto, G.; Chiappa, C.; Maritan, E.; Villa, F.; Carcano, G.; Dionigi, R. Impact of static cold storage VS hypothermic machine preservation on ischemic kidney graft: Inflammatory cytokines and adhesion molecules as markers of ischemia/reperfusion tissue damage. Our preliminary results. Int. J. Surg. 2013, 11 (Suppl. S1), S110–S114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.F.; Dong, Q.; Zhang, T. Effects of Static Cold Storage and Hypothermic Machine Perfusion on Oxidative Stress Factors, Adhesion Molecules, and Zinc Finger Transcription Factor Proteins Before and after Liver Transplantation. Ann. Transplant. 2017, 22, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef] [PubMed]
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350. [Google Scholar] [CrossRef]
- Gallinat, A.; Moers, C.; Treckmann, J.; Smits, J.M.; Leuvenink, H.G.; Lefering, R.; van Heurn, E.; Kirste, G.R.; Squifflet, J.P.; Rahmel, A.; et al. Machine perfusion versus cold storage for the preservation of kidneys from donors >/= 65 years allocated in the Eurotransplant Senior Programme. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 4458–4463. [Google Scholar] [CrossRef]
- Groen, H.; Moers, C.; Smits, J.M.; Treckmann, J.; Monbaliu, D.; Rahmel, A.; Paul, A.; Pirenne, J.; Ploeg, R.J.; Buskens, E. Cost-effectiveness of hypothermic machine preservation versus static cold storage in renal transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2012, 12, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Gallinat, A.; Paul, A.; Efferz, P.; Luer, B.; Kaiser, G.; Wohlschlaeger, J.; Treckmann, J.; Minor, T. Hypothermic reconditioning of porcine kidney grafts by short-term preimplantation machine perfusion. Transplantation 2012, 93, 787–793. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; de Rougemont, O.; Oberkofler, C.E.; Clavien, P.A.; Dutkowski, P. Short, Cool, and Well Oxygenated - HOPE for Kidney Transplantation in a Rodent Model. Ann. Surg. 2016, 264, 815–822. [Google Scholar] [CrossRef]
- Thuillier, R.; Allain, G.; Celhay, O.; Hebrard, W.; Barrou, B.; Badet, L.; Leuvenink, H.; Hauet, T. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J. Surg. Res. 2013, 184, 1174–1181. [Google Scholar] [CrossRef]
- Koetting, M.; Frotscher, C.; Minor, T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2010, 23, 538–542. [Google Scholar] [CrossRef]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia-reperfusion autotransplant model. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2019, 19, 752–762. [Google Scholar] [CrossRef]
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 6063. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Vergauwen, M.; Smith, T.; Patel, K.; Craps, J.; Joris, V.; Aydin, S.; Ury, B.; Buemi, A.; De Meyer, M.; et al. Influence of different partial pressures of oxygen during continuous hypothermic machine perfusion in a pig kidney ischemia-reperfusion autotransplant model. Transplantation 2019, 104, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant: A Randomized Clinical Trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef]
- Epstein, F.H. Oxygen and renal metabolism. Kidney Int. 1997, 51, 381–385. [Google Scholar] [CrossRef]
- Lawson, D.S.; Smigla, G.R.; McRobb, C.M.; Walczak, R.; Kaemmer, D.; Shearer, I.R.; Lodge, A.; Jaggers, J. A clinical evaluation of the Dideco Kids D100 neonatal oxygenator. Perfusion 2008, 23, 39–42. [Google Scholar] [CrossRef]
- Darius, T.; Devresse, A.; Buemi, A.; Kanaan, N.; De Meyer, M.; Mourad, M. First kidneys transplanted in man after brief bubble and subsequent surface oxygenation as alternative for membrane oxygenation during hypothermic machine perfusion. Artif. Organs 2023, 47, 777–785. [Google Scholar] [CrossRef]
- Bodewes, S.B.; van Leeuwen, O.B.; Thorne, A.M.; Lascaris, B.; Ubbink, R.; Lisman, T.; Monbaliu, D.; De Meijer, V.E.; Nijsten, M.W.N.; Porte, R.J. Oxygen Transport during Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells or Artificial Oxygen Carriers. Int. J. Mol. Sci. 2020, 22, 235. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, Z.; Lan, J.; Li, M.; Wang, W.; Yang, J.; Tang, C.; Wang, J.; Ye, S.; Xiong, Y.; et al. Mechanisms of Hypothermic Machine Perfusion to Decrease Donation after Cardiac Death Graft Inflammation: Through the Pathway of Upregulating Expression of KLF2 and Inhibiting TGF-beta Signaling. Artif. Organs 2017, 41, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Brown, R.J.; Nicholson, M.L. Advances in kidney preservation techniques and their application in clinical practice. Transplantation 2021, 105, e202–e214. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Tortorici, F.; Amabile, M.I.; D’Andrea, V. Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion. Int. J. Mol. Sci. 2021, 22, 1121. [Google Scholar] [CrossRef]
- Moers, C.; Varnav, O.C.; van Heurn, E.; Jochmans, I.; Kirste, G.R.; Rahmel, A.; Leuvenink, H.G.; Squifflet, J.P.; Paul, A.; Pirenne, J.; et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 2010, 90, 966–973. [Google Scholar] [CrossRef]
- Guzzi, F.; Knight, S.R.; Ploeg, R.J.; Hunter, J.P. A systematic review to identify whether perfusate biomarkers produced during hypothermic machine perfusion can predict graft outcomes in kidney transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2020, 33, 590–602. [Google Scholar] [CrossRef]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Wurdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Sousa Da Silva, R.X.; Darius, T.; Mancina, L.; Eden, J.; Wernlé, K.; Ghoneima, A.S.; Barlow, A.D.; Clavien, P.-A.; Dutkowski, P.; Kron, P. Real-time assessment of kidney allografts during HOPE using flavin mononucleotide (FMN)—A preclinical study. Front. Transplant. 2023, 2, 1132673. [Google Scholar] [CrossRef]
- Meister, F.A.; Czigany, Z.; Bednarsch, J.; Boecker, J.; Wiltberger, G.; Rohlfs, W.; Neumann, U.P.; Lurje, G. Hypothermic oxygenated machine perfusion-Preliminary experience with end-ischemic reconditioning of marginal kidney allografts. Clin. Transplant. 2019, 33, e13673. [Google Scholar] [CrossRef] [PubMed]
- Houtzager, J.H.E.; Hemelrijk, S.D.; Post, I.; Idu, M.M.; Bemelman, F.J.; van Gulik, T.M. The Use of the Oxygenated AirdriveTM Machine Perfusion System in Kidney Graft Preservation: A Clinical Pilot Study. Eur. Surg. Res. Eur. Chir. Forschung. Rech. Chir. Eur. 2020, 61, 153–162. [Google Scholar] [CrossRef]
- Pravisani, R.; Baccarani, U.; Molinari, E.; Cherchi, V.; Bacchetti, S.; Terrosu, G.; Avital, I.; Ekser, B.; Adani, G.L. PO(2) 21% oxygenated hypothermic machine perfusion in kidney transplantation: Any clinical benefit? Int. J. Artif. Organs 2022, 45, 666–671. [Google Scholar] [CrossRef]
- Lindbergh, C.A. An apparatus for the culture of whole organs. J. Exp. Med. 1935, 62, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, J.R.; Hosgood, S.A.; Moore, T.; Ferro, A.; Ward, C.J.; Castro-Dopico, T.; Nicholson, M.L.; Clatworthy, M.R. Cytokine absorption during human kidney perfusion reduces delayed graft function-associated inflammatory gene signature. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 2188–2199. [Google Scholar] [CrossRef]
- McEvoy, C.M.; Clotet-Freixas, S.; Tokar, T.; Pastrello, C.; Reid, S.; Batruch, I.; RaoPeters, A.A.E.; Kaths, J.M.; Urbanellis, P.; Farkona, S.; et al. Normothermic Ex-vivo Kidney Perfusion in a Porcine Auto-Transplantation Model Preserves the Expression of Key Mitochondrial Proteins: An Unbiased Proteomics Analysis. Mol Cell Proteom. 2021, 20, 100101. [Google Scholar] [CrossRef] [PubMed]
- Urbanellis, P.; McEvoy, C.M.; Škrtić, M.; Kaths, J.M.; Kollmann, D.; Linares, I.; Ganesh, S.; Oquendo, F.; Sharma, M.; Mazilescu, L.; et al. Transcriptome Analysis of Kidney Grafts Subjected to Normothermic Ex Vivo Perfusion Demonstrates an Enrichment of Mitochondrial Metabolism Genes. Transplant. Direct 2021, 7, e719. [Google Scholar] [CrossRef] [PubMed]
- Bagul, A.; Hosgood, S.A.; Kaushik, M.; Kay, M.D.; Waller, H.L.; Nicholson, M.L. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br. J. Surg. 2008, 95, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Maessen, J.G.; van der Vusse, G.J.; Vork, M.; Kootstra, G. The beneficial effect of intermediate normothermic perfusion during cold storage of ischemically injured kidneys. A study of renal nucleotide homeostasis during hypothermia in the dog. Transplantation 1989, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Rijkmans, B.G.; Buurman, W.A.; Kootstra, G. Six-day canine kidney preservation. Hypothermic perfusion combined with isolated blood perfusion. Transplantation 1984, 37, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Stubenitsky, B.M.; Booster, M.H.; Brasile, L.; Araneda, D.; Haisch, C.E.; Kootstra, G. Exsanguinous metabolic support perfusion--a new strategy to improve graft function after kidney transplantation. Transplantation 2000, 70, 1254–1258. [Google Scholar] [CrossRef]
- van der Wijk, J.; Slooff, M.J.; Rijkmans, B.G.; Kootstra, G. Successful 96- and 144-hour experimental kidney preservation: A combination of standard machine preservation and newly developed normothermic ex vivo perfusion. Cryobiology 1980, 17, 473–477. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 2011, 92, 735–738. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Patel, M.; Nicholson, M.L. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J. Surg. Res. 2013, 182, 153–160. [Google Scholar] [CrossRef]
- Rijkse, E.; Bouari, S.; Kimenai, H.; de Jonge, J.; de Bruin, R.W.F.; Slagter, J.S.; van den Hoogen, M.W.F.; JNM, I.J.; Hoogduijn, M.J.; Minnee, R.C. Additional Normothermic Machine Perfusion Versus Hypothermic Machine Perfusion in Suboptimal Donor Kidney Transplantation: Protocol of a Randomized, Controlled, Open-Label Trial. Int. J. Surg. Protoc. 2021, 25, 227–237. [Google Scholar] [CrossRef]
- Dennis, S.C.; Gevers, W.; Opie, L.H. Protons in ischemia: Where do they come from; where do they go to? J. Mol. Cell. Cardiol. 1991, 23, 1077–1086. [Google Scholar] [CrossRef]
- Devarajan, P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 2006, 17, 1503–1520. [Google Scholar] [CrossRef]
- Chandak, P.; Phillips, B.L.; Uwechue, R.; Thompson, E.; Bates, L.; Ibrahim, I.; Sewpaul, A.; Figueiredo, R.; Olsburgh, J.; Hosgood, S.; et al. Dissemination of a novel organ perfusion technique: Ex vivo normothermic perfusion of deceased donor kidneys. Artif. Organs 2019, 43, E308–E319. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.; Pearson, R.; Lathan, R.; Mark, P.B.; Clancy, M.J. Perfusate Composition and Duration of Ex-Vivo Normothermic Perfusion in Kidney Transplantation: A Systematic Review. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2022, 35, 10236. [Google Scholar] [CrossRef] [PubMed]
- Pool, M.B.F.; Hamelink, T.L.; van Goor, H.; van den Heuvel, M.C.; Leuvenink, H.G.D.; Moers, C. Prolonged ex-vivo normothermic kidney perfusion: The impact of perfusate composition. PLoS ONE 2021, 16, e0251595. [Google Scholar] [CrossRef]
- Venema, L.H.; van Leeuwen, L.L.; Posma, R.A.; van Goor, H.; Ploeg, R.J.; Hannaert, P.; Hauet, T.; Minor, T.; Leuvenink, H.G.D. Impact of Red Blood Cells on Function and Metabolism of Porcine Deceased Donor Kidneys During Normothermic Machine Perfusion. Transplantation 2022, 106, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Barlow, A.D.; Hunter, J.P.; Nicholson, M.L. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br. J. Surg. 2015, 102, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, M.L. An Assessment of Urinary Biomarkers in a Series of Declined Human Kidneys Measured During ex-vivo Normothermic Kidney Perfusion. Transplantation 2016, 101, 2120–2125. [Google Scholar] [CrossRef]
- Georgiades, F.; Hosgood, S.A.; Butler, A.J.; Nicholson, M.L. Use of ex vivo normothermic machine perfusion after normothermic regional perfusion to salvage a poorly perfused DCD kidney. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2019, 19, 3415–3419. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2018, 105, 388–394. [Google Scholar] [CrossRef]
- Hunter, J.P.; Faro, L.L.; Rozenberg, K.; Dengu, F.; Ogbemudia, A.; Weissenbacher, A.; Mulvey, J.F.; Knijff, L.; Gopalakrishnan, K.; Ploeg, R.J. Assessment of Mitochondrial Function and Oxygen Consumption Measured During Ex Vivo Normothermic Machine Perfusion of Injured Pig Kidneys Helps to Monitor Organ Viability. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2022, 35, 10420. [Google Scholar] [CrossRef]
- Markgraf, W.; Feistel, P.; Thiele, C.; Malberg, H. Algorithms for mapping kidney tissue oxygenation during normothermic machine perfusion using hyperspectral imaging. Biomed. Tech. 2018, 63, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tetschke, F.; Markgraf, W.; Gransow, M.; Koch, S.; Thiele, C.; Kulcke, A.; Malberg, H. Hyperspectral imaging for monitoring oxygen saturation levels during normothermic kidney perfusion. J. Sens. Sens. Syst. 2016, 5, 313–318. [Google Scholar] [CrossRef]
- Markgraf, W.; Mühle, R.; Lilienthal, J.; Kromnik, S.; Thiele, C.; Malberg, H.; Janssen, M.; Putz, J. Inulin Clearance During Ex vivo Normothermic Machine Perfusion as a Marker of Renal Function. ASAIO J. 2022, 68, 1211–1218. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Elliott, T.R.; Jordan, N.P.; Nicholson, M.L. The Effects of Free Heme on Functional and Molecular Changes During Ex Vivo Normothermic Machine Perfusion of Human Kidneys. Front. Immunol. 2022, 13, 849742. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.E.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin Mononucleotide as a Biomarker of Organ Quality-A Pilot Study. Transplant. Direct 2020, 6, e600. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Stone, J.P.; Lo Faro, M.L.; Hunter, J.P.; Ploeg, R.J.; Coussios, C.C.; Fildes, J.E.; Friend, P.J. Hemodynamics and Metabolic Parameters in Normothermic Kidney Preservation Are Linked With Donor Factors, Perfusate Cells, and Cytokines. Front. Med. 2021, 8, 801098. [Google Scholar] [CrossRef]
- Woud, W.W.; Arykbaeva, A.S.; Alwayn, I.P.J.; Baan, C.C.; Minnee, R.C.; Hoogduijn, M.J.; Boer, K. Extracellular Vesicles Released During Normothermic Machine Perfusion Are Associated With Human Donor Kidney Characteristics. Transplantation 2022, 106, 2360–2369. [Google Scholar] [CrossRef]
- Ashcroft, J.; Leighton, P.; Elliott, T.R.; Hosgood, S.A.; Nicholson, M.L.; Kosmoliaptsis, V. Extracellular vesicles in kidney transplantation: A state-of-the-art review. Kidney Int. 2022, 101, 485–497. [Google Scholar] [CrossRef]
- He, X.; Chen, G.; Zhu, Z.; Zhang, Z.; Yuan, X.; Han, M.; Zhao, Q.; Zheng, Y.; Tang, Y.; Huang, S.; et al. The First Case of Ischemia-Free Kidney Transplantation in Humans. Front. Med. 2019, 6, 276. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.L.; Hosgood, S.A. Renal transplantation after ex vivo normothermic perfusion: The first clinical study. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2013, 13, 1246–1252. [Google Scholar] [CrossRef]
- Rijkse, E.; de Jonge, J.; Kimenai, H.; Hoogduijn, M.J.; de Bruin, R.W.F.; van den Hoogen, M.W.F.; JNM, I.J.; Minnee, R.C. Safety and feasibility of 2 h of normothermic machine perfusion of donor kidneys in the Eurotransplant Senior Program. BJS Open 2021, 5, zraa024. [Google Scholar] [CrossRef]
- Mazilescu, L.I.; Urbanellis, P.; Kim, S.J.; Goto, T.; Noguchi, Y.; Konvalinka, A.; Reichman, T.W.; Sayed, B.A.; Mucsi, I.; Lee, J.Y.; et al. Normothermic Ex Vivo Kidney Perfusion for Human Kidney Transplantation: First North American Results. Transplantation 2022, 106, 1852–1859. [Google Scholar] [CrossRef]
- Arcolino, F.O.; Hosgood, S.; Akalay, S.; Jordan, N.; Herman, J.; Elliott, T.; Veys, K.; Vermeire, K.; Sprangers, B.; Nicholson, M.; et al. De novo SIX2 activation in human kidneys treated with neonatal kidney stem/progenitor cells. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22, 2791–2803. [Google Scholar] [CrossRef]
- Brasile, L.; Henry, N.; Orlando, G.; Stubenitsky, B. Potentiating Renal Regeneration Using Mesenchymal Stem Cells. Transplantation 2019, 103, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, S.; Eijken, M.; Møldrup, U.; Møller, B.K.; Hunter, J.; Moers, C.; Leuvenink, H.; Ploeg, R.J.; Clahsen-van Groningen, M.C.; Hoogduijn, M.; et al. Ex Vivo Administration of Mesenchymal Stromal Cells in Kidney Grafts Against Ischemia-reperfusion Injury-Effective Delivery Without Kidney Function Improvement Posttransplant. Transplantation 2021, 105, 517–528. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Vos, J.; Eijken, M.; van Pel, M.; Reinders, M.E.J.; Ploeg, R.J.; Hoogduijn, M.J.; Jespersen, B.; Leuvenink, H.G.D.; Moers, C. Treating Ischemically Damaged Porcine Kidneys with Human Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stromal Cells During Ex Vivo Normothermic Machine Perfusion. Stem Cells Dev. 2020, 29, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Parraga, J.M.; Eijken, M.; Hunter, J.; Moers, C.; Leuvenink, H.; Moller, B.; Ploeg, R.J.; Baan, C.C.; Jespersen, B.; Hoogduijn, M.J. Mesenchymal Stromal Cells as Anti-Inflammatory and Regenerative Mediators for Donor Kidneys During Normothermic Machine Perfusion. Stem Cells Dev. 2017, 26, 1162–1170. [Google Scholar] [CrossRef]
- Thompson, E.R.; Bates, L.; Ibrahim, I.K.; Sewpaul, A.; Stenberg, B.; McNeill, A.; Figueiredo, R.; Girdlestone, T.; Wilkins, G.C.; Wang, L.; et al. Novel delivery of cellular therapy to reduce ischemia reperfusion injury in kidney transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- DiRito, J.R.; Hosgood, S.A.; Reschke, M.; Albert, C.; Bracaglia, L.G.; Ferdinand, J.R.; Stewart, B.J.; Edwards, C.M.; Vaish, A.G.; Thiru, S.; et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human kidneys. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.M.; Lu, D.B.; Burns, H.; Byrne, N.; Chew, Y.V.; Julovi, S.; Ghimire, K.; Zanjani, N.T.; P’Ng, C.H.; Meijles, D.; et al. Pharmacologic targeting of renal ischemia-reperfusion injury using a normothermic machine perfusion platform. Sci. Rep. 2020, 10, 6930. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, G.T.; Hosgood, S.A.; DiRito, J.; Cui, J.; Deep, D.; Song, E.; Kraehling, J.R.; Piotrowski-Daspit, A.S.; Kirkiles-Smith, N.C.; Al-Lamki, R.; et al. Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Sci. Transl. Med. 2017, 9, eaam6764. [Google Scholar] [CrossRef]
- Thompson, E.R.; Sewpaul, A.; Figuereido, R.; Bates, L.; Tingle, S.J.; Ferdinand, J.R.; Situmorang, G.R.; Ladak, S.S.; Connelly, C.M.; Hosgood, S.A.; et al. MicroRNA antagonist therapy during normothermic machine perfusion of donor kidneys. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22, 1088–1100. [Google Scholar] [CrossRef]
- MacMillan, S.; Hosgood, S.A.; Nicholson, M.L. Enzymatic blood group conversion of human kidneys during ex vivo normothermic machine perfusion. Br. J. Surg. 2023, 110, 133–137. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. The evolution of donation after circulatory death donor kidney repair in the United Kingdom. Curr. Opin. Organ Transplant. 2017, 23, 130–135. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Barlow, A.D.; Yates, P.J.; Snoeijs, M.G.; van Heurn, E.L.; Nicholson, M.L. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys. J. Surg. Res. 2011, 171, 283–290. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Saeb-Parsy, K.; Wilson, C.; Callaghan, C.; Collett, D.; Nicholson, M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017, 7, e012237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foguenne, M.; MacMillan, S.; Kron, P.; Nath, J.; Devresse, A.; De Meyer, M.; Michel, M.; Hosgood, S.; Darius, T. Current Evidence and Future Perspectives to Implement Continuous and End-Ischemic Use of Normothermic and Oxygenated Hypothermic Machine Perfusion in Clinical Practice. J. Clin. Med. 2023, 12, 3207. https://doi.org/10.3390/jcm12093207
Foguenne M, MacMillan S, Kron P, Nath J, Devresse A, De Meyer M, Michel M, Hosgood S, Darius T. Current Evidence and Future Perspectives to Implement Continuous and End-Ischemic Use of Normothermic and Oxygenated Hypothermic Machine Perfusion in Clinical Practice. Journal of Clinical Medicine. 2023; 12(9):3207. https://doi.org/10.3390/jcm12093207
Chicago/Turabian StyleFoguenne, Maxime, Serena MacMillan, Philipp Kron, Jay Nath, Arnaud Devresse, Martine De Meyer, Mourad Michel, Sarah Hosgood, and Tom Darius. 2023. "Current Evidence and Future Perspectives to Implement Continuous and End-Ischemic Use of Normothermic and Oxygenated Hypothermic Machine Perfusion in Clinical Practice" Journal of Clinical Medicine 12, no. 9: 3207. https://doi.org/10.3390/jcm12093207
APA StyleFoguenne, M., MacMillan, S., Kron, P., Nath, J., Devresse, A., De Meyer, M., Michel, M., Hosgood, S., & Darius, T. (2023). Current Evidence and Future Perspectives to Implement Continuous and End-Ischemic Use of Normothermic and Oxygenated Hypothermic Machine Perfusion in Clinical Practice. Journal of Clinical Medicine, 12(9), 3207. https://doi.org/10.3390/jcm12093207






