Cardiometabolic Effects of Cabergoline and Combined Oral Contraceptive Pills in Young Women with Hyperprolactinemia: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
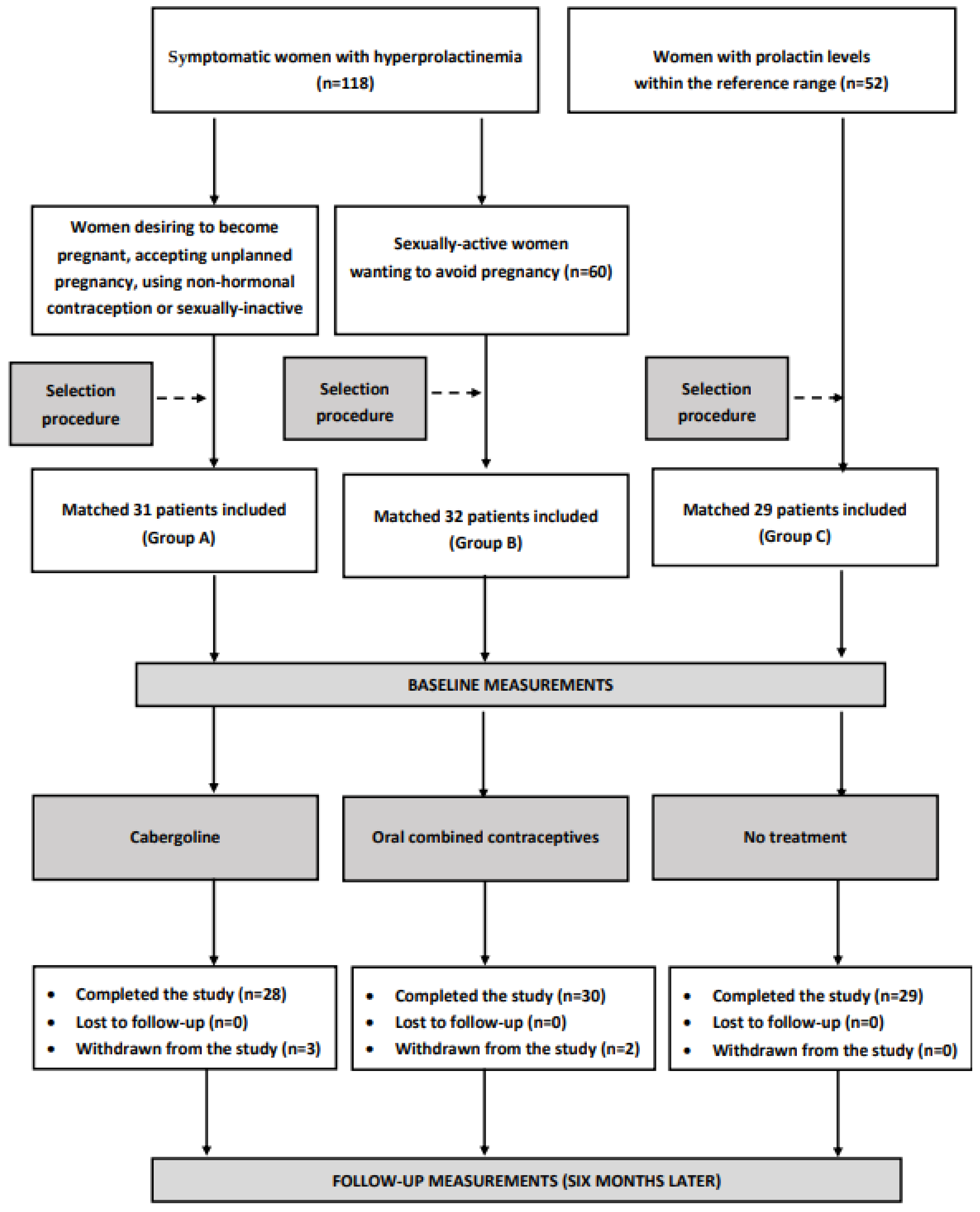
2.1. Study Population
2.2. Study Design
2.3. Laboratory Assays
2.4. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Samperi, I.; Lithgow, K.; Karavitaki, N. Hyperprolactinaemia. J. Clin. Med. 2019, 8, 2203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.B.; Li, C.L.; He, D.S.; Mao, Z.G.; Liu, D.H.; Fan, X.; Hu, B.; Zhu, Y.H.; Wang, H.J. Increased carotid intima media thickness is associated with prolactin levels in subjects with untreated prolactinoma: A pilot study. Pituitary 2014, 17, 232–239. [Google Scholar] [CrossRef]
- Arslan, M.S.; Topaloglu, O.; Sahin, M.; Tutal, E.; Gungunes, A.; Cakir, E.; Ozturk, I.U.; Karbek, B.; Ucan, B.; Ginis, Z.; et al. Preclinical atherosclerosis in patients with prolactinoma. Endocr. Pract. 2014, 20, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, D.; Deyneli, O.; Akpinar, I.; Yildiz, E.; Gözü, H.; Sezgin, O.; Haklar, G.; Akalin, S. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic premenopausal women. Eur. J. Endocrinol. 2003, 149, 187–193. [Google Scholar] [CrossRef]
- Reuwer, A.Q.; van Eijk, M.; Houttuijn-Bloemendaal, F.M.; van der Loos, C.M.; Claessen, N.; Teeling, P.; Kastelein, J.J.; Hamann, J.; Goffin, V.; von der Thüsen, J.H.; et al. The prolactin receptor is expressed in macrophages within human carotid atherosclerotic plaques: A role for prolactin in atherogenesis? J. Endocrinol. 2011, 208, 107–117. [Google Scholar] [CrossRef]
- Erem, C.; Kocak, M.; Nuhoglu, I.; Yılmaz, M.; Ucuncu, O. Blood coagulation, fibrinolysis and lipid profile in patients with prolactinoma. Clin. Endocrinol. 2010, 73, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Bruska-Sikorska, M.; Rojek, M.; Junik, R. Hyperprolactinemia and insulin resistance. Endokrynol. Pol. 2022, 73, 959–967. [Google Scholar] [CrossRef]
- Auriemma, R.S.; De Alcubierre, D.; Pirchio, R.; Pivonello, R.; Colao, A. Glucose abnormalities associated to prolactin secreting pituitary adenomas. Front. Endocrinol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Stumpe, K.O.; Kolloch, R.; Higuchi, M.; Kruck, F.; Vetter, H. Hyperprolactinaemia and antihypertensive effect of bromocriptine in essential hypertension. Identification of abnormal central dopamine control. Lancet 1977, 2, 211–214. [Google Scholar]
- Raaz, D.; Wallaschofski, H.; Stumpf, C.; Yilmaz, A.; Cicha, I.; Klinghammer, L. Increased prolactin in acute coronary syndromes as putative co-activator of ADP-stimulated P-selectin expression. Horm. Metab. Res. 2006, 38, 767–772. [Google Scholar] [CrossRef]
- Wallaschofski, H.; Lohmann, T.; Hild, E.; Kobsar, A.; Siegemund, A.; Spilcke-Liss, E.; Hentschel, B.; Stumpf, C.; Daniel, W.G.; Garlichs, C.D.; et al. Enhanced platelet activation by prolactin in patients with ischemic stroke. Thromb. Haemost. 2006, 96, 38–44. [Google Scholar] [CrossRef]
- Wallaschofski, H.; Eigenthaler, M.; Kiefer, M.; Donné, M.; Hentschel, B.; Gertz, H.J.; Lohmann, T. Hyperprolactinemia in patients on antipsychotic drugs causes ADP-stimulated platelet activation that might explain the increased risk for venous thromboembolism: Pilot study. J. Clin. Psychopharmacol. 2003, 23, 479–483. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Pirchio, R.; De Alcubierre, D.; Pivonello, R.; Colao, A. Dopamine agonists: From the 1970s to today. Neuroendocrinology 2019, 109, 34–41. [Google Scholar] [CrossRef]
- dos Santos Silva, C.M.; Barbosa, F.R.; Lima, G.A.; Warszawski, L.; Fontes, R.; Domingues, R.C.; Gadelha, M.R. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity 2011, 19, 800–805. [Google Scholar] [CrossRef]
- Ciresi, A.; Amato, M.C.; Guarnotta, V.; Lo Castro, F.; Giordano, C. Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clin. Endocrinol. 2013, 79, 845–852. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Galdiero, M.; Vitale, P.; Granieri, L.; Lo Calzo, F.; Salzano, C.; Ferreri, L.; Pivonello, C.; CariatI, F.; Coppola, G.; et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology 2013, 98, 299–310. [Google Scholar] [CrossRef]
- Auriemma, R.S.; De Alcubierre, D.; Pirchio, R.; Pivonello, R.; Colao, A. The effects of hyperprolactinemia and its control on metabolic diseases. Expert Rev. Endocrinol. Metab. 2018, 13, 99–106. [Google Scholar] [CrossRef]
- Greenman, Y.; Tordjman, K.; Stern, N. Increased body weight associated with prolactin secreting pituitary adenomas: Weight loss with normalization of prolactin levels. Clin. Endocrinol. 1998, 48, 547–553. [Google Scholar] [CrossRef]
- Berinder, K.; Nyström, T.; Höybye, C.; Hall, K.; Hulting, A.L. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary 2011, 14, 199–207. [Google Scholar] [CrossRef]
- Inancli, S.S.; Usluogullari, A.; Ustu, Y.; Caner, S.; Tam, A.A.; Ersoy, R.; Cakir, B. Effect of cabergoline on insulin sensitivity, inflammation, and carotid intima media thickness in patients with prolactinoma. Endocrine 2013, 44, 193–199. [Google Scholar] [CrossRef]
- Doğan, B.A.; Arduç, A.; Tuna, M.M.; Nasıroğlu, N.I.; Işık, S.; Berker, D.; Güler, S. Evaluation of atherosclerosis after cessation of cabergoline therapy in patients with prolactinoma. Anatol. J. Cardiol. 2016, 16, 440–447. [Google Scholar] [PubMed]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society Clinical Practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [PubMed]
- Foy, M.C.; Vaishnav, J.; Sperati, C.J. Drug-induced hypertension. Endocrinol. Metab. Clin. N. Am. 2019, 48, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Nyska, A.; Spectre, G. Drug-induced thrombosis: An update. Drug Saf. 2013, 36, 585–603. [Google Scholar] [CrossRef]
- Nader, S.; Diamanti-Kandarakis, E. Polycystic ovary syndrome, oral contraceptives and metabolic issues: New perspectives and a unifying hypothesis. Hum. Reprod. 2007, 22, 317–322. [Google Scholar] [CrossRef]
- Luciano, A.A.; Sherman, B.M.; Chapler, F.K.; Hauser, K.S.; Wallace, R.B. Hyperprolactinemia and contraception: A prospective study. Obstet. Gynecol. 1985, 65, 506–510. [Google Scholar]
- Kotsis, V.; Antza, C.; Doundoulakis, I.; Stabouli, S. Markers of early vascular ageing. Curr. Pharm. Des. 2017, 23, 3200–3204. [Google Scholar] [CrossRef]
- Tracy, R.P. Inflammation, the metabolic syndrome and cardiovascular risk. Int. J. Clin. Pract. Suppl. 2003, 134, 10–17. [Google Scholar]
- Moutachakkir, M.; Lamrani Hanchi, A.; Baraou, A.; Boukhira, A.; Chellak, S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann. Biol. Clin. 2017, 75, 225–229. [Google Scholar] [CrossRef]
- Herrick, S.; Blanc-Brude, O.; Gray, A.; Laurent, G. Fibrinogen. Int. J. Biochem. Cell Biol. 1999, 31, 741–746. [Google Scholar] [CrossRef]
- Duran-Salgado, M.B.; Rubio-Guerra, A.F. Diabetic nephropathy and inflammation. World J. Diabetes 2014, 5, 393–398. [Google Scholar] [CrossRef]
- Prasad, K. C-reactive protein (CRP)-lowering agents. Cardiovasc. Drug Rev. 2006, 24, 32–50. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Shadoan, M.K.; Zhang, L.; Wagner, J.D. Effects of hormone therapy on insulin signaling proteins in skeletal muscle of cynomolgus monkeys. Steroids 2004, 69, 313–318. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef]
- Mormile, R. Induction of GLUT4 by inhibiting IFN-γ: A winning move to halt type 2 diabetes? Int. J. Colorectal Dis. 2016, 31, 1387. [Google Scholar] [CrossRef]
- Peraldi, P.; Spiegelman, B. TNF-alpha and insulin resistance: Summary and future prospects. Mol. Cell. Biochem. 1998, 182, 169–175. [Google Scholar] [CrossRef]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and autoimmunity. Front. Immunol. 2018, 9, 73. [Google Scholar] [CrossRef]
- Tavares, G.; Marques, D.; Barra, C.; Rosendo-Silva, D.; Costa, A.; Rodrigues, T.; Gasparini, P.; Melo, B.F.; Sacramento, J.F.; Seica, R.; et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol. Metab. 2021, 51, 101241. [Google Scholar] [CrossRef]
- Kars, M.; Pereira, A.M.; Bax, J.J.; Romijn, J.A. Cabergoline and cardiac valve disease in prolactinoma patients: Additional studies during long-term treatment are required. Eur. J. Endocrinol. 2008, 159, 363–367. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Pivonello, R.; Ferreri, L.; Priscitelli, P.; Colao, A. Cabergoline use for pituitary tumors and valvular disorders. Endocrinol. Metab. Clin. N. Am. 2015, 44, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bogazzi, F.; Manetti, L.; Raffaelli, V.; Lombardi, M.; Rossi, G.; Martino, E. Cabergoline therapy and the risk of cardiac valve regurgitation in patients with hyperprolactinemia: A meta-analysis from clinical studies. J. Endocrinol. Investig. 2008, 31, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; van der Pols, J.C.; Dobson, A.J. Regression to the mean: What it is and how to deal with it. Int. J. Epidemiol. 2005, 34, 215–220. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group A | Group B | Group C |
|---|---|---|---|
| Number (n) | 28 | 30 | 29 |
| Age (years) | 34 ± 7 | 35 ± 8 | 35 ± 7 |
| Smokers (%)/Number of cigarettes a day (n)/Duration of smoking (months) | 25/10 ± 6/110 ± 32 | 23/9 ± 6/114 ± 28 | 28/9 ± 5/118 ± 35 |
| Reasons for prolactin excess (%): microprolactinoma/drug-induced hyperprolactinemia/traumatic brain injury/empty sella syndrome/idiopathic | 14/36/25/18/7 | 13/40/23/17/7 | 17/41/24/14/4 |
| Duration of hyperprolactinemia symptoms (months) | 8 ± 5 | 7 ± 6 | - |
| BMI (kg/m2) | 24.4 ± 4.0 | 24.2 ± 3.5 | 23.7 ± 4.2 |
| Waist circumference (cm) | 84 ± 8 | 84 ± 7 | 82 ± 8 |
| Systolic blood pressure (mmHg) | 131 ± 12 | 129 ± 14 | 127 ± 14 |
| Diastolic blood pressure (mmHg) | 83 ± 6 | 82 ± 5 | 81 ± 6 |
| Variable | Group A | Group B | Group C |
|---|---|---|---|
| Total prolactin (ng/mL) | |||
| Baseline | 57.0 ± 11.0 # | 55.5 ± 12.0 # | 15.0 ± 7.1 |
| Follow-up | 15.2 ± 7.0 $ | 56.8 ± 10.6 *# | 14.6 ± 7.5 |
| Monomeric prolactin (ng/mL) | |||
| Baseline | 54.2 ± 10.8 # | 52.5 ± 11.9 # | 12.4 ± 6.4 |
| Follow-up | 12.3 ± 6.4 $ | 54.1 ± 10.5 *# | 12.1 ± 6.8 |
| Macroprolactin (ng/mL) | |||
| Baseline | 2.8 ± 1.2 | 3.0 ± 1.4 | 2.6 ± 1.1 |
| Follow-up | 2.9 ± 1.0 | 2.7 ± 1.2 | 2.5 ± 1.0 |
| Glucose (mg/dL) | |||
| Baseline | 95 ± 12 # | 94 ± 13 # | 86 ± 12 |
| Follow-up | 87 ± 10 $ | 95 ± 11 *# | 85 ± 11 |
| HOMA1-IR | |||
| Baseline | 3.0 ± 0.8 # | 2.9 ± 0.8 # | 1.5 ± 0.6 |
| Follow-up | 1.7 ± 0.5 $ | 3.5 ± 1.1 *#$ | 1.6 ± 0.5 |
| Glycated hemoglobin (%) | |||
| Baseline | 5.5 ± 0.2 # | 5.5 ± 0.3 # | 5.2 ± 0.3 |
| Follow-up | 5.2 ± 0.2 $ | 5.5 ± 0.2 *# | 5.2 ± 0.2 |
| Total cholesterol (mg/dL) | |||
| Baseline | 201 ± 50 | 203 ± 42 | 194 ± 46 |
| Follow-up | 194 ± 46 | 205 ± 43 | 195 ± 47 |
| HDL-cholesterol (mg/dL) | |||
| Baseline | 48 ± 9 # | 48 ± 8 # | 56 ± 10 |
| Follow-up | 55 ± 10 $ | 46 ± 8 *# | 55 ± 11 |
| LDL-cholesterol (mg/dL) | |||
| Baseline | 118 ± 32 | 120 ± 26 | 115 ± 34 |
| Follow-up | 110 ± 29 | 121 ± 30 | 112 ± 28 |
| Triglycerides (mg/dL) | |||
| Baseline | 152 ± 48 # | 148 ± 42 # | 118 ± 32 |
| Follow-up | 120 ± 35 $ | 174 ± 47 *#$ | 121 ± 38 |
| Uric acid (mg/dL) | |||
| Baseline | 4.8 ± 1.3 # | 4.9 ± 1.2 # | 4.2 ± 1.1 |
| Follow-up | 4.0 ± 1.0 $ | 5.0 ± 1.5 *# | 4.4 ± 1.3 |
| hsCRP (mg/L) | |||
| Baselin | 2.6 ± 1.0 # | 2.8 ± 0.9 # | 1.2 ± 0.3 |
| Follow-up | 1.2 ± 0.4 $ | 3.6 ± 1.0 *#$ | 1.1 ± 0.4 |
| Fibrinogen (mg/dL) | |||
| Baseline | 372 ± 75 # | 358 ± 95 # | 288 ± 70 |
| Follow-up | 302 ± 83 $ | 455 ± 105 *#$ | 294 ± 64 |
| Homocysteine (μmol/L) | |||
| Baseline | 25.6 ± 10.1 # | 24.2 ± 10.2 # | 11.2 ± 4.3 |
| Follow-up | 12.7 ± 5.2 $ | 26.5 ± 11.4 *# | 11.6 ± 5.0 |
| UACR (mg/g) | |||
| Baseline | 31.5 ± 8.3 # | 30.4 ± 8.8 # | 8.5 ± 2.3 |
| Follow-up | 10.0 ± 4.8 $ | 36.8 ± 9.2 *#$ | 9.0 ± 4.2 |
| Variable | Group A | Group B |
|---|---|---|
| Δ Total prolactin | −73 ± 12 | 2 ± 8 |
| Δ Monomeric prolactin | −77 ± 10 * | 3 ± 7 |
| Δ Macroprolactin | −9 ± 10 | −8 ± 9 |
| Δ Glucose | −8 ± 5 * | 1 ± 2 |
| Δ HOMA1-IR | −43 ± 12 * | 21 ± 8 |
| Δ Glycated hemoglobin | −5 ± 3 * | 0 ± 5 |
| Δ Total cholesterol | −4 ± 8 | 1 ± 7 |
| Δ HDL-cholesterol | 15 ± 6 * | −4 ± 6 |
| Δ LDL-cholesterol | −7 ± 12 | 1 ± 8 |
| Δ Triglycerides | −21 ± 12 * | 18 ± 10 |
| Δ Uric acid | −17 ± 10 * | 2 ± 12 |
| Δ hsCRP | −54 ± 18 * | 29 ± 14 |
| Δ Fibrinogen | −19 ± 12 * | 27 ± 15 |
| Δ Homocysteine | −50 ± 18 * | 10 ± 15 |
| Δ UACR | −68 ± 20 * | 21 ± 10 |
| Variable | Baseline | Follow-Up (Six Months Later) |
|---|---|---|
| Total prolactin (ng/mL) | 57.8 ± 159 | 58.9 ± 17.8 |
| Glucose (mg/dL) | 96 ± 13 | 95 ± 12 |
| HOMA1-IR | 3.1 ± 1.1 | 2.9 ± 1.0 |
| Total cholesterol (mg/dL) | 204 ± 52 | 208 ± 49 |
| HDL-cholesterol (mg/dL) | 48 ± 12 | 50 ± 14 |
| LDL-cholesterol (mg/dL) | 122 ± 40 | 125 ± 38 |
| Triglycerides (mg/dL) | 160 ± 55 | 148 ± 52 |
| Uric acid (mg/dL) | 5.0 ± 2.0 | 4.8 ± 1.8 |
| hsCRP (mg/L) | 2.8 ± 1.2 | 2.8 ± 1.4 |
| Fibrinogen (mg/dL) | 380 ± 140 | 406 ± 165 |
| Homocysteine (μmol/L) | 23.8 ± 12.4 | 23.1 ± 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, R.; Kowalcze, K.; Okopień, B. Cardiometabolic Effects of Cabergoline and Combined Oral Contraceptive Pills in Young Women with Hyperprolactinemia: A Pilot Study. J. Clin. Med. 2023, 12, 3208. https://doi.org/10.3390/jcm12093208
Krysiak R, Kowalcze K, Okopień B. Cardiometabolic Effects of Cabergoline and Combined Oral Contraceptive Pills in Young Women with Hyperprolactinemia: A Pilot Study. Journal of Clinical Medicine. 2023; 12(9):3208. https://doi.org/10.3390/jcm12093208
Chicago/Turabian StyleKrysiak, Robert, Karolina Kowalcze, and Bogusław Okopień. 2023. "Cardiometabolic Effects of Cabergoline and Combined Oral Contraceptive Pills in Young Women with Hyperprolactinemia: A Pilot Study" Journal of Clinical Medicine 12, no. 9: 3208. https://doi.org/10.3390/jcm12093208
APA StyleKrysiak, R., Kowalcze, K., & Okopień, B. (2023). Cardiometabolic Effects of Cabergoline and Combined Oral Contraceptive Pills in Young Women with Hyperprolactinemia: A Pilot Study. Journal of Clinical Medicine, 12(9), 3208. https://doi.org/10.3390/jcm12093208







