Abstract
Perihilar cholangiocarcinomas (pCCA) are rare yet aggressive tumors originating from the bile ducts. While surgery remains the mainstay of treatment, only a minority of patients are amenable to curative resection, and the prognosis of unresectable patients is dismal. The introduction of liver transplantation (LT) after neoadjuvant chemoradiation for unresectable pCCA in 1993 represented a major breakthrough, and it has been associated with 5-year survival rates consistently >50%. Despite these encouraging results, pCCA has remained a niche indication for LT, which is most likely due to the need for stringent candidate selection and the challenges in preoperative and surgical management. Machine perfusion (MP) has recently been reintroduced as an alternative to static cold storage to improve liver preservation from extended criteria donors. Aside from being associated with superior graft preservation, MP technology allows for the safe extension of preservation time and the testing of liver viability prior to implantation, which are characteristics that may be especially useful in the setting of LT for pCCA. This review summarizes current surgical strategies for pCCA treatment, with a focus on unmet needs that have contributed to the limited spread of LT for pCCA and how MP could be used in this setting, with a particular emphasis on the possibility of expanding the donor pool and improving transplant logistics.
1. Introduction
Perihilar cholangiocarcinomas (pCCA) are epithelial tumors originating from the biliary tree below second-order bile ducts and proximally to the confluence of the cystic duct, and they represent 50–70% of the tumors arising from the biliary tree [1]. They are relatively rare [2] but aggressive tumors, and surgical resection is generally considered the only potentially curative treatment [3,4]. However, most patients with pCCA are diagnosed at an advanced stage, and only 15–35% are amenable to curative resection [3,5,6], which is associated with a 15–40% 5-year survival [7,8,9]. The 5-year survival of patients suffering from unresectable pCCA is 2% [10].
The dismal prognosis of unresectable pCCA led to exploring liver transplantation (LT) following neoadjuvant treatment with external beam irradiation, brachytherapy, and 5-fluorouracil (5-FU) and/or oral capecitabine as a potential treatment. The first series from the Mayo Clinic reported an impressive intention-to-treat 54% 5-year survival and a 82% 5-year survival after transplantation [11]. However, although the survival benefit of this approach has been confirmed in subsequent series [12], LT for pCCA has not gained widespread acceptance due to the difficulties in applying the neoadjuvant protocol, patient selection and the lack of clear allocation rules in this setting.
The term “transplant oncology” refers to the application of oncology along with transplant medicine and surgery to improve the survival and quality of life of cancer patients [13]. This includes considering LT for patients affected by malignancies that classically represented contraindications for LT, such as liver metastases from colorectal cancer [14], hepatocellular carcinoma beyond the most widely adopted selection criteria, pCCA and intrahepatic cholangiocarcinoma [15]. The prerequisite to successfully implement LT as a treatment for these diseases is the availability of suitable liver grafts. Although the introduction of direct acting antivirals against hepatitis C virus has profoundly changed the landscape of indications for LT [16], increasing the number of available grafts for alternative indications, the supply–demand gap for liver grafts remains an unresolved issue. The two main strategies to expand the donor pool are currently represented by the utilization of extended criteria donors (ECD) and by living donation. In most cases, ECD are represented by donors whose death has been determined by circulatory criteria (DCD), elderly donors, or liver grafts with significant macrovesicular steatosis [17,18]. While utilizations of these grafts may allow expanding the donor pool, their use has been associated with inferior outcomes as compared to those of LT using standard donors.
In the last decade, machine perfusion (MP) has been re-introduced in clinical practice, which is prompted by the need to cope with the increased risks associated with the use of ECD grafts [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Several MP techniques exist, which are characterized by different principles and mechanisms of graft protection [55]. Apart from improving graft preservation and allowing for longer preservation times, MP has a very interesting feature: it allows testing the viability of a liver graft prior to implantation (so-called “viability assessment”) [18,56]. Although normothermic MP (NMP) has been most frequently used as a tool for viability assessment, information about liver viability can be obtained also during hypothermic perfusion [33,57]. Assessing the viability of a graft should ideally allow for an increase in the number of transplanted grafts while minimizing recipient risk and avoiding discarding potentially usable grafts solely based on donor characteristics. In addition, other aspects of machine perfusion technology make its application in the setting of LT for pCCA appealing.
This review will summarize some important aspects of pCCA surgical management, emphasizing the need to improve the oncologic outcome of both resectable and unresectable patients. Literature on the results of LT for pCCA will be reviewed, discussing the limitations of current approaches. Finally, potential applications of MP in pCCA treatment of will be reviewed.
2. The Challenge of Perihilar Cholangiocarcinoma
As the international classification of cholangiocarcinoma does not distinguish between perihepatic and distal cholangiocarcinoma [4], estimating the true incidence of pCCA is difficult. In the West, age standardized incidence rates range between 0.5 and 2 per 100,000 individuals, whereas in eastern Asia, incidence is higher due to endemic liver flukes (Opisthorchis viverrini and Clonorchis sinensis) infection as well as a higher incidence of hepatolithiasis. Worldwide, the incidence of pCCA has increased in recent years, which has been linked to the increased incidence of metabolic syndrome, especially in countries with historically low incidence rates [2].
Perihilar CCA is an aggressive disease. A large study from the Netherlands on 2031 patients showed an overall median survival of 5.2 months [58]. Patients undergoing palliative systemic treatment, loco-regional treatment or best supportive care had a median survival of 12.2, 14.5 and 2.9 months, respectively. Notably, only 15% of patients underwent curative resection, which was associated with a median survival of 29.6 months [58].
2.1. Surgery for Perihilar Cholangiocarcinoma
The outcome of patients suffering from pCCA is primarily determined by the possibility to undergo curative resection. However, only a minority of patients are eligible for surgical resection due to several factors. Early diagnosis is infrequent in pCCA because most patients with early disease are asymptomatic or symptoms are poorly specific (dyspepsia, abdominal discomfort, fatigue, weight loss) [3]. Furthermore, pCCA are desmoplastic and paucicellular tumors, which complicates obtaining histological confirmation once the clinical diagnosis becomes more evident [59]. At this stage, most patients will present with jaundice and/or cholangitis and will frequently require preoperative biliary drainage (PBD). In patients undergoing surgery for pCCA, preoperative cholangitis is associated with increased mortality, overall morbidity, incidence of liver failure, and sepsis, and it is an absolute indication for PBD [60]. In patients with jaundice but not cholangitis, PBD is still frequently indicated due to the concerns for impaired liver regeneration capability, as pCCA patients are frequently candidate for major liver resections. However, PBD has been associated with higher overall morbidity, perioperative transfusion, cholangitis, infection and bile leakage [61,62], suggesting that it could be reasonably avoided in patients with sufficient future liver remnant (≥50%). It is significant that regardless of the technique used for PBD (endoscopic versus percutaneous transhepatic biliary drainage), about 15% of patients will fail to proceed to surgery because of PBD complications and progressive deterioration [63]. Another factor complicating the surgical approach is the necessity to perform an oncologically adequate (R0) surgery, which frequently involves an extended hepatectomy associated with the resection of the biliary confluence and the reconstruction by an hepaticojejunostomy while preserving a sufficient portion of liver parenchyma. Portal vein embolization has traditionally been used to induce future liver remnant hypertrophy. Associating liver partition and portal vein ligation for stage hepatectomy (ALPPS) represents an alternative approach [64]. However, ALPPS is still debated in the setting of pCCA [65,66]. In patients who do not develop sufficient liver hypertrophy after portal vein embolization alone, associating hepatic vein embolization (so-called liver venous deprivation) could contribute to enhancing the growth of future liver remnants and improve access to curative resection [67].
Patients who can access resection with curative intent are exposed to an overall major morbidity rate of 43–65%, whereas postoperative mortality rates as high as 17% have been reported [68,69]. In a study evaluating outcomes of pCCA resection in 708 low-risk patients at 24 high-volume centers, the benchmark values (i.e., the 75% or 25% percentiles of the medians of each center) for Clavien–Dindo ≥ 3 complications rate and in-hospital mortality were ≤70% and ≤8%, respectively [70].
About 80% of patients will experience recurrence after resection, in most cases within 2 years from surgery [71,72]. Overall 5-year survival is 11–44% and appears to be strongly influenced by the radicality of surgical resection, being ~60% in patients undergoing R0 resection versus <10% after R1 resection [69]. Interestingly, benchmark value for R1 resection has been set at ≤43% [70].
Overall, surgery with curative intent appears to be an option only in a minority of patients suffering from pCCA, and it is burdened by a complicated preoperative management, high postoperative morbidity and mortality, and high recurrence rates, which highlights the urgent need for alternative strategies to improve the outcome in these patients.
2.2. Liver Transplantation as a Treatment for Perihilar Cholangiocarcinoma
In theory, LT is an interesting option for patients with pCCA because it allows for the radical excision of the tumor while avoiding the issue of residual hepatic functional reserve. Unfortunately, early results of LT performed in patients with pCCA were burdened by high recurrence rates, leading to pCCA being considered a contraindication for LT. [73,74]. However, observations that long-term survival could be achieved in patients with limited tumor burden, negative resection margins and no lymph node involvement opened to reconsider pCCA as a possible indication for LT in selected patients [75]. As aforementioned, the early experiences from the Mayo Clinic (Rochester, MN, USA) team showed that by stringent patient selection and by applying a neoadjuvant protocol of external beam radiotherapy, brachytherapy and 5-FU, excellent results could be achieved [11,76,77]. Table 1 summarizes the results of LT for pCCA [11,12,76,78,79,80,81,82,83,84,85,86,87,88,89,90].

Table 1.
Results of LT for pCCA.
In the absence of a neoadjuvant protocol, LT has been associated with 5-year overall survival rates ranging from 20% to 36%, whereas using a pre-transplant chemoradiation protocol has resulted in 5-year survival rates ranging from 52% to 82%. These positive outcomes have come at the expense of strict patient selection and the morbidity of the neoadjuvant treatment itself. Indeed, 25–42% of patients initially candidate to LT after chemoradiation will not be transplanted due to inability to tolerate the treatment, complications, or tumor progression. Furthermore, LT can be technically complicated due to the effects of radiotherapy on the hepatic hilum. Since the early reports [11], an increased incidence of hepatic artery and portal vein thrombosis has been reported, leading to the frequent choice of utilizing an interposition graft anastomosed to infrarenal aorta for arterial vascularization. Early postoperative outcomes have been marked by a higher rate of complications, sometimes directly related to preoperative radiation therapy. Another element of difficulty may be represented by the presence of adhesions. Indeed, a staging laparotomy is indicated to rule out peritoneal disease or extrahepatic lymphnodes involvement before the patient can be considered eligible for LT. In the setting of deceased donor LT, considerable time can separate the staging laparotomy from LT operation, further complicating an already difficult dissection. An alternative option, which has been adopted by some centers, is performing the staging laparotomy simultaneously with LT, to avoid a repeat operation and peritoneal adhesions. While this is a viable option in living donor liver transplantation, in deceased donor LT, it necessitates the availability of a back-up recipient and has the disadvantage of significantly prolonging preservation time, which may have a negative impact on postoperative graft function.
In summary, although excellent outcomes have been reported, LT for pCCA has not gained widespread adoption. This is likely explained by the limited number of eligible patients, the difficulties in preoperative management and the technical and logistical difficulties linked to the neoadjuvant chemoradiation protocol.
3. Machine Perfusion in Liver Transplantation for Perihilar Cholangiocarcinoma: A Game Changer?
Based on the available evidence, there are two aspects of MP technology that could be of particular interest in the setting of LT for pCCA: (1) the possibility of expanding donor pool by improving the preservation of grafts from ECD and by testing their viability; (2) the possibility of improving transplant logistic by prolonging preservation time (Figure 1).
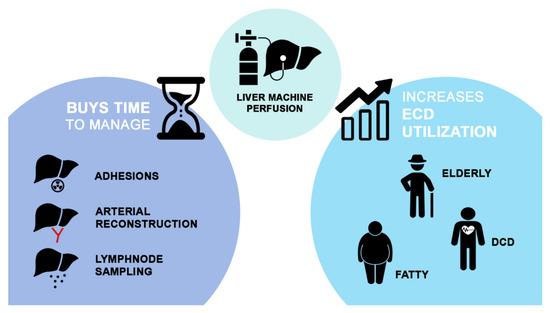
Figure 1.
A visual representation of the possible advantages of MP technology in the setting of liver transplantation for perihilar cholangiocarcinoma.
3.1. Expanding Donor Pool and Viability Assessment
In the Italian liver allocation system, patients with pCCA can benefit from a priority allocation based on a multidisciplinary discussion involving transplant surgeons, hepatologists and intensive care anesthetists [94]. It has been proposed that 5% of organ donor pool could be allocated to novel indications for which strong scientific evidence is lacking. However, in a system already stressed by a chronic organ donor shortage, this may be difficult to achieve. Campaigns promoting organ donation, the use of extended criteria donors, and living donations are all effective ways to increase the donor pool.
Despite the widespread gap between organ demand and supply, there is a significant disparity in many countries between the number of offered organs and the number of those that are eventually transplanted. In 2021, 23% of signaled livers in Italy were not transplanted because of general contraindications to organ donation or because they were judged unsuitable for LT. The situation is similar in other countries, such as the UK [29] or USA [95]. Traditionally, the choice of accepting an organ offer has been based on donor and recipient characteristics. Appropriately weighing the risk profile associated with each donor–recipient match is a fine art, and many scores have been proposed to help transplant surgeons make the difficult decision of accepting an organ for a specific recipient [96,97,98].
Machine perfusion is associated with a significant reduction in ischemia–reperfusion injury associated with LT, as demonstrated by randomized controlled trials [20,24,31,38,45,99] and retrospective studies [19,21,22,25,26,32,34,35,36,37,40,42,43,44,47,48,95,100,101,102,103,104,105,106,107]. Although clinical indication for its use is still heterogeneous [108], many groups have now implemented this technology into routine clinical practice and others are enthusiastically starting to adopt it [109]. Implementing MP technology can effectively lead to a donor pool expansion by changing the perceived risk profile associated with a specific organ offer and allowing for the successful use of a greater number of ECD grafts. As a result, transplant professionals may be more willing to consider higher-risk offers and to use ECD grafts that would otherwise be discarded.
However, this decision would still be based on a presumed risk, similarly to what happens when the graft is preserved by static cold storage. The possibility of testing liver viability during preservation challenges this concept. Indeed, one fundamental aspect of MP is the possibility to assess the function and metabolism of the liver to be transplanted ex situ, after the damage sustained during procurement and initial cold preservation. MP represents an unbiased environment, in which objective parameters guiding graft acceptance can be gathered [110,111]. While this property has classically been referred to normothermic machine perfusion [29,54,112,113], recent studies suggest that precious information about liver viability and post-LT can be obtained also during cold preservation [33,57,114]. The most widely adopted criteria for viability assessment during normothermic MP are based on lactate and glucose metabolism, pH homeostasis, vascular flows, perfusate transaminases and bile production and composition. However, at least in theory, any metabolic function can be tested during MP and serve as a further element to assess liver viability. When used on livers that were previously deemed unsuitable for LT, NMP has allowed for the successful transplantation of 46% to 100% of them, confirming its enormous potential in expanding the donor pool. However, the primary non-function of normothermic MP-treated livers has been reported [48,115]. Furthermore, normothermic MP, especially when applied after a period of cold preservation (so-called “back-to-base” approach) has been shown to be suboptimal in preventing the development of non-anastomotic biliary strictures [29]. As a result, some centers have included parameters to assess cholangiocyte viability in their protocols, which has improved the ability to predict the development of ischemic cholangiopathy. These criteria have been criticized as they might be too restrictive, and the debate about how high-risk livers should be evaluated during NMP is still ongoing. Evaluation protocols are heterogeneous and constantly evolving. While an element of subjectivity in the complex decision of accepting a liver graft appears to be unavoidable, MP appears to have enormous potential for increasing ECD utilization and expanding donor pool, thereby improving access to LT for patients suffering from pCCA.
Table 2 summarized studies on liver viability assessment during MP.

Table 2.
Studies on liver viability assessment during MP.
3.2. Improving Transplant Logistics
Time is a critical issue in LT for pCCA. The transplanting surgeon must deal with several issues at once, including adhesions from the previous staging laparotomy, fibrosis and tissue thickening from radiation therapy at the hepatic hilum, and the frequent need to perform a complex hepatic artery reconstruction using an interposition graft anastomosed to the abdominal aorta. In patients suffering from primary sclerosing cholangitis, concomitant portal hypertension may further complicate LT operation. One option for avoiding adhesions is to perform lymphnode sampling concurrently with LT; however, even in this case, the time required to confirm the absence of lymphnode involvement may prolong preservation time. Additionally, in the unfortunate case of lymph node involvement, preservation time would become prohibitively long, exposing the back-up recipient to a high risk of post-LT graft dysfunction. While liver grafts from optimal donors may tolerate longer preservation time, those from ECD are more susceptible to severe ischemia–reperfusion injury when cold ischemia time is prolonged.
MP technology can be used to safely prolong preservation time [46,54,101,112,116], possibly facilitating transplant organization and transforming it into a semi-elective procedure. In the randomized controlled trial by Nasralla et al. [31], improved postoperative outcomes were observed despite significantly longer preservation time in the MP group. With regard to normothermic MP, undoubtedly, the experience from the Innsbruck group represents a model of organization [101]. At this center, the liver is handed over to the intensive care unit team after it has been connected to the MP device by the on-call surgeon. In the ICU, the liver is monitored like a patient. At the end of the preservation, device and perfusate parameters are reviewed and, if the liver is confirmed as transplantable, LT is scheduled. The positive impact of NMP on transplant logistics has also been highlighted by the Birmingham group in the recently published NAPLES study [117]. In this study, outcomes of repeat LT performed using NMP-preserved suboptimal liver grafts were compared to those performed with optimal livers preserved by static cold storage. As the outcomes in both cohorts were comparable, the authors concluded that NMP enabled them to achieve comparable outcomes despite using grafts from extended-criteria donors, thereby improving access to LT. Another important aspect of MP in repeat LT is the possibility of relieving time pressure from the transplanting surgeon having to perform a difficult recipient hepatectomy. This approach, which can be applied also based on logistical aspects and recipient characteristics, could be of value in the setting of pCCA. The ability to extend preservation time without compromising post-LT graft function could allow the transplanting surgeon to perform an accurate lymphadenectomy, wait for the pathologist’s response, and then proceed with a difficult hepatectomy and complex arterial reconstruction. Obviously, given the time constraints of LT for pCCA, it would be unwise to abuse MP technology and begin LT with an already extended preservation time with the risk of discarding the graft should the recipient be ultimately not transplantable.
It should be noted that the possibility of prolonging preservation time is not exclusive of NMP. A recent multicenter European study has highlighted that a period of hypothermic oxygenated machine perfusion ≥ 4 h has no detrimental consequences for graft function and patient outcome [118]. Based on these results, the Groningen group designed a randomized controlled trial to test the safety of pronged hypothermic oxygenated MP, in which livers procured after 4 p.m. and 4 a.m. will be treated with prolonged dual hypothermic oxygenated machine perfusion and transplanted the following day [119]. The trial has completed recruitment, and results are expected soon [109].
It is worth noting that some of the benefits of MP, particularly the ability to extend preservation time, could potentially apply to other indications of transplant oncology, such as LT for colorectal cancer hepatic metastases, where patients frequently undergo LT after repeated hepatic resections and recipient hepatectomy can be challenging.
4. Conclusions
LT represents a potentially curative treatment for patients suffering from pCCA. The reported outcomes of LT in this setting, which compare favorably to surgical resection, have raised the question of whether LT should be offered to selected patients with resectable disease [120]. MP technology could help overcome some of the obstacles complicating this approach.
Thanks to a better understanding of genetics and molecular biology [4,121], it is likely that in the upcoming years, the armamentarium of treatments for intrahepatic and perihilar cholangiocarcinoma will expand significantly [4,122,123]. Hopefully, this will result in a greater number of previously unresectable patients becoming eligible for surgical resection or LT. To be sustainable, any expansion of the indications for LT must be accompanied by an increase in the number of available grafts. Even if more effective target- and immunotherapy will possibly avoid the need for neoadjuvant radiation and the complications related to this approach, the problem of organ supply will remain. In this view, MP will be instrumental in optimizing preservation of ECD livers and allowing a safe donor pool expansion.
Author Contributions
Conceptualization, D.P., F.C. and M.C.; writing—original draft preparation, D.P., F.C. and M.C.; writing—review and editing, E.M., S.C., N.D.S., A.L.A., G.R., S.M. and R.R.; supervision, D.P. and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.N. Hilar cholangiocarcinoma: Expert consen-sus statement. HPB 2015, 17, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; McGlynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001, 234, 507–517; discussion 509–517. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Harmsen, W.S.; Marrero, C.R.; Aboelsoud, M.M.; Ndzengue, A.; Kaiya, J.; Therneau, T.M.; Sanchez, W.; Gores, G.J.; Roberts, L.R. A new clinically based staging system for perihilar cholangiocarcinoma. Am. J. Gastroenterol. 2014, 109, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Wiggers, J.K.; Gonen, M.; Doussot, A.; Allen, P.J.; Besselink, M.G.H.; Blumgart, L.H.; Busch, O.R.C.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann. Oncol. 2015, 26, 1930–1935. [Google Scholar] [CrossRef]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M.; et al. Surgery for cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 143–155. [Google Scholar] [CrossRef]
- Lee, S.G.; Song, G.W.; Hwang, S.; Ha, T.Y.; Moon, D.B.; Jung, D.H.; Kim, K.H.; Ahn, C.S.; Kim, M.H.; Lee, S.K.; et al. Surgical treatment of hilar cholangiocarcinoma in the new era: The Asan experience. J. Hepatobiliary Pancreat. Sci. 2010, 17, 476–489. [Google Scholar] [CrossRef]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef]
- Gaspersz, M.P.; Buettner, S.; van Vugt, J.L.A.; Roos, E.; Coelen, R.J.S.; Vugts, J.; Belt, E.J.; de Jonge, J.; Polak, W.G.; Willemssen, F.; et al. Conditional survival in patients with unresectable perihilar cholangiocarcinoma. HPB 2017, 19, 966–971. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Darwish Murad, S.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98.e3, quiz e14. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Saharia, A.; McMillan, R.; Victor, D.; Kodali, S.; Shetty, A.; Nolte Fong, J.V.; et al. Transplant Oncology: An Evolving Field in Cancer Care. Cancers 2021, 13, 4911. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjornbeth, B.A.; Hagness, M.; Line, P.D. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.R.; Javle, M.; Kodali, S.; Saharia, A.; Mobley, C.; Heyne, K.; Hobeika, M.J.; Lunsford, K.E.; Victor, D.W., 3rd; Shetty, A.; et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transplant. 2022, 22, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, A.; Gimson, A.; Agarwal, K.; Aldersley, M.; Bathgate, A.; MacDonald, D.; McPherson, S.; Mutimer, D.; Gelson, W. Liver transplant listing for hepatitis C-associated cirrhosis and hepatocellular carcinoma has fallen in the United Kingdom since the introduction of direct-acting antiviral therapy. J. Viral Hepat. 2019, 26, 231–235. [Google Scholar] [CrossRef]
- Attia, M.; Silva, M.A.; Mirza, D.F. The marginal liver donor—An update. Transpl. Int. 2008, 21, 713–724. [Google Scholar] [CrossRef]
- Salvi, M.; Molinaro, L.; Metovic, J.; Patrono, D.; Romagnoli, R.; Papotti, M.; Molinari, F. Fully automated quantitative assessment of hepatic steatosis in liver transplants. Comput. Biol. Med. 2020, 123, 103836. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Nasralla, D.; Coussios, C.C.; Friend, P.J. The case for normothermic machine perfusion in liver transplantation. Liver Transplant. 2018, 24, 269–275. [Google Scholar] [CrossRef]
- Czigany, Z.; Pratschke, J.; Fronek, J.; Guba, M.; Schoning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]
- Dutkowski, P.; Polak, W.G.; Muiesan, P.; Schlegel, A.; Verhoeven, C.J.; Scalera, I.; DeOliveira, M.L.; Kron, P.; Clavien, P.A. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann. Surg. 2015, 262, 764–770; discussion 761–770. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Cardini, B.; Peter, W.; Weissenbacher, A.; Oberhuber, R.; Hautz, T.; Otarashvili, G.; Margreiter, C.; Maglione, M.; Resch, T.; et al. Static cold storage compared with normothermic machine perfusion of the liver and effect on ischaemic-type biliary lesions after transplantation: A propensity score-matched study. Br. J. Surg. 2021, 108, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Friend, P.J.; Imber, C.; St Peter, S.; Lopez, I.; Butler, A.J.; Rees, M.A. Normothermic perfusion of the isolated liver. Transplant. Proc. 2001, 33, 3436–3438. [Google Scholar] [CrossRef]
- Ghinolfi, D.; Rreka, E.; De Tata, V.; Franzini, M.; Pezzati, D.; Fierabracci, V.; Masini, M.; Cacciatoinsilla, A.; Bindi, M.L.; Marselli, L.; et al. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transplant. 2019, 25, 436–449. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, R.S., Jr.; et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am. J. Transplant. 2010, 10, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Reznik, E.; Musat, C.; Lukose, T.I.; Ratner, L.E.; Brown, R.S., Jr.; Kato, T.; Emond, J.C. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am. J. Transplant. 2015, 15, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, J.; Huang, S.; Yin, M.; Zhao, Q.; Ju, W.; Wang, D.; Gao, N.; Huang, C.; Yang, L.; et al. Abrogation of graft ischemia-reperfusion injury in ischemia-free liver transplantation. Clin. Transl. Med. 2022, 12, e546. [Google Scholar] [CrossRef]
- He, X.; Guo, Z.; Zhao, Q.; Ju, W.; Wang, D.; Wu, L.; Yang, L.; Ji, F.; Tang, Y.; Zhang, Z.; et al. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am. J. Transplant. 2018, 18, 737–744. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef]
- Mergental, H.; Perera, M.T.; Laing, R.W.; Muiesan, P.; Isaac, J.R.; Smith, A.; Stephenson, B.T.; Cilliers, H.; Neil, D.A.; Hubscher, S.G.; et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transplant. 2016, 16, 3235–3245. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; Garcia-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Op den Dries, S.; Karimian, N.; Porte, R.J. Normothermic machine perfusion of discarded liver grafts. Am. J. Transplant. 2013, 13, 2504. [Google Scholar] [CrossRef]
- Patrono, D.; Catalano, G.; Rizza, G.; Lavorato, N.; Berchialla, P.; Gambella, A.; Caropreso, P.; Mengozzi, G.; Romagnoli, R. Perfusate Analysis During Dual Hypothermic Oxygenated Machine Perfusion of Liver Grafts: Correlations With Donor Factors and Early Outcomes. Transplantation 2020, 104, 1929–1942. [Google Scholar] [CrossRef]
- Patrono, D.; Cussa, D.; Sciannameo, V.; Montanari, E.; Panconesi, R.; Berchialla, P.; Lepore, M.; Gambella, A.; Rizza, G.; Catalano, G.; et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am. J. Transplant. 2022, 22, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Lavezzo, B.; Molinaro, L.; Rizza, G.; Catalano, G.; Gonella, F.; Salizzoni, M.; Romagnoli, R. Hypothermic Oxygenated Machine Perfusion for Liver Transplantation: An Initial Experience. Exp. Clin. Transplant. 2018, 16, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Surra, A.; Catalano, G.; Rizza, G.; Berchialla, P.; Martini, S.; Tandoi, F.; Lupo, F.; Mirabella, S.; Stratta, C.; et al. Hypothermic Oxygenated Machine Perfusion of Liver Grafts from Brain-Dead Donors. Sci. Rep. 2019, 9, 9337. [Google Scholar] [CrossRef] [PubMed]
- Quintini, C.; Del Prete, L.; Simioni, A.; Del Angel, L.; Diago Uso, T.; D’Amico, G.; Hashimoto, K.; Aucejo, F.; Fujiki, M.; Eghtesad, B.; et al. Transplantation of declined livers after normothermic perfusion. Surgery 2022, 171, 747–756. [Google Scholar] [CrossRef]
- Ravaioli, M.; Germinario, G.; Dajti, G.; Sessa, M.; Vasuri, F.; Siniscalchi, A.; Morelli, M.C.; Serenari, M.; Del Gaudio, M.; Zanfi, C.; et al. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am. J. Transplant. 2022, 22, 2401–2408. [Google Scholar] [CrossRef]
- Schlegel, A.; de Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef]
- Sousa Da Silva, R.X.; Weber, A.; Dutkowski, P.; Clavien, P.A. Machine perfusion in liver transplantation. Hepatology 2022, 76, 1531–1549. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, O.B.; Bodewes, S.B.; Lantinga, V.A.; Haring, M.P.D.; Thorne, A.M.; Bruggenwirth, I.M.A.; van den Berg, A.P.; de Boer, M.T.; de Jong, I.E.M.; de Kleine, R.H.J.; et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am. J. Transplant. 2022, 22, 1658–1670. [Google Scholar] [CrossRef]
- van Leeuwen, O.B.; de Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.N.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.M.; Reyntjens, K.; van den Berg, A.P.; de Boer, M.T.; et al. Transplantation of High-risk Donor Livers After Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial. Ann. Surg. 2019, 270, 906–914. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; Karimian, N.; Matton, A.P.M.; Burlage, L.C.; Westerkamp, A.C.; van den Berg, A.P.; de Kleine, R.H.J.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 104, 907–917. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef]
- Vogel, T.; Brockmann, J.G.; Quaglia, A.; Morovat, A.; Jassem, W.; Heaton, N.D.; Coussios, C.C.; Friend, P.J. The 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transplant. 2017, 23, 207–220. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Gaurav, R.; Fear, C.; Swift, L.; Selves, L.; Ceresa, C.D.L.; Upponi, S.S.; Brais, R.; Allison, M.; Macdonald-Wallis, C.; et al. Predicting Early Allograft Function After Normothermic Machine Perfusion. Transplantation 2022, 106, 2391–2398. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef]
- de Vries, Y.; Matton, A.P.M.; Nijsten, M.W.N.; Werner, M.J.M.; van den Berg, A.P.; de Boer, M.T.; Buis, C.I.; Fujiyoshi, M.; de Kleine, R.H.J.; van Leeuwen, O.B.; et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am. J. Transplant. 2019, 19, 1202–1211. [Google Scholar] [CrossRef]
- Matton, A.P.M.; de Vries, Y.; Burlage, L.C.; van Rijn, R.; Fujiyoshi, M.; de Meijer, V.E.; de Boer, M.T.; de Kleine, R.H.J.; Verkade, H.J.; Gouw, A.S.H.; et al. Biliary Bicarbonate, pH, and Glucose Are Suitable Biomarkers of Biliary Viability During Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2019, 103, 1405–1413. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Kosmoliaptsis, V.; Randle, L.V.; Gimson, A.E.; Brais, R.; Klinck, J.R.; Hamed, M.; Tsyben, A.; Butler, A.J. Normothermic Perfusion in the Assessment and Preservation of Declined Livers Before Transplantation: Hyperoxia and Vasoplegia-Important Lessons From the First 12 Cases. Transplantation 2017, 101, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Reiling, J.; Butler, N.; Simpson, A.; Hodgkinson, P.; Campbell, C.; Lockwood, D.; Bridle, K.; Santrampurwala, N.; Britton, L.; Crawford, D.; et al. Assessment and Transplantation of Orphan Donor Livers: A Back-to-Base Approach to Normothermic Machine Perfusion. Liver Transpl. 2020, 26, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Hann, A.; Lembach, H.; Nutu, A.; Mergental, H.; Isaac, J.L.; Isaac, J.R.; Oo, Y.H.; Armstrong, M.J.; Rajoriya, N.; Afford, S.; et al. Assessment of Deceased Brain Dead Donor Liver Grafts via Normothermic Machine Perfusion: Lactate Clearance Time Threshold Can Be Safely Extended to 6 Hours. Liver Transpl. 2022, 28, 493–496. [Google Scholar] [CrossRef]
- Clavien, P.A.; Dutkowski, P.; Mueller, M.; Eshmuminov, D.; Bautista Borrego, L.; Weber, A.; Muellhaupt, B.; Sousa Da Silva, R.X.; Burg, B.R.; Rudolf von Rohr, P.; et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat. Biotechnol. 2022, 40, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Vrakas, G.; Nasralla, D.; Ceresa, C.D.L. The future of organ perfusion and re-conditioning. Transpl. Int. 2019, 32, 586–597. [Google Scholar] [CrossRef]
- Patrono, D.; Lonati, C.; Romagnoli, R. Viability testing during liver preservation. Curr. Opin. Organ. Transplant. 2022, 27, 454–465. [Google Scholar] [CrossRef]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Wurdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- van Keulen, A.M.; Franssen, S.; van der Geest, L.G.; de Boer, M.T.; Coenraad, M.; van Driel, L.; Erdmann, J.I.; Haj Mohammad, N.; Heij, L.; Klumpen, H.J.; et al. Nationwide treatment and outcomes of perihilar cholangiocarcinoma. Liver Int. 2021, 41, 1945–1953. [Google Scholar] [CrossRef]
- Barr Fritcher, E.G.; Voss, J.S.; Brankley, S.M.; Campion, M.B.; Jenkins, S.M.; Keeney, M.E.; Henry, M.R.; Kerr, S.M.; Chaiteerakij, R.; Pestova, E.V.; et al. An Optimized Set of Fluorescence In Situ Hybridization Probes for Detection of Pancreatobiliary Tract Cancer in Cytology Brush Samples. Gastroenterology 2015, 149, 1813–1824.e1. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, W.; Tang, Z.; Meng, W.; Zhou, W.; Li, X. Effect of preoperative cholangitis on prognosis of patients with hilar cholangiocarcinoma: A systematic review and meta-analysis. Medicine 2018, 97, e12025. [Google Scholar] [CrossRef]
- Celotti, A.; Solaini, L.; Montori, G.; Coccolini, F.; Tognali, D.; Baiocchi, G. Preoperative biliary drainage in hilar cholangiocarcinoma: Systematic review and meta-analysis. Eur. J. Surg. Oncol. 2017, 43, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Tang, Y.Y.; Dai, J.L.; Li, Y.; Chen, Z.Y. The effect and safety of preoperative biliary drainage in patients with hilar cholangiocarcinoma: An updated meta-analysis. World J. Surg. Oncol. 2020, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Pang, T.; Chiou, J.; Pleass, H.; Lam, V.; Hollands, M.; Johnston, E.; Richardson, A.; Yuen, L. Percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma—A systematic review and meta-analysis. HPB 2016, 18, 400–410. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Horbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Balci, D.; Sakamoto, Y.; Li, J.; Di Benedetto, F.; Kirimker, E.O.; Petrowsky, H. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure for cholangiocarcinoma. Int. J. Surg. 2020, 82S, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Olthof, P.B.; Coelen, R.J.S.; Wiggers, J.K.; Groot Koerkamp, B.; Malago, M.; Hernandez-Alejandro, R.; Topp, S.A.; Vivarelli, M.; Aldrighetti, L.A.; Robles Campos, R.; et al. High mortality after ALPPS for perihilar cholangiocarcinoma: Case-control analysis including the first series from the international ALPPS registry. HPB 2017, 19, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hocquelet, A.; Sotiriadis, C.; Duran, R.; Guiu, B.; Yamaguchi, T.; Halkic, N.; Melloul, E.; Demartines, N.; Denys, A. Preoperative Portal Vein Embolization Alone with Biliary Drainage Compared to a Combination of Simultaneous Portal Vein, Right Hepatic Vein Embolization and Biliary Drainage in Klatskin Tumor. Cardiovasc. Interv. Radiol. 2018, 41, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Launois, B.; Reding, R.; Lebeau, G.; Buard, J.L. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J. Hepatobiliary Pancreat. Surg. 2000, 7, 128–134. [Google Scholar] [CrossRef]
- Soares, K.C.; Jarnagin, W.R. The Landmark Series: Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2021, 28, 4158–4170. [Google Scholar] [CrossRef]
- Mueller, M.; Breuer, E.; Mizuno, T.; Bartsch, F.; Ratti, F.; Benzing, C.; Ammar-Khodja, N.; Sugiura, T.; Takayashiki, T.; Hessheimer, A.; et al. Perihilar Cholangiocarcinoma—Novel Benchmark Values for Surgical and Oncological Outcomes From 24 Expert Centers. Ann. Surg. 2021, 274, 780–788. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Beal, E.W.; Chakedis, J.; Chen, Q.; Lv, Y.; Ethun, C.G.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; et al. Defining Early Recurrence of Hilar Cholangiocarcinoma After Curative-intent Surgery: A Multi-institutional Study from the US Extrahepatic Biliary Malignancy Consortium. World J. Surg. 2018, 42, 2919–2929. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, S.; Todo, S.; Marsh, J.W.; Madariaga, J.R.; Lee, R.G.; Dvorchik, I.; Fung, J.J.; Starzl, T.E. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J. Am. Coll. Surg. 1998, 187, 358–364. [Google Scholar] [CrossRef]
- Jeyarajah, D.R.; Klintmalm, G.B. Is liver transplantation indicated for cholangiocarcinoma? J. Hepatobiliary Pancreat. Surg. 1998, 5, 48–51. [Google Scholar] [CrossRef]
- Shimoda, M.; Farmer, D.G.; Colquhoun, S.D.; Rosove, M.; Ghobrial, R.M.; Yersiz, H.; Chen, P.; Busuttil, R.W. Liver transplantation for cholangiocellular carcinoma: Analysis of a single-center experience and review of the literature. Liver Transplant. 2001, 7, 1023–1033. [Google Scholar] [CrossRef]
- De Vreede, I.; Steers, J.L.; Burch, P.A.; Rosen, C.B.; Gunderson, L.L.; Haddock, M.G.; Burgart, L.; Gores, G.J. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transplant. 2000, 6, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B.; Gores, G.J. Transplantation for hilar cholangiocarcinoma. Liver Transplant. 2004, 10, S65–S68. [Google Scholar] [CrossRef]
- Ahmed, O.; Vachharajani, N.; Chang, S.H.; Park, Y.; Khan, A.S.; Chapman, W.C.; Doyle, M.B.M. Single-center experience of liver transplantation for perihilar cholangiocarcinoma. HPB 2022, 24, 461–469. [Google Scholar] [CrossRef]
- Axelrod, D.; Koffron, A.; Kulik, L.; Al-Saden, P.; Mulcahy, M.; Baker, T.; Fryer, J.; Abecassis, M. Living donor liver transplant for malignancy. Transplantation 2005, 79, 363–366. [Google Scholar] [CrossRef]
- Dondorf, F.; Utebeta, F.; Fahrner, R.; Felgendreff, P.; Ardelt, M.; Tautenhahn, H.M.; Settmacher, U.; Rauchfubeta, F. Liver Transplant for Perihilar Cholangiocarcinoma (Klatskin Tumor): The Essential Role of Patient Selection. Exp. Clin. Transplant. 2019, 17, 363–369. [Google Scholar] [CrossRef]
- Duignan, S.; Maguire, D.; Ravichand, C.S.; Geoghegan, J.; Hoti, E.; Fennelly, D.; Armstrong, J.; Rock, K.; Mohan, H.; Traynor, O. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: A single-centre national experience. HPB 2014, 16, 91–98. [Google Scholar] [CrossRef]
- Ethun, C.G.; Lopez-Aguiar, A.G.; Anderson, D.J.; Adams, A.B.; Fields, R.C.; Doyle, M.B.; Chapman, W.C.; Krasnick, B.A.; Weber, S.M.; Mezrich, J.D.; et al. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann. Surg. 2018, 267, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Figueras, J.; Llado, L.; Valls, C.; Serrano, T.; Ramos, E.; Fabregat, J.; Rafecas, A.; Torras, J.; Jaurrieta, E. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transplant. 2000, 6, 786–794. [Google Scholar] [CrossRef]
- Hidalgo, E.; Asthana, S.; Nishio, H.; Wyatt, J.; Toogood, G.J.; Prasad, K.R.; Lodge, J.P. Surgery for hilar cholangiocarcinoma: The Leeds experience. Eur. J. Surg. Oncol. 2008, 34, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Mittler, J.; Pascher, A.; Theruvath, T.; Thelen, A.; Klupp, J.; Langrehr, J.M.; Neuhaus, P. Extended indications in living-donor liver transplantation: Bile duct cancer. Transplantation 2005, 80, S101–S104. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, G.M.; Sotiropoulos, G.C.; Jauch, K.W.; Lohe, F.; Hirner, A.; Kalff, J.C.; Konigsrainer, A.; Steurer, W.; Senninger, N.; Brockmann, J.G.; et al. Liver transplantation for hilar cholangiocarcinoma: A German survey. Transplant. Proc. 2008, 40, 3191–3193. [Google Scholar] [CrossRef] [PubMed]
- Marchan, E.M.; Landry, J.C. Neoadjuvant chemoradiation followed by orthotopic liver transplantation in cholangiocarcinomas: The emory experience. J. Gastrointest. Oncol. 2016, 7, 248–254. [Google Scholar] [CrossRef]
- Robles, R.; Figueras, J.; Turrion, V.S.; Margarit, C.; Moya, A.; Varo, E.; Calleja, J.; Valdivieso, A.; Valdecasas, J.C.; Lopez, P.; et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann. Surg. 2004, 239, 265–271. [Google Scholar] [CrossRef]
- Schule, S.; Altendorf-Hofmann, A.; Utess, F.; Rauchfuss, F.; Freesmeyer, M.; Knosel, T.; Dittmar, Y.; Settmacher, U. Liver transplantation for hilar cholangiocarcinoma--a single-centre experience. Langenbecks Arch. Surg. 2013, 398, 71–77. [Google Scholar] [CrossRef]
- Zaborowski, A.; Heneghan, H.M.; Fiore, B.; Stafford, A.; Gallagher, T.; Geoghegan, J.; Maguire, D.; Hoti, E. Neoadjuvant Chemoradiotherapy and Liver Transplantation for Unresectable Hilar Cholangiocarcinoma: The Irish Experience of the Mayo Protocol. Transplantation 2020, 104, 2097–2104. [Google Scholar] [CrossRef]
- Sudan, D.; DeRoover, A.; Chinnakotla, S.; Fox, I.; Shaw, B., Jr.; McCashland, T.; Sorrell, M.; Tempero, M.; Langnas, A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am. J. Transplant. 2002, 2, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.B.; Darwish Murad, S.; Heimbach, J.K.; Nyberg, S.L.; Nagorney, D.M.; Gores, G.J. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: Is pretreatment pathological confirmation of diagnosis necessary? J. Am. Coll. Surg. 2012, 215, 31–38; discussion 38–40. [Google Scholar] [CrossRef]
- Welling, T.H.; Feng, M.; Wan, S.; Hwang, S.Y.; Volk, M.L.; Lawrence, T.S.; Zalupski, M.M.; Sonnenday, C.J. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transpl. 2014, 20, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Burra, P.; Mazzaferro, V.; Belli, L.; Pinna, A.D.; Spada, M.; Nanni Costa, A.; Toniutto, P. A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a “Blended Principle Model”. Am. J. Transplant. 2015, 15, 2552–2561. [Google Scholar] [CrossRef]
- MacConmara, M.; Hanish, S.I.; Hwang, C.S.; De Gregorio, L.; Desai, D.M.; Feizpour, C.A.; Tanriover, B.; Markmann, J.F.; Zeh, H., 3rd; Vagefi, P.A. Making Every Liver Count: Increased Transplant Yield of Donor Livers Through Normothermic Machine Perfusion. Ann. Surg. 2020, 272, 397–401. [Google Scholar] [CrossRef]
- Dutkowski, P.; Oberkofler, C.E.; Slankamenac, K.; Puhan, M.A.; Schadde, E.; Mullhaupt, B.; Geier, A.; Clavien, P.A. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann. Surg. 2011, 254, 745–753; discussion 753. [Google Scholar] [CrossRef] [PubMed]
- Halldorson, J.B.; Bakthavatsalam, R.; Fix, O.; Reyes, J.D.; Perkins, J.D. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am. J. Transplant. 2009, 9, 318–326. [Google Scholar] [CrossRef]
- Rana, A.; Hardy, M.A.; Halazun, K.J.; Woodland, D.C.; Ratner, L.E.; Samstein, B.; Guarrera, J.V.; Brown, R.S., Jr.; Emond, J.C. Survival outcomes following liver transplantation (SOFT) score: A novel method to predict patient survival following liver transplantation. Am. J. Transplant. 2008, 8, 2537–2546. [Google Scholar] [CrossRef]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef]
- Agopian, V.G.; Harlander-Locke, M.P.; Markovic, D.; Dumronggittigule, W.; Xia, V.; Kaldas, F.M.; Zarrinpar, A.; Yersiz, H.; Farmer, D.G.; Hiatt, J.R.; et al. Evaluation of Early Allograft Function Using the Liver Graft Assessment Following Transplantation Risk Score Model. JAMA Surg. 2018, 153, 436–444. [Google Scholar] [CrossRef]
- Cardini, B.; Oberhuber, R.; Fodor, M.; Hautz, T.; Margreiter, C.; Resch, T.; Scheidl, S.; Maglione, M.; Bosmuller, C.; Mair, H.; et al. Clinical Implementation of Prolonged Liver Preservation and Monitoring Through Normothermic Machine Perfusion in Liver Transplantation. Transplantation 2020, 104, 1917–1928. [Google Scholar] [CrossRef]
- Cussa, D.; Patrono, D.; Catalano, G.; Rizza, G.; Catalano, S.; Gambella, A.; Tandoi, F.; Romagnoli, R. Use of Dual Hypothermic Oxygenated Machine Perfusion to Recover Extended Criteria Pediatric Liver Grafts. Liver Transplant. 2020, 26, 835–839. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, R.; Schlegel, A.; Frassoni, S.; Olivieri, T.; Ravaioli, M.; Camagni, S.; Patrono, D.; Bassi, D.; Pagano, D.; Di Sandro, S.; et al. How to Preserve Liver Grafts From Circulatory Death With Long Warm Ischemia? A Retrospective Italian Cohort Study With Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation 2021, 105, 2385–2396. [Google Scholar] [CrossRef]
- Dondossola, D.; Ravaioli, M.; Lonati, C.; Maroni, L.; Pini, A.; Accardo, C.; Germinario, G.; Antonelli, B.; Odaldi, F.; Zanella, A.; et al. The Role of Ex Situ Hypothermic Oxygenated Machine Perfusion and Cold Preservation Time in Extended Criteria Donation After Circulatory Death and Donation After Brain Death. Liver Transplant. 2021, 27, 1130–1143. [Google Scholar] [CrossRef]
- Ghinolfi, D.; Dondossola, D.; Rreka, E.; Lonati, C.; Pezzati, D.; Cacciatoinsilla, A.; Kersik, A.; Lazzeri, C.; Zanella, A.; Peris, A.; et al. Sequential Use of Normothermic Regional and Ex Situ Machine Perfusion in Donation After Circulatory Death Liver Transplant. Liver Transplant. 2021, 27, 385–402. [Google Scholar] [CrossRef]
- Patrono, D.; Zanierato, M.; Vergano, M.; Magaton, C.; Diale, E.; Rizza, G.; Catalano, S.; Mirabella, S.; Cocchis, D.; Potenza, R.; et al. Normothermic Regional Perfusion and Hypothermic Oxygenated Machine Perfusion for Livers Donated After Controlled Circulatory Death With Prolonged Warm Ischemia Time: A Matched Comparison With Livers From Brain-Dead Donors. Transpl. Int. 2022, 35, 10390. [Google Scholar] [CrossRef] [PubMed]
- Rayar, M.; Beaurepaire, J.M.; Bajeux, E.; Hamonic, S.; Renard, T.; Locher, C.; Desfourneaux, V.; Merdrignac, A.; Bergeat, D.; Lakehal, M.; et al. Hypothermic Oxygenated Perfusion Improves Extended Criteria Donor Liver Graft Function and Reduces Duration of Hospitalization Without Extra Cost: The PERPHO Study. Liver Transplant. 2021, 27, 349–362. [Google Scholar] [CrossRef]
- Patrono, D.; Cussa, D.; Rigo, F.; Romagnoli, R.; Liver Machine Perfusion Survey, G. Heterogeneous indications and the need for viability assessment: An international survey on the use of machine perfusion in liver transplantation. Artif. Organs 2022, 46, 296–305. [Google Scholar] [CrossRef]
- Patrono, D.; De Stefano, N.; Martins, P.N.; Romagnoli, R.; Meeting, C. Highlights from the Turin international workshop on liver machine perfusion. Artif. Organs 2022, 46, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Bruggenwirth, I.M.A.; de Meijer, V.E.; Porte, R.J.; Martins, P.N. Viability criteria assessment during liver machine perfusion. Nat. Biotechnol. 2020, 38, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Bruggenwirth, I.M.A.; van Leeuwen, O.B.; Porte, R.J.; Martins, P.N. The Emerging Role of Viability Testing During Liver Machine Perfusion. Liver Transplant. 2022, 28, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Jassem, W.; Mergental, H.; Heaton, N.; Mirza, D.; Perera, M.T.; Quaglia, A.; Holroyd, D.; Vogel, T.; Coussios, C.C.; et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am. J. Transplant. 2016, 16, 1779–1787. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Bogensperger, C.; Oberhuber, R.; Meszaros, A.; Gasteiger, S.; Ulmer, H.; Berchtold, V.; Krendl, F.J.; Fodor, M.; Messner, F.; et al. Perfusate Enzymes and Platelets Indicate Early Allograft Dysfunction After Transplantation of Normothermically Preserved Livers. Transplantation 2022, 106, 792–805. [Google Scholar] [CrossRef]
- Patrono, D.; Roggio, D.; Mazzeo, A.T.; Catalano, G.; Mazza, E.; Rizza, G.; Gambella, A.; Rigo, F.; Leone, N.; Elia, V.; et al. Clinical assessment of liver metabolism during hypothermic oxygenated machine perfusion using microdialysis. Artif. Organs 2022, 46, 281–295. [Google Scholar] [CrossRef]
- Patrono, D.; De Carlis, R.; Gambella, A.; Farnesi, F.; Podesta, A.; Lauterio, A.; Tandoi, F.; De Carlis, L.; Romagnoli, R. Viability assessment and transplantation of fatty liver grafts using end-ischemic normothermic machine perfusion. Liver Transplant. 2022. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Bautista Borrego, L.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hann, A.; Lembach, H.; Nutu, A.; Dassanayake, B.; Tillakaratne, S.; McKay, S.C.; Boteon, A.; Boteon, Y.L.; Mergental, H.; Murphy, N.; et al. Outcomes of normothermic machine perfusion of liver grafts in repeat liver transplantation (NAPLES initiative). Br. J. Surg. 2022, 109, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Bruggenwirth, I.M.A.; Mueller, M.; Lantinga, V.A.; Camagni, S.; De Carlis, R.; De Carlis, L.; Colledan, M.; Dondossola, D.; Drefs, M.; Eden, J.; et al. Prolonged preservation by hypothermic machine perfusion facilitates logistics in liver transplantation: A European observational cohort study. Am. J. Transplant. 2022, 22, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Bruggenwirth, I.M.A.; Lantinga, V.A.; Rayar, M.; van den Berg, A.P.; Blokzijl, H.; Reyntjens, K.; Porte, R.J.; de Meijer, V.E.; Investigators, D.-P.T. Prolonged dual hypothermic oxygenated machine preservation (DHOPE-PRO) in liver transplantation: Study protocol for a stage 2, prospective, dual-arm, safety and feasibility clinical trial. BMJ Open. Gastroenterol. 2022, 9, e000842. [Google Scholar] [CrossRef]
- Breuer, E.; Mueller, M.; Doyle, M.B.; Yang, L.; Darwish Murad, S.; Anwar, I.J.; Merani, S.; Limkemann, A.; Jeddou, H.; Kim, S.C.; et al. Liver Transplantation as a New Standard of Care in Patients With Perihilar Cholangiocarcinoma? Results From an International Benchmark Study. Ann. Surg. 2022, 276, 846–853. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Tu, J.; Zheng, Y. Identification and Validation of Novel Biomarkers and Potential Targeted Drugs in Cholangiocarcinoma: Bioinformatics, Virtual Screening, and Biological Evaluation. J. Microbiol. Biotechnol. 2022, 32, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Frosio, F.; Mocchegiani, F.; Conte, G.; Bona, E.D.; Vecchi, A.; Nicolini, D.; Vivarelli, M. Neoadjuvant therapy in the treatment of hilar cholangiocarcinoma: Review of the literature. World J. Gastrointest. Surg. 2019, 11, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ge, F.; Yuan, T.; Qian, M.; Yan, F.; Yang, B.; He, Q.; Zhu, H. The molecular mechanisms and targeting strategies of transcription factors in cholangiocarcinoma. Expert. Opin. Ther. Targets 2022, 26, 781–789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).