Enteral Nutrition during Radiotherapy for Oropharyngeal Cancers: Prevalence and Prognostic Factors Based on HPV Status (A GETTEC Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- Previously untreated OPSCC diagnosed between 2009 and 2014;
- OPSCC p16 status determined through immunohistochemistry;
- Curative intent treatment;
- Treatment based on definitive radiotherapy.
- The exclusion criteria were as follows:
- Prior surgical treatment of the tumor or lymph nodes;
- Metastatic OPSCC;
- Undetermined p16 tumor status;
- Medical history of head and neck cancer;
- Medical history of head and neck radiotherapy;
- Feeding tube before treatment initiation.
2.2. Ethical Considerations
2.3. Study Measurements
- - The absence of enteral nutrition during or after treatment;
- - The implementation of a Reactive Feeding Tube (RFT) during or after treatment.
2.4. Statistical Analyses
3. Results
3.1. Patients Clinical Characteristics
3.2. Risk and Predictive Factors of RFT Implementation According to Initial Features
3.3. Risk and Predictive Factors of RFT Implementation According to Therapeutic Modalities
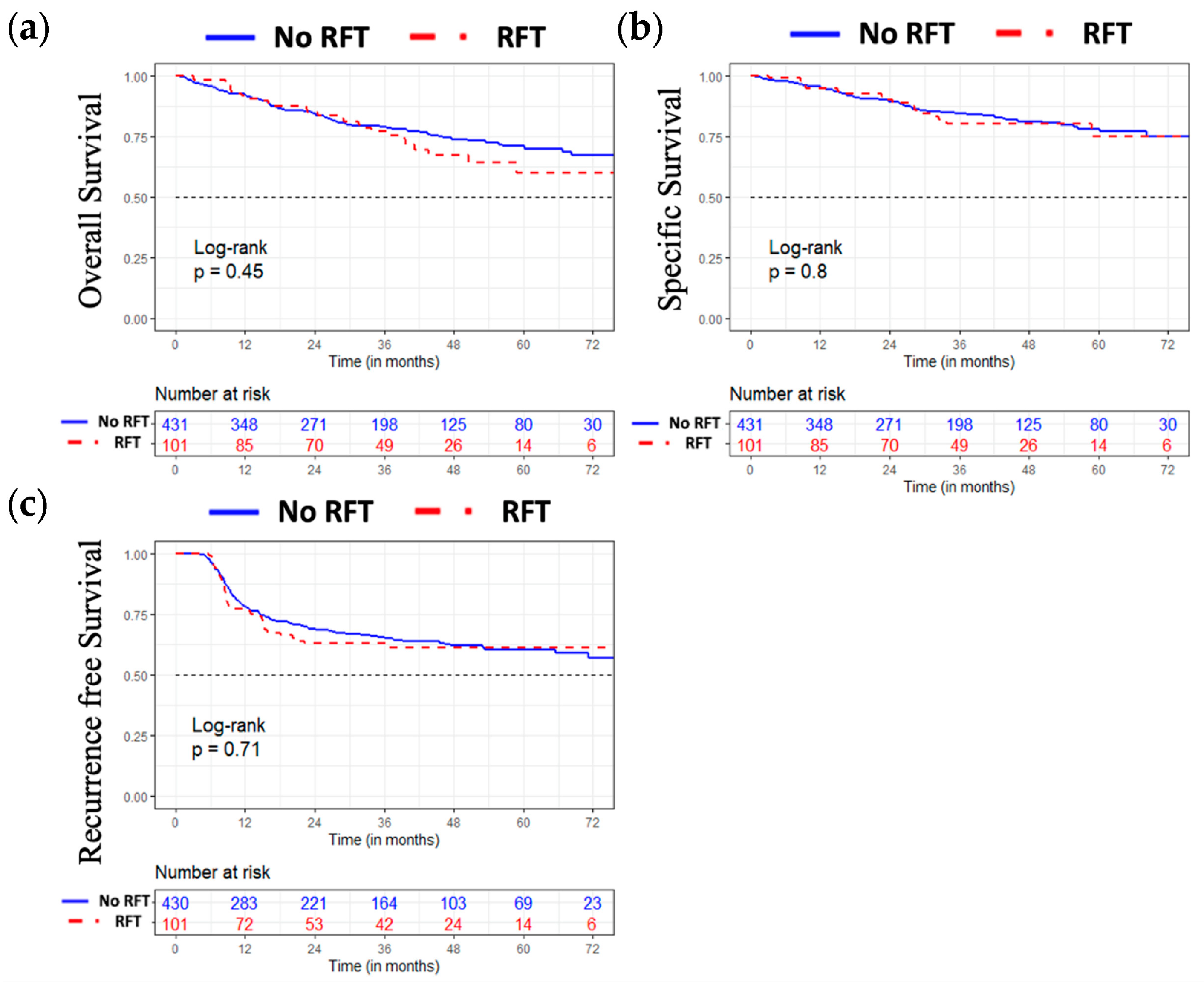
3.4. Oncological Results
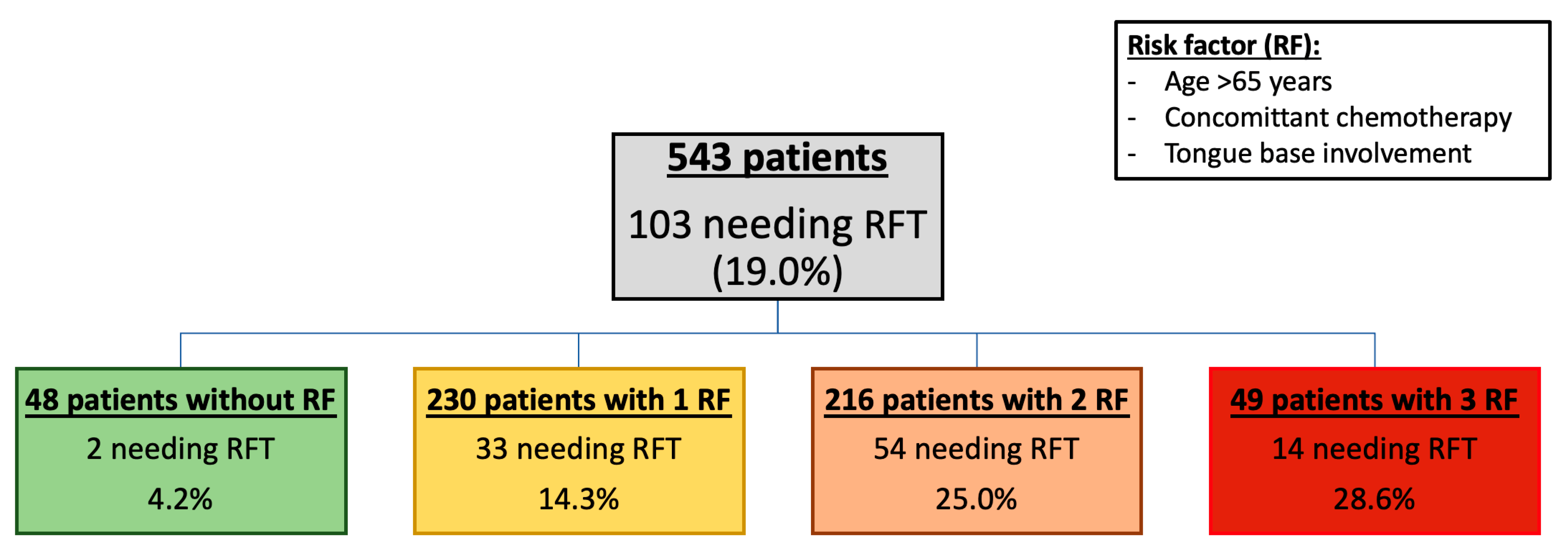
3.5. Proportion of RFT According to the Number of Risk Factors (Figure 2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Alibay, T.A.; Merad, M.; DiPalma, M.; Raynard, B.; Antoun, S. Detection and evaluation of malnutrition in oncology: What tools, what type of cancer and for what purposes? Bull. Cancer 2016, 103, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Alhambra Expósito, M.R.; Herrera-Martínez, A.D.; Manzano García, G.; Espinosa Calvo, M.; Bueno Serrano, C.M.; Gálvez Moreno, M.Á. Early nutrition support therapy in patients with head-neck cancer. Nutr. Hosp. 2018, 35, 505–510. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, W.R.; Sood, A.J.; Nguyen, S.A.; Day, T.A. Initial Symptoms in Patients With HPV-Positive and HPV-Negative Oropharyngeal Cancer. JAMA Otolaryngol.–Head Neck Surg. 2014, 140, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Nugent, B.; Lewis, S.; O’Sullivan, J.M. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst. Rev. 2013, 2013, CD007904. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.E.; Bakker, S.J.; Rietveld, D.H.F.; Kruizenga, H.M.; Langendijk, J.A.; Weijs, P.J.M.; Leemans, C.R. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br. J. Cancer 2013, 109, 1093–1099. [Google Scholar] [CrossRef]
- Patel, R.R.; Ludmir, E.B.; Augustyn, A.; Zaorsky, N.G.; Lehrer, E.J.; Ryali, R.; Trifiletti, D.M.; Adeberg, S.; Amini, A.; Verma, V. De-intensification of therapy in human papillomavirus associated oropharyngeal cancer: A systematic review of prospective trials. Oral Oncol. 2020, 103, 104608. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Gilbert, J.; Gillison, M.L.; Haddad, R.I. NCCN Guidelines Version 2.2016 Panel Members Head and Neck Cancers. 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 4 October 2017).
- Brewczyński, A.; Jabłońska, B.; Mrowiec, S.; Składowski, K.; Rutkowski, T. Nutritional Support in Head and Neck Radiotherapy Patients Considering HPV Status. Nutrients 2021, 13, 57. [Google Scholar] [CrossRef]
- Vangelov, B.; Kotevski, D.P.; Williams, J.R.; Smee, R.I. The impact of HPV status on weight loss and feeding tube use in oropharyngeal carcinoma. Oral Oncol. 2018, 79, 33–39. [Google Scholar] [CrossRef]
- Naik, M.; Ward, M.; Bledsoe, T.; Kumar, A.; Rybicki, L.; Saxton, J.; Burkey, B.; Greskovich, J.; Adelstein, D.; Koyfman, S. It is not just IMRT: Human papillomavirus related oropharynx squamous cell carcinoma is associated with better swallowing outcomes after definitive chemoradiotherapy. Oral Oncol. 2015, 51, 800–804. [Google Scholar] [CrossRef]
- Larsen, C.G.; Gyldenløve, M.; Jensen, D.H.; Therkildsen, M.H.; Kiss, K.; Norrild, B.; Konge, L.; von Buchwald, C. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br. J. Cancer 2014, 110, 1587–1594. [Google Scholar] [CrossRef]
- Arends, J.; Bodoky, G.; Bozzetti, F.; Fearon, K.; Muscaritoli, M.; Selga, G.; van Bokhorst-de van der Schueren, M.; Von Meyenfeldt, M.; Zürcher, G.; Fietkau, R.; et al. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin. Nutr. 2006, 25, 245–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Ling, Y.; Zhang, L.; Wan, H. Comparative effects of different enteral feeding methods in head and neck cancer patients receiving radiotherapy or chemoradiotherapy: A network meta-analysis. OncoTargets Ther. 2016, 9, 2897–2909. [Google Scholar] [CrossRef]
- Pohar, S.; Demarcantonio, M.; Whiting, P.; Crandley, E.; Wadsworth, J.; Karakla, D. Percutaneous endoscopic gastrostomy tube dependence following chemoradiation in head and neck cancer patients. Laryngoscope 2015, 125, 1366–1371. [Google Scholar] [CrossRef]
- Hujala, K.; Sipilä, J.; Pulkkinen, J.; Grenman, R. Early percutaneous endoscopic gastrostomy nutrition in head and neck cancer patients. Acta Oto-Laryngol. 2004, 124, 847–850. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; Van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.-K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Paccagnella, A.; Morello, M.; Da Mosto, M.C.; Baruffi, C.; Marcon, M.L.; Gava, A.; Baggio, V.; Lamon, S.; Babare, R.; Rosti, G.; et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support. Care Cancer 2010, 18, 837–845. [Google Scholar] [CrossRef]
- Jovanovic, N.; Dreyer, C.; Hawkins, S.; Thouless, K.; Palma, D.; Doyle, P.C.; Theurer, J.A. The natural history of weight and swallowing outcomes in oropharyngeal cancer patients following radiation or concurrent chemoradiation therapy. Support. Care Cancer 2021, 29, 1597–1607. [Google Scholar] [CrossRef]
- Calais, G.; Alfonsi, M.; Bardet, E.; Sire, C.; Germain, T.; Bergerot, P.; Rhein, B.; Tortochaux, J.; Oudinot, P.; Bertrand, P. Randomized Trial of Radiation Therapy Versus Concomitant Chemotherapy and Radiation Therapy for Advanced-Stage Oropharynx Carcinoma. J. Natl. Cancer Inst. 1999, 91, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; van Rooij, P.; Verduijn, G.M.; Mehilal, R.; Kerrebijn, J.D.; Levendag, P.C. The impact of treatment modality and radiation technique on outcomes and toxicity of patients with locally advanced oropharyngeal cancer. Laryngoscope 2013, 123, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Sasegbon, A.; Hamdy, S. The anatomy and physiology of normal and abnormal swallowing in oropharyngeal dysphagia. Neurogastroenterol. Motil. 2017, 29, e13100. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Palmer, J.B. Anatomy and Physiology of Feeding and Swallowing: Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.; Landry, A.; Deshpande, A.; Marchica, C.; Cooley, A.; Raol, N. Posterior Tongue Tie, Base of Tongue Movement, and Pharyngeal Dysphagia: What is the Connection? Dysphagia 2020, 35, 129–132. [Google Scholar] [CrossRef]
- Rosen, S.P.; Jones, C.A.; McCulloch, T.M. Pharyngeal swallowing pressures in the base-of-tongue and hypopharynx regions identified with three-dimensional manometry. Laryngoscope 2017, 127, 1989–1995. [Google Scholar] [CrossRef]
- King, S.N.; Dunlap, N.E.; Tennant, P.A.; Pitts, T. Pathophysiology of Radiation-Induced Dysphagia in Head and Neck Cancer. Dysphagia 2016, 31, 339–351. [Google Scholar] [CrossRef]
- Bozzetti, F. Tube feeding in the elderly cancer patient. Nutrition 2015, 31, 608–609. [Google Scholar] [CrossRef]
- Lescut, N.; Personeni, E.; Desmarets, M.; Puyraveau, M.; Hamlaoui, R.; Servagi-Vernat, S.; Bosset, J.-F.; Nguyen, F. Evaluation of a predictive score for determining indications of prophylactic enteral nutrition in patients treated by irradiation for head and neck cancer: A retrospective study in 127 patients. Cancer/Radiotherapie 2013, 17, 649–655. [Google Scholar] [CrossRef]
- Van Der Linden, N.C.; Kok, A.; Leermakers-Vermeer, M.J.; De Roos, N.M.; De Bree, R.; Van Cruijsen, H.; Terhaard, C.H.J. Indicators for Enteral Nutrition Use and Prophylactic Percutaneous Endoscopic Gastrostomy Placement in Patients With Head and Neck Cancer Undergoing Chemoradiotherapy. Nutr. Clin. Pract. 2017, 32, 225–232. [Google Scholar] [CrossRef]
- Anderson, N.J.; Wada, M.; Schneider-Kolsky, M.; Rolfo, M.; Joon, D.L.; Khoo, V. Dose-volume response in acute dysphagia toxicity: Validating QUANTEC recommendations into clinical practice for head and neck radiotherapy. Acta Oncol. 2014, 53, 1305–1311. [Google Scholar] [CrossRef]
- Grepl, J.; Sirak, I.; Vosmik, M.; Tichy, A. The Changes in Pharyngeal Constrictor Muscles Related to Head and Neck Radiotherapy: A Systematic Review. Technol. Cancer Res. Treat. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Jackson, J.E.; Anderson, N.J.; Wada, M.; Schneider, M.; Poulsen, M.; Rolfo, M.; Fahandej, M.; Gan, H.; Joon, D.L.; Khoo, V. Clinical and dosimetric risk stratification for patients at high-risk of feeding tube use during definitive IMRT for head and neck cancer. Tech. Innov. Patient Support Radiat. Oncol. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Pignon, J.-P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Blanchard, P.; Baujat, B.; Holostenco, V.; Bourredjem, A.; Baey, C.; Bourhis, J.; Pignon, J.-P.; The MACH-CH Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother. Oncol. 2011, 100, 33–40. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Metreau, A.; Louvel, G.; Godey, B.; Le Clech, G.; Jegoux, F. Long-term functional and quality of life evaluation after treatment for advanced pharyngolaryngeal carcinoma. Head Neck 2014, 36, 1604–1610. [Google Scholar] [CrossRef]
- Hutcheson, K.A.; Lewin, J. Functional Outcomes after Chemoradiotherapy of Laryngeal and Pharyngeal Cancers. Curr. Oncol. Rep. 2012, 14, 158–165. [Google Scholar] [CrossRef]
- Bossola, M. Nutritional Interventions in Head and Neck Cancer Patients Undergoing Chemoradiotherapy: A Narrative Review. Nutrients 2015, 7, 265–276. [Google Scholar] [CrossRef]
- O’shea, R.; Byrne, H.; Tuckett, J.; O’leary, G.; Sheahan, P. Impact of Current Smoking and Alcohol Consumption on Gastrostomy Duration in Patients With Head and Neck Cancer Undergoing Definitive Chemoradiotherapy. JAMA Otolaryngol.-Head Neck Surg. 2015, 141, 463–469. [Google Scholar] [CrossRef]
- Vangelov, B.; Smee, R.I. Clinical predictors for reactive tube feeding in patients with advanced oropharynx cancer receiving radiotherapy ± chemotherapy. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3741–3749. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; McNutt, T.R.; Dudley, S.A.; Kumar, R.; Starmer, H.M.; Gourin, C.G.; Moore, J.A.; Evans, K.; Allen, M.; Agrawal, N.; et al. Predictive Factors for Prophylactic Percutaneous Endoscopic Gastrostomy (PEG) Tube Placement and Use in Head and Neck Patients Following Intensity-Modulated Radiation Therapy (IMRT) Treatment: Concordance, Discrepancies, and the Role of Gabapentin. Dysphagia 2016, 31, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Vatca, M.; Lucas, J.; Laudadio, J.; D’agostino, R.; Waltonen, J.; Sullivan, C.; Rouchard-Plasser, R.; Matsangou, M.; Browne, J.; Greven, K.; et al. Retrospective analysis of the impact of HPV status and smoking on mucositis in patients with oropharyngeal squamous cell carcinoma treated with concurrent chemotherapy and radiotherapy. Oral Oncol. 2014, 50, 869–876. [Google Scholar] [CrossRef]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Harrowfield, J.; Isenring, E.; Kiss, N.; Laing, E.; Lipson-Smith, R.; Britton, B. The Impact of Human Papillomavirus (HPV) Associated Oropharyngeal Squamous Cell Carcinoma (OPSCC) on Nutritional Outcomes. Nutrients 2021, 13, 514. [Google Scholar] [CrossRef]
- Raykher, A.; Correa, L.; Russo, L.; Brown, P.; Lee, N.; Pfister, D.; Gerdes, H.; Shah, J.; Kraus, D.; Schattner, M.; et al. The Role of Pretreatment Percutaneous Endoscopic Gastrostomy in Facilitating Therapy of Head and Neck Cancer and Optimizing the Body Mass Index of the Obese Patient. JPEN J. Parenter. Enter. Nutr. 2009, 33, 404–410. [Google Scholar] [CrossRef]
- Locher, J.L.; Bonner, J.A.; Carroll, W.R.; Caudell, J.J.; Keith, J.N.; Kilgore, M.L.; Ritchie, C.S.; Roth, D.L.; Tajeu, G.S.; Allison, J.J. Prophylactic Percutaneous Endoscopic Gastrostomy Tube Placement in Treatment of Head and Neck Cancer: A comprehensive review and call for evidence-based medicine. J. Parenter. Enter. Nutr. 2011, 35, 365–374. [Google Scholar] [CrossRef]
- Cady, J. Nutritional Support During Radiotherapy for Head and Neck Cancer: The Role of Prophylactic Feeding Tube Placement. Clin. J. Oncol. Nurs. 2007, 11, 875–880. [Google Scholar] [CrossRef]
- Xiang, M.; Gensheimer, M.F.; Pollom, E.L.; Holsinger, F.C.; Colevas, A.D.; Le, Q.-T.; Beadle, B.M. Prolongation of definitive head and neck cancer radiotherapy: Survival impact and predisposing factors. Radiother. Oncol. 2021, 156, 201–208. [Google Scholar] [CrossRef]
- Goff, D.; Coward, S.; Fitzgerald, A.; Paleri, V.; Moor, J.; Patterson, J. Swallowing outcomes for patients with oropharyngeal squamous cell carcinoma treated with primary (chemo)radiation therapy receiving either prophylactic gastrostomy or reactive nasogastric tube: A prospective cohort study. Clin. Otolaryngol. 2017, 42, 1135–1140. [Google Scholar] [CrossRef]
- Wiggenraad, R.; Flierman, L.; Goossens, A.; Brand, R.; Verschuur, H.; Croll, G.; Moser, L.; Vriesendorp, R. Prophylactic gastrostomy placement and early tube feeding may limit loss of weight during chemoradiotherapy for advanced head and neck cancer, a preliminary study. Clin. Otolaryngol. 2007, 32, 384–390. [Google Scholar] [CrossRef]
- Brown, T.E.; Getliffe, V.; Banks, M.D.; Hughes, B.G.M.; Lin, C.Y.; Kenny, L.M.; Bauer, J.D. Validation of an updated evidence-based protocol for proactive gastrostomy tube insertion in patients with head and neck cancer. Eur. J. Clin. Nutr. 2016, 70, 574–581. [Google Scholar] [CrossRef]
- Koyfman, S.A.; Adelstein, D.J. Enteral Feeding Tubes in Patients Undergoing Definitive Chemoradiation Therapy for Head-and-Neck Cancer: A Critical Review. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 581–589. [Google Scholar] [CrossRef]
| All Patients N = 543 (%) | p16+ Patients N = 250 (%) | p16− Patients N = 293 (%) | p Values (UA) | |
|---|---|---|---|---|
| Initial clinical features | ||||
| Gender: male/female | 403 (74.2)/140 (25.8) | 181 (72.4)/69 (27.6) | 222 (75.8)/71 (24.2) | NS |
| Age: average ± SD | 61.08 ± 9.91 | 61.87 ± 9.88 | 60.45 ± 9.86 | NS |
| Age: >65/≥65 years | 369 (68)/174 (32) | 156 (62)/94 (38) | 213 (73)/80 (27) | 0.01 |
| ASA score: </= 3 | 392 (77.8)/112 (22.2) | 200 (80.0)/36 (14.4) | 192 (65.6)/76 (25.9) | NS |
| KFI score: | 153C(28.4)/270 (50)/ | 105 (42.2)/104 (42)/ | 48 (16.7)/166 (57)/ | NS |
| 0/1/2/3 | 80 (15)/40 (7.4) | 29 (12)/12 (4.8) | 51 (17)/28 (9.6) | |
| Tobacco use: </≥10 PY | 189 (38.3)/304 (61.7) | 104 (41.6)/146 (58.4) | 85 (29.0)/208 (71.0) | 0.03 |
| Tumor site: BOT/LPW/GTS/PPW/SP | 199 (36.7)/346 (63.7)/156 (28.7)/35 (6.5)/139 (25.6) | 95 (38.0)/188 (75.2)/71 (28.4)/3 (1.2)/47 (18.8) | 104 (35.5)/158 (53.9)/85 (29.0)/32 (10.9)/92 (31.4) | NS/0.02/NS/<0.001/<0.001 |
| T stage: </≥ 3 | 225 (41.5)/317 (58.5) | 123 (49.2)/127 (50.8) | 102 (34.8)/190 (64.8) | NS |
| N stage: </≥ 2a | 207 (38.6)/330 (61.5) | 79 (31.6)/169 (67.6) | 128 (43.7)/161 (54.9) | 0.06 |
| Treatment modalities | ||||
| Year of treatment: | NS | |||
| 2009 | 43 (7.9) | 19 (7.6) | 24 (8.2) | |
| 2010 | 61 (11.2) | 17 (6.8) | 44 (15.0) | |
| 2011 | 93 (17.1) | 43 (17.2) | 50 (17.1) | |
| 2012 | 106 (19.5) | 47 (18.8) | 59 (20.1) | |
| 2013 | 107 (19.7) | 52 (20.8) | 55 (18.8) | |
| 2014 | 122 (22.5) | 67 (26.8) | 55 (18.8) | |
| Induction CT: yes/no | 116 (21)/427 (79) | 44 (18)/206 (82) | 72 (25)/221 (75) | 0.048 |
| Conco-CT: yes/no | 435 (80.1)/108 (19.1) | 210 (84.0)/40 (16.0) | 225 (76.8)/68 (23.2) | 0.04 |
| RT interruption: Mean days ± SD | 0.78 ± 3.8 | 0.74 ± 3.8 | 0.73 ± 3.8 | NS |
| Mucositis toxicity *: <2/≥2 | 322 (59.3)/221 (40.7) | 135 (54.0)/115 (46.0) | 187 (63.8)/106 (36.2) | 0.02 |
| Dysphagia toxicity *: <2/≥2 | 412 (75.9)/131 (24.1) | 179 (71.6)/71 (28.4) | 233 (79.5)/60 (20.5) | 0.03 |
| Dyspnea toxicity *: <2/≥2 | 540 (99.4)/3 (0.6) | 249 (99.6)/1 (0.4) | 291 (99.3)/2 (0.7) | NS |
| Clinical Features | No RFT N = 440 (%) | RFT N = 103 (%) | p Values (UA) | p Values (MA) |
|---|---|---|---|---|
| Center: Nice/Nîmes/ Villejuif/Marseille/ Montpellier/Toulon/Toulouse | 80 (70.8)/57 (89.1)/100 (67.6) /37 (94.9)/40 (90.9)/ 38 (95)/88 (92.6) | 33 (29.2)/7 (10.9)/48 (32.4)/2 (5.1)/4 (9.1)/ 2 (5)/7 (7.4) | <0.001 | NS |
| Gender: male/female | 326 (80.9)/114 (81.4) | 77 (19.1)/26 (18.6) | 0.989 | - |
| Age: average ± SD | 60.71 ± 9.83 | 62.65 ± 10.15 | 0.076 | NS |
| Age: >65/≥65 years | 305 (69)/135 (31) | 64 (62)/39 (38) | 0.2 | - |
| ASA score: </=3 | 311 (79.3)/92 (82.1) | 81 (20.7)/20 (17.9) | 0.603 | - |
| KFI score: /0/1/2/3 | 124 (27.7)/217 (49)/ 62 (14)/36 (8.4) | 29 (28)/53 (51)/ 18 (17)/3 (2.9) | 0.675 | - |
| Tobacco use: </≥10 PY | 154 (81.5)/241 (81.4) | 50 (18.6)/52 (19.4) | 0.892 | - |
| Tumor site: BOT/LPW/GTS/PPW/SP | 151 (75.9)/278 (80.3)/117 (75)/28 (80)/116 (83.5) | 48 (24.1)/68 (19.7)/39 (25)/7 (20) /23 (16.5) | 0.027/0.671/0.031/1/0.472 | 0.01/-/NS/-/- |
| T stage: </≥3 | 187 (83.1)/252 (79.5) | 38 (16.9)/65 (20.5) | 0.344 | - |
| N stage: </≥2a | 179 (86.5)/256 (77.6) | 28 (13.5)/74 (22.4) | 0.014 | NS |
| p16 status: positive/negative | 196 (78.4)/244 (83.3) | 54 (21.6)/49 (16.7) | 0.182 | NS |
| Therapeutic Modalities | No RFT N = 440 (%) | RFT N = 103 (%) | p Values (UA) | p Values (MA) |
|---|---|---|---|---|
| Year of treatment: | <0.001 | NS | ||
| 2009 | 40 (9.1) | 4 (3.9) | ||
| 2010 | 57 (13) | 5 (4.8) | ||
| 2011 | 79 (18) | 17 (16.3) | ||
| 2012 | 92 (20.9) | 16 (15.4) | ||
| 2013 | 89 (20.2) | 20 (19.2) | ||
| 2014 | 82 (18.6) | 41 (39.4) | ||
| Induction CT: Yes/No | 95 (22)/345 (78) | 21 (20)/82 (80) | NS | - |
| Concomitant CT: Yes/No | 340 (78.2)/100 (92.6) | 95 (21.8)/8 (7.4) | 0.001 | 0.01 |
| Concomitant CT: | - | |||
| Platinum salts | 200 (75.2) | 66 (24.8) | 0.626 | |
| Cetuximab | 81 (81) | 19 (19) | 0.231 | |
| Other | 15 (65.2) | 8 (34.8) | 0.313 | |
| RT interruption: Mean days ± SD | 0.54 ± 3.2 | 1.7 ± 6.07 | 0.01 | - |
| Mucositis toxicity *: <2/≥2 | 72 (80)/142 (64.3) | 18 (20)/79 (35.7) | 0.01 | - |
| Dysphagia toxicity *: <2/≥2 | 148 (82.2)/66 (50.4) | 32 (17.8)/65 (49.6) | <0.001 | <0.0001 |
| Dyspnea toxicity *: <2/≥2 | 212 (69.3)/2 (66.7) | 94 (30.7)/1 (33.3) | 1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culié, D.; Schiappa, R.; Pace-Loscos, T.; Guelfucci, B.; Vergez, S.; Garrel, R.; Fakhry, N.; Dassonville, O.; Poissonnet, G.; Lallemant, B.; et al. Enteral Nutrition during Radiotherapy for Oropharyngeal Cancers: Prevalence and Prognostic Factors Based on HPV Status (A GETTEC Study). J. Clin. Med. 2023, 12, 3169. https://doi.org/10.3390/jcm12093169
Culié D, Schiappa R, Pace-Loscos T, Guelfucci B, Vergez S, Garrel R, Fakhry N, Dassonville O, Poissonnet G, Lallemant B, et al. Enteral Nutrition during Radiotherapy for Oropharyngeal Cancers: Prevalence and Prognostic Factors Based on HPV Status (A GETTEC Study). Journal of Clinical Medicine. 2023; 12(9):3169. https://doi.org/10.3390/jcm12093169
Chicago/Turabian StyleCulié, Dorian, Renaud Schiappa, Tanguy Pace-Loscos, Bruno Guelfucci, Sebastien Vergez, Renaud Garrel, Nicolas Fakhry, Olivier Dassonville, Gilles Poissonnet, Benjamin Lallemant, and et al. 2023. "Enteral Nutrition during Radiotherapy for Oropharyngeal Cancers: Prevalence and Prognostic Factors Based on HPV Status (A GETTEC Study)" Journal of Clinical Medicine 12, no. 9: 3169. https://doi.org/10.3390/jcm12093169
APA StyleCulié, D., Schiappa, R., Pace-Loscos, T., Guelfucci, B., Vergez, S., Garrel, R., Fakhry, N., Dassonville, O., Poissonnet, G., Lallemant, B., Sudaka, A., Saada-Bouzid, E., Benezery, K., Temam, S., Gorphe, P., Chamorey, E., & Bozec, A. (2023). Enteral Nutrition during Radiotherapy for Oropharyngeal Cancers: Prevalence and Prognostic Factors Based on HPV Status (A GETTEC Study). Journal of Clinical Medicine, 12(9), 3169. https://doi.org/10.3390/jcm12093169







