Abstract
Hypertensive heart disease (HHD) develops in response to the chronic exposure of the left ventricle and left atrium to elevated systemic blood pressure. Left ventricular structural changes include hypertrophy and interstitial fibrosis that in turn lead to functional changes including diastolic dysfunction and impaired left atrial and LV mechanical function. Ultimately, these changes can lead to heart failure with a preserved (HFpEF) or reduced (HFrEF) ejection fraction. This review will outline the clinical evaluation of a patient with hypertension and/or suspected HHD, with a particular emphasis on the role and recent advances of multimodality imaging in both diagnosis and differential diagnosis.
1. Introduction
Worldwide, the age-standardised prevalence of hypertension continues to rise and has reached 30% in the general population and up to two-thirds in those over the age of 60 [1,2]. Hypertension is the most frequent cardiovascular risk factor that is associated with cardiac remodelling [1,2]. Hypertensive heart disease (HHD) is the spectrum of cardiovascular abnormalities developing in response to the chronic exposure of the left ventricle (LV) and left atrium (LA) to elevated systemic blood pressure. Hypertension is an important vascular risk factor for the development of atherosclerosis and its sequelae.
Left ventricular hypertrophy (LVH)—defined as an increase in the LV wall thickness or mass—is widely regarded as a cardinal feature and phase in the evolution of HHD [3]. Traditional models of the pathophysiological response to chronic hypertension describe the development of LVH as an initial adaptive response of the LV to reduce wall stress. According to these models, systolic heart failure then develops via the intermediate step of LVH when the ability of the ventricle to compensate for elevated wall stress is exhausted or the ventricle is exposed to another additional insult [3]. However, while this transition can doubtless occur in some individuals, it is increasingly appreciated that these models are not supported by empirical evidence in humans and that concentric LVH does not commonly transition into dilated systolic heart failure in the absence of another myocardial insult (e.g., intercurrent myocardial infarction) [3]. Some patients with hypertension also appear to be able to develop dilated systolic heart failure in the absence of antecedent concentric LVH or an additional intercurrent myocardial insult, and indeed, eccentric remodelling rather than concentric hypertrophy may occur in patients with hypertension [4].
LV structural changes include hypertrophy and interstitial fibrosis that in turn lead to functional changes including diastolic dysfunction and impaired LA and LV mechanical function. Ultimately, these changes can lead to heart failure with a preserved (HFpEF) or reduced (HFrEF) ejection fraction [5]. It should be noted that while hypertrophy is a hallmark of HHD, interstitial fibrosis and its functional consequences may precede the development of hypertrophy. Synergistically, diabetes, obesity, dyslipidaemia, metabolic syndrome, obstructive sleep apnoea, and renal failure add to more advanced LV dysfunction and structural impairment [6]. A further challenge to clinicians is the differential diagnosis of LVH, particularly where dual pathology may exist. This review will outline the clinical evaluation of a patient with hypertension and/or suspected hypertensive heart disease, with a particular emphasis on the role of multimodality imaging in both diagnosis and differential diagnosis.
2. History and Examination
Most patients with hypertension are asymptomatic and, as such, the condition has often been described as a silent killer [7]. History taking should therefore focus on symptoms that may result from a hypertensive crisis (headache, dizziness, visual disturbance, chest pain, dyspnoea), conditions which may predispose to or contribute to hypertension (e.g., phaeochromocytoma, Cushing’s disease/syndrome, acromegaly, coarctation of the aorta, etc.) and detecting hypertensive heart failure (exertional dyspnoea, orthopnoea, paroxysmal nocturnal dyspnoea, oedema, paroxysmal palpitations/atrial fibrillation). Patients should be comprehensively examined to diagnose hypertension, identify syndromic causes (Table 1) or risk factors for secondary hypertension, and screen for cardiovascular sequelae [8]. Table 2 summarises the key clinical features to assess.

Table 1.
Comprehensive examination of patients with arterial hypertension for identification of syndromic causes, risk factors for secondary hypertension, and to screen for cardiovascular sequelae.

Table 2.
Key clinical features to assess in patients with arterial hypertension.
3. Investigations
All patients should have a baseline 12-lead Electrocardiogram to screen for LVH/end-organ damage although it has a low sensitivity for detecting LVH [8]. All patients should also be offered basic blood chemistry to screen for renal disease and markers that might point towards a secondary cause (e.g., unexplained hypokalaemia). Most patients over the age of 40 who have confirmed hypertension and who respond to treatment do not need any further investigations. Patients under the age of 40 without a clear cause for their hypertension should be screened for secondary causes. Those over the age of 40 who have suggestive features on history taking, examination, or initial biochemical screening should also be considered for further investigation. Testing for adrenal secondary causes for hypertension should focus on a biochemical confirmation of diagnosis first before any imaging is undertaken given the high incidence of often unrelated adrenal adenomas in the general population. Non-invasive imaging of the renal arteries with MRI or CT should be considered to screen for fibromuscular dysplasia, and in older patients who experience significant declines in renal function on ACE-inhibitor/angiotensin II receptor blocker therapy, to screen for atherosclerotic renal artery stenosis. A urine sample should be obtained to screen for microalbuminuria/proteinuria and glycosuria. A 24 h collection can be of value to assess sodium excretion (and thereby identify dietary sodium excess) or separately for catecholamines where a phaeochromocytoma is suspected.
4. Echocardiography
Given its availability and low cost, transthoracic echocardiography (TTE) is the first-line diagnostic imaging test to detect structural and functional changes in HHD. Left ventricular mass index (LVMI), left ventricular diastolic dysfunction (LVDD), and impaired global longitudinal strain (GLS) have prognostic implications in patients with arterial hypertension [9,10,11]. However, since large outcome trials show lower cardiovascular event rates that parallel pharmacologic blood pressure lowering, antihypertensive therapy is warranted irrespective of the presence of HHD and so routine echocardiography in all patients is not indicated.
Echocardiographic assessment of LVH and thus left ventricular mass (LVM) relies on linear measurements of LV dimensions on 2-dimensional images. Calculation of myocardial mass then uses geometric assumptions with respect to LV shape [12], which, together with the dependence on image quality, represents a major drawback of this technique. Thus, while echocardiographic assessment of LVM in populations can detect, for example, differences in subpopulations, its sensitivity to detect changes on an individual level is reduced. When compared to cardiovascular magnetic resonance (CMR) imaging, 2-dimensional echocardiography tends to overestimate LVM in patients with good image quality and underestimate LVM in those with limited image quality [13]. The aforementioned limitations regarding geometric assumptions can be overcome by using 3-dimensional echocardiography, and it has been shown that this leads to LVH measurements that reduce underestimation compared to CMR [14]. However, 3D echocardiography heavily depends on optimal image quality. Since LVM is related to body size, indexing values to parameters of body size such as height or body surface area is necessary for comparison to population-based reference values. Whereas LVM/body surface area ratios underestimate the prevalence of LVH in obese patients, LVM/height ratios may need to rely on additional allometric scaling for accuracy [15]. Depending on the relation between LVM and LV dimensions, LVH can be further divided into concentric and eccentric forms (Figure 1). Together with LV remodelling without LVH, which represents an initial stage of HHD, these parameters are associated with increased cardiovascular risk.
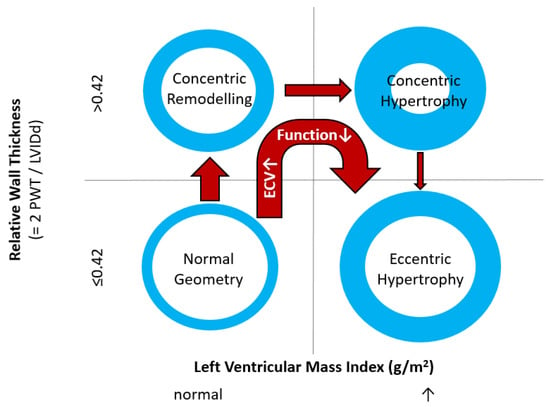
Figure 1.
Commonly used classification of left ventricular geometry and typical geometry changes occurring in patients with uncontrolled hypertension/hypertensive heart disease. PWT denotes posterior wall thickness; LVIDd denotes Left Ventricular Inner Diastolic diameter; ECV denotes myocardial extracellular volume. Deteriorating function implies both parameters of systolic and diastolic function. Normal values for Left Ventricular Mass Index depend on gender and imaging modality used.
LV systolic function is maintained at normal or near-normal values until the late stages of HHD. The most commonly used surrogate marker of LV systolic function is left ventricular ejection fraction (LVEF), which depends not only on LV contractility but also on pre- and afterload and left atrial function. In clinical practice, LVEF is measured on 2D echocardiography using Simpson’s biplane method or on 3D echocardiography using manually assisted semi-automated endocardial border tracking [12]. Of note, fully automated artificial-intelligence-supported software tools have recently been shown to accurately reproduce LVEF measurements performed by human experts [16].
While LVEF may remain normal along the natural history of HHD, echocardiographic techniques such as speckle tracking strain have been shown to be able to detect subtler impairments in myocardial mechanics such as reduced global longitudinal strain (GLS) [17]. In hypertensive patients with normal ejection fraction, reduced GLS has been shown to predict cardiovascular events independent of clinical parameters and the presence of increased LVM [11]. The myocardial consequences of chronic systolic and diastolic hypertension are not confined to myocyte hypertrophy but also include perivascular and intramyocardial diffuse fibrosis. The LV strain has emerged as a sensitive marker of LV function and may provide a long-term prognostic assessment over traditional echocardiographic parameters [18]. In patients with HHD, the LV strain (specifically GLS) may predict MACE (death and admission because of heart failure, myocardial infarction, and strokes), independent of and incremental to clinical parameters and concentric hypertrophy [11]. While GLS represents the best validated and most easily measured parameter of LV mechanics, multidirectional strain and LV twist/untwist are also affected during the pathogenesis of HHD with an early increase in twist followed by deterioration in later stages [19].
Inherent to the LV structural changes including hypertrophy and interstitial fibrosis, LV elasticity, compliance, and stiffness are compromised in hypertensive patients and lead to LVDD. While a complete description of an echocardiographic assessment of diastolic function is beyond the scope of the present review, measurements include Doppler and tissue Doppler measurements of mitral inflow and mitral annular velocities [20]. These measurements are then integrated with LA volumes and systolic pulmonary pressure to diagnose LVDD. However, the accuracy of these parameters is controversial, with some reports showing a good correlation with invasive pressure measurements [21], while others have reported only modest relationships [22].
The LA dilates as a consequence of LVDD in HHD, and the LA volume index (LAVI) has been shown to independently predict cardiovascular events [23]. Recently, speckle tracking strain imaging has enabled accurate assessment of LA mechanics throughout the cardiac cycle. This includes LA reservoir function during LV contraction and isovolumic relaxation, conduit function during early and mid-diastole, and active atrial contraction following atrial systole. In hypertensive patients, limited data indicate that reservoir and conduit function decreases, while, likely as a compensatory mechanism to maintain stroke volume, active atrial contraction increases [24].
Furthermore, arterial hypertension is a risk factor for developing an abdominal aortic aneurysm. Screening for abdominal aortic aneurysms can easily be integrated into a routine echocardiography assessment and is indicated in patients with long-standing hypertension and individuals over 65 years of age (female smokers and males irrespective of smoking status) [25].
Serial echocardiography can be used in principle to monitor longitudinal changes in LV mass in response to treatment, which may have prognostic implications; however, the higher accuracy and precision of CMR make this more suited for this application.
5. Cardiovascular Magnetic Resonance
Hypertensive patients with LV hypertrophy disproportionate to the degree of hypertension may be referred for CMR for further examination and differential diagnosis. CMR is regarded as the non-invasive gold standard for the evaluation of LV volumes, systolic function, LV mass, and myocardial tissue characterisation [26]. Image acquisition can be achieved largely independent of body habitus, imaging windows, and without ionising radiation exposure [26]. Cardiac images are usually acquired in four-, three-, and two-chamber views, as well as a full stack of 10–15 short-axis images. Its three-dimensional nature with excellent spatial resolution and high tissue contrast enables accurate measurement of cardiac function and morphology: LV volumes, mass, and the ejection fraction, as well as an assessment of regional wall motion abnormalities without relying on geometrical assumptions (such as Simpson’s biplane method) [27]. CMR provides the potential to also assess and quantify focal and diffuse myocardial fibrosis caused by hypertension (hypertension-mediated organ damage) and to screen for secondary causes of hypertension [28]. The nascent technique of cardiac MR elastography can also allow the non-invasive measurement of myocardial stiffness [29]. Importantly, the high accuracy and precision of CMR allow prognostically important longitudinal changes in LV mass in response to therapy to be readily appreciated [30]
Table 3 summarises a suggested CMR scan protocol for the diagnosis of presumed HHD and differential diagnosis of patients with a “thickened left ventricle”.

Table 3.
The cardiovascular magnetic resonance protocol used for assessment of hypertensive patients. The described protocol can be acquired within 45–50 min. Images are examples from patients with hypertensive heart disease.
Similar to echocardiography, LV geometry can be assessed using relative wall thickness (RWT) and CMR-specific normal values for LV myocardial mass (Figure 2). LA dimensions can be assessed with Simpson’s biplane or area-length methods from the standard views, or more precisely with a volumetric assessment using an atrial short-axis stack. LA enlargement is a reliable marker of diastolic dysfunction (chronically elevated LV filling pressures) in the absence of mitral valve disease [31]. The recently introduced left atrial coupling index (LACI) is a ratio of the indexed left atrial end-diastolic volume (LAVI) in relation to the left ventricular end-diastolic volume (LVEDVI) [32]. This ratio may offer prognostic information regarding cardiovascular events such as atrial fibrillation, heart failure, and coronary artery disease-related death.
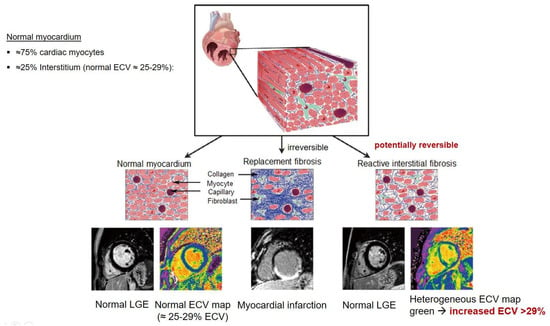
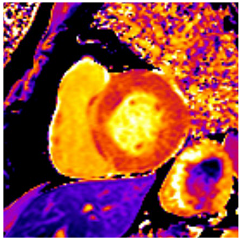
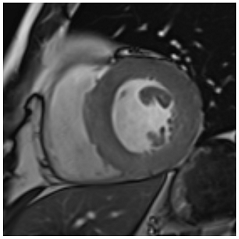
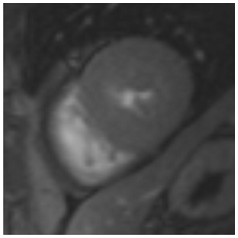
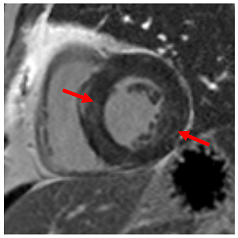
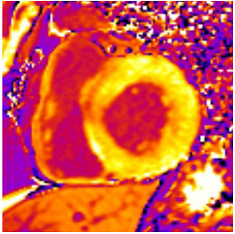
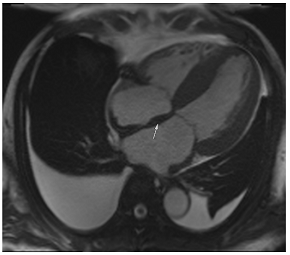
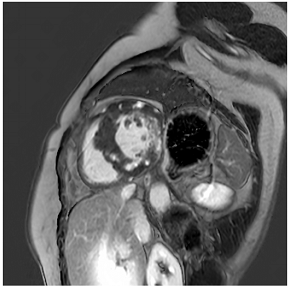
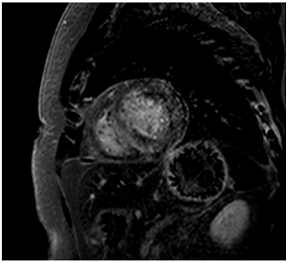
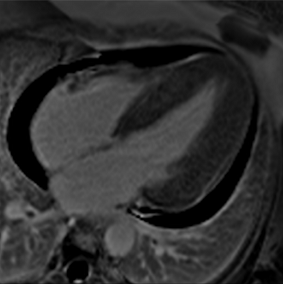
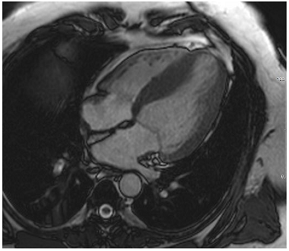
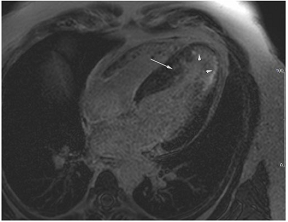
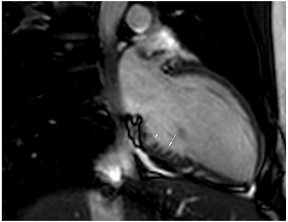
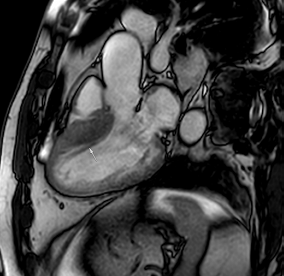
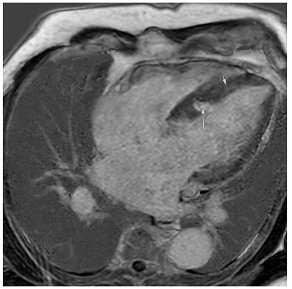
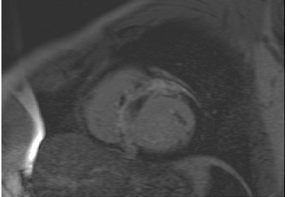
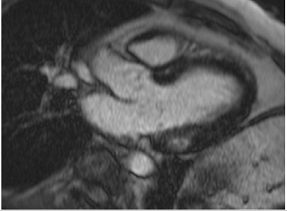
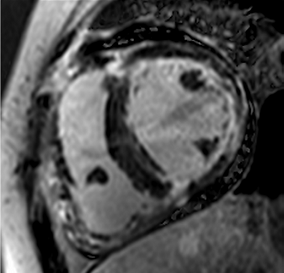
Figure 2.
Tissue characterization with Late Gadolinium Enhancement (LGE) and Extracellular Volume (ECV) Map. Adapted with permission from [33].
6. CMR Strain
Feature-tracking strain imaging can also be performed in CMR with the advantage of better image quality, lack of angle dependency or impediment from difficult acoustic windows, and less observer dependency [34]. Myocardial strain exhibited good reproducibility, declined prior to changes in LVEF or volumes, and correlated with mean arterial pressure in hypertensive patients [34]. LV longitudinal strain exhibits better reproducibility and less vendor dependency compared to the other strain types [6]. Furthermore, the CMR strain was shown to correlate with myocardial fibrosis detected by CMR [35].
7. CMR Stress Perfusion
Hypertension is a well-established atherosclerotic risk factor for the evolution of coronary artery disease (CAD). Both macrovascular CAD of the epicardial coronary arteries and microvascular CAD (small vessel disease with endothelial dysfunction) are detectable by CMR stress perfusion [36]. There is an increased frequency of hypertension in patients with chest pain, angiographically normal coronary arteries, and microvascular CAD, which may be detected by CMR using visual assessment or perfusion quantification techniques [31].
CMR stress perfusion essentially visualizes myocardial first-pass perfusion during pharmacological stress. Images are interpreted in conjunction with rest perfusion images and late gadolinium enhancement (LGE) images. In the presence of a significant epicardial coronary stenosis, the myocardial contrast uptake is reduced in a specific coronary territory (coronary pattern). Conversely, microvascular dysfunction displays a more diffuse and delayed but synchronous myocardial contrast uptake during stress. Stress CMR offers excellent sensitivity and specificity for the detection of anatomically and functionally significant CAD [37] and allows risk stratification irrespective of LVEF, the presence of CAD, symptoms, and LGE [38]. The assessment of quantitative myocardial blood flow has prognostic value and is likely to be used in the clinical routine in the near future [39].
8. Tissue Characterisation with CMR
To avoid the low but important risks of endomyocardial biopsy, which can have an overall complication rate of up to 6% [2], myocardial fibrosis can be assessed non-invasively using CMR: LGE is suitable for detecting irreversible replacement fibrosis and myocardial scarring and T1/ECV mapping for detection of potentially reversible (reactive) interstitial and more diffuse fibrosis (not detectable by LGE) (Figure 2).
9. Tissue Characterisation with Late Gadolinium Enhancement
LGE has become the reference standard for non-invasive imaging of myocardial scar and focal fibrosis [40]. Gadolinium chelates are interstitial agents that cannot penetrate healthy intact cell membranes. Therefore, they remain in the interstitial space and accumulate in areas of cell injury/necrosis and focal fibrosis where this is expanded, while in healthy regions, contrast more readily washes out [41]. Specific LGE patterns are seen in different diseases (e.g., subendocardial fibrosis in CAD, patchy epicardial/mid-wall fibrosis in areas of hypertrophy in HCM). Minor areas of LGE can be detected in up to 50% of patients with HHD, but there is no specific pattern (in 95% of the non-ischaemic LGE distribution) [42]. If present, LGE is often found in the basal to mid-septal, inferior, and inferolateral segments in patients with HHD [42]. The severity of diastolic dysfunction increases with the extent of fibrosis by LGE [25,31]. Furthermore, focal fibrosis/LGE may be a substrate for ventricular arrhythmia and is associated with sudden cardiac death [31].
10. Tissue Characterisation with T1 and Extracellular Volume (ECV) Mapping
LGE allows the detection of focal alterations in the myocardium, but diffuse fibrosis may go undetected on LGE imaging. Tissue characterisation with parametric mapping methods such as T1 and ECV mapping has the potential to detect and quantify both focal and diffuse alterations in the myocardial structure. Furthermore, changes in the myocardium over time may be assessed longitudinally [40]. Estimation of myocardial ECV (interstitium and extracellular matrix) requires the measurement of myocardial and blood T1 before and after the administration of contrast agents, as well as the patient’s haematocrit. ECV can then be calculated using the formula:
Myocytes account for approximately one-third of all cells in normal myocardium. The remaining two-thirds of cells include endothelial and vascular smooth muscle cells and fibroblasts in interstitial/perivascular spaces [2] (Figure 2). Normal CMR ECV values vary between 25.3 and 3.5% [43]. Ideally, age- and sex-corrected normal values for ECV should be used [44]. Hypertension affects both the cellular and extracellular compartments of the myocardium. In addition to cardiomyocyte hypertrophy, in HHD, fibrous tissue (primarily type I fibrillar collagen) is deposited in the extracellular matrix over time and leads to increased tissue stiffness (i.e., diastolic dysfunction) [2]. ECV values are higher in hypertensive patients with LVH than in patients without LVH, and eccentric forms of hypertrophy seem to have the most fibrosis and highest ECV values, together with more pronounced systolic impairment and are associated with a poor cardiovascular prognosis (Figure 1) [2,6]. CMR-derived T1 mapping and strain analysis seem to be related, but an adequate comparison of the performance of these parameters is often limited due to the lack of harmonization of measurement methods [35]. Furthermore, ECV values seem to correlate with many blood biomarkers associated with (i) systemic inflammation; (ii) metabolism; (iii) fibrosis; (iv) chemotaxis; and (v) platelet activation [6]. This may suggest that an increase in ECV in hypertensive patients is a (non-specific) imaging biomarker of inflammation, tissue remodelling, atherogenesis, or metabolic disorder in patients with HHD [6]. Given the clinical consequences of myocardial fibrosis in HHD and considering the potential for recovery of fibrosis with appropriate treatment, the need for an accurate diagnosis of myocardial fibrosis is apparent.
Although tissue characterisation with native T1 and ECV has been shown to have incremental diagnostic benefits even in very early disease stages (e.g., diffuse fibrosis not detectable by LGE), there is an overlap between different cardiomyopathies and some overlap with normal T1 values. The difference in ECV between normal subjects and patients with HHD is small (0.29 ± 0.03 vs. 0.27 ± 0.02) [2]. Furthermore, other pathologies in addition to fibrosis increase ECV values such as myocardial inflammation or amyloid deposition. As with all medical parameters, abnormalities in native T1 and ECV need to be interpreted within their clinical context and pre-test probabilities and in conjunction with established CMR techniques such as LGE. Nevertheless, native T1 and myocardial ECV mapping seem to be promising imaging biomarkers to characterise HHD and eventually may even help to guide and monitor treatment response with antifibrotic agents in certain hypertensive individuals. Non-ischaemic LGE is associated with adverse LV remodelling, worse function, and elevated markers of wall stress and myocardial injury. Reactive interstitial fibrosis is potentially reversible with targeted therapies [45]. It is increasingly appreciated that the identification of focal or diffuse fibrosis may have significant independent prognostic implications and may help in monitoring disease progression and guiding anti-fibrotic therapies in the future [46].
11. Phenocopies and Differential Diagnosis of Hypertensive Heart Disease
The differential diagnosis of HHD includes hypertrophic cardiomyopathy and its phenocopies (e.g., Fabry’s disease, mitochondrial disease); valvular heart disease (sub-valvular, valvular, and supra-valvular aortic stenosis); pseudohypertrophy (amyloidosis (Table 4A); sarcoidosis (Table 4B)); and the so-called athletic heart, among others. Imaging can plan an important role in elucidating the cause of LVH [47], especially in patients with dual-presence arterial hypertension/HHD and another cardiomyopathy associated with LV hypertrophy.

Table 4.
Example images of various differential diagnoses of hypertensive heart disease.
Echocardiography allows the detailed evaluation of the left ventricular outflow tract, aortic valve, and supra-valvular structures. The combination with Doppler imaging allows haemodynamically significant obstructions to be readily detected and quantified. Valvular contributions to LVH can therefore be readily identified. However, CMR or DPD-scintigraphy can offer added value as ATTR amyloidosis and aortic stenosis frequently coexist [48].
Cardiovascular magnetic resonance (CMR) uniquely allows tissue characterisation to be undertaken and as such, can readily differentiate hypertensive LVH from causes of pseudohypertrophy or thickening of the left ventricle due to myocardial infiltration with amyloid (Table 4A) or sarcoid (Table 4B) [47].
All imaging findings should be assessed in their clinical context; patient history, cardiac blood biomarkers, and a 12-lead ECG can be very helpful in the differential diagnosis of LV hypertrophy (Table 5 and Supplement Table S1).

Table 5.
Differential diagnosis in LV hypertrophy. Other rare causes of LV hypertrophy not considered in this table such as rare storage disorders, mitochondrial myopathy, aortic coarctation, etc.
12. Cardiac Amyloid
Cardiac amyloid is typically characterised by the thickening of the ventricular walls, atria, and valvular structures (Table 4A). There is usually reduced long-axis motion. Native T1 and extracellular volume (ECV) are increased, and the latter may potentially be used as a marker of the degree of infiltration, and to monitor response to therapy. The findings on late gadolinium enhancement imaging are characteristic of abnormal gadolinium kinetics (the myocardium nulling in advance of the blood pool), difficulty in achieving a suitable null time, and widespread circumferential subendocardial enhancement [49]. When the latter involves the LV sub-endocardium and the RV aspect of the septum, it can give rise to the so-called zebra-stripe sign (Table 4C) [49]. The LV thickening seen in amyloid is often described as concentric, but asymmetrical patterns of thickening not too dissimilar to the patterns seen in hypertrophic cardiomyopathy are not infrequently encountered, and so the pattern of thickening alone cannot be used for differential diagnosis [50]. CMR findings are often accompanied by signs of fluid overload in the form of pleural or pericardial effusions (Table 4A,C), but these may not be seen in early disease. Efforts to differentiate between ATTR and AL using CMR have been made, and comparisons show more extensive LGE, LGE of the RV, and higher LV mass in ATTR versus less extensive more subendocardial LGE in AL [51]. The overlap between AL and ATTR amyloidosis, though, remains substantial. ATTR cardiac amyloid can also be readily identified by 99mDPD bone scintigraphy, particularly in the absence of light chain excess or evidence of a paraprotein or plasma cell dyscrasia [52]. Emerging PET tracers, such as delayed [18F]-florbetapen cardiac uptake, represent promising biomarkers, which may discriminate cardiac amyloid infiltration due to AL from ATTR and in future may further obviate the need for endomyocardial biopsy [53].
13. Hypertrophic Cardiomyopathy
In clinical practice, the differentiation of hypertrophic cardiomyopathy from hypertensive LVH is a frequent and difficult challenge, especially as the two pathologies frequently coexist. Hypertrophic cardiomyopathy is typically characterised by asymmetrical LVH, usually involving the septum, but any myocardial segment can be involved [54]. Hypertensive LVH often causes concentric LV remodelling, but asymmetrical LVH is not infrequent, and concentric hypertrophic cardiomyopathy is well-described particularly in non-sarcomeric phenocopies (Table 4D), so the pattern of remodelling alone may not be helpful unless it disproportionately affects, for instance, apical segments that do not hypertrophy in isolation in patients with HHD [55,56]. Patients with sarcomeric HCM also frequently develop a reverse septal curvature morphology with a loss of concavity of the septal endocardial surface (Table 4E,F) [57]. Other features that may point towards HCM as opposed to hypertensive heart disease include ancillary abnormalities such as elongation of the anterior leaflet of the mitral valve [58]; protrusion of the anterior leaflet into the LV cavity–26 mm or more (“night cap” mitral valve) [59]; the presence of numerous myocardial crypts [Table 4G] [59] (isolated myocardial crypts are likely within normal limits [60]); apical displacement of the papillary muscles; anteromedial displacement or duplication of the anterolateral papillary muscles; apico-septal muscle bundles (Table 4H) [61]; and accessory papillary muscles or anomalous direct insertion of papillary muscles onto the mitral valve [62]. Systolic anterior motion of the anterior leaflet of the mitral valve and the associated posteriorly directed MR and left ventricular outflow tract obstruction are more frequently seen in HCM than hypertensive heart disease but can occur in both settings, particularly if there is hypertension and isolated basal septal hypertrophy with hyperdynamic LV contractility in the elderly [63]. Some degree of LGE can be seen in approximately two-thirds of patients with HCM, most often in the areas of maximum hypertrophy [64]. The pattern and extent of enhancement are often very heterogeneous within as well as between patients [65]. Diffuse patchy mid-wall enhancement is frequently seen in advanced hypertensive heart disease with poor blood pressure control (Table 4I) but equally can be seen in HCM [47]. However, very dense organised fibrosis is more commonly seen in the latter, particularly in the so-called burned-out phase (Table 4J).
14. Fabry’s Disease
CMR can also be used to identify several HCM spectrum disorders or phenocopies. Patients with Fabry’s disease in the early phases of cardiac involvement can display a drop in native T1 as glycosphingolipids accumulate in the myocardium (in contrast to normal or increased native T1 typically seen in sarcomeric HCM or hypertensive heart disease) [66]. However, as the disease progresses, these values can pseudonormalise and as overt fibrosis supervenes, late enhancement can develop, usually in the basal inferolateral wall (Table 4K) (an unusual location for fibrosis in sarcomeric HCM) [66]. Fabry’s disease can be diagnosed by measuring alpha galactosidase enzyme activity in male patients, but genetic testing is required to confirm the diagnosis and reliably detect carrier status in females [67]. CMR can be useful to raise the suspicion of Fabry’s and to detect cardiac involvement in hemizygous males or female carriers but should not be relied upon in isolation for diagnosis or exclusion.
15. Danon Disease
Other X-linked storage disorders such as Danon disease also have characteristic LGE findings (dense LV endocardial enhancement) that allow their differentiation from sarcomeric HCM (Table 4L) [68], although the latter can be readily diagnosed by clinical/neurological evaluation of the patient as well as by diagnostic genetic testing.
16. Cardiac Sarcoidosis
Sarcoid can occasionally cause LV thickening when there is focal or extensive granulomatous infiltration of the myocardium [69]. If there is significant myocardial oedema, this can itself give rise to pseudohypertrophy [69]. The identification of ancillary imaging findings (e.g., significant mediastinal lymphadenopathy, unexplained hepatosplenomegaly) and myocardial oedema imaging (T2W-spin echo sequences or parametric mapping), as well as clinical findings or FDG-PET-CT, can achieve a differential diagnosis [41].
17. Athlete’s Heart
The extent/severity of hypertrophy can be of value in a differential diagnosis. It is unusual for hypertensive heart disease to produce very severe hypertrophy (>20 mm) and never extreme hypertrophy (>30 mm). Similarly, it is often suggested that high levels of athletic activity can be associated with LVH. However, most patients who experience athletic remodelling develop mild symmetrical cavity dilatation and modest increases in LV mass. Increases in LV wall thickness are uncommon and, if present, tend to be very mild (13–15 mm). Such changes are more commonly seen in athletes with black African ancestry. Increases in LV wall thickness can often be seen in recreational bodybuilders who take androgens or an exogenous growth hormone [70]. Interestingly, recreational bodybuilders who undertake similar levels of isometric exercise but who do not use exogenous trophic substances seldom appear to demonstrate increases in wall thickness [70].
As LVH increases, there is a reduction in ECV in athletes (cellular hypertrophy) but an increase in ECV in patients with HCM (cellular disarray and extracellular matrix expansion). Based on this divergent finding, ECV may be helpful to distinguish HCM and athletic remodelling, particularly in subjects with indeterminate maximal wall thickness (13–15 mm) [71].
18. Calcium Score and Computed Tomography Coronary Angiography in HHD
The relationship between hypertension grade and coronary atherosclerosis was evaluated in 8238 patients who underwent calcium scoring and computed tomography coronary angiography (CTCA) for screening purposes. The prevalence of calcified and non-calcified plaques increased with the grade of hypertension and was 17%, 28%, 34%, and 40% in normotensive, pre-hypertension, stage 1 hypertension, and stage 2 hypertension, respectively (p < 0.05) [72].
CTCA has the highest negative predictive value of all imaging techniques regarding the exclusion of macrovascular CAD. Often coronary artery tortuosity can be documented (Table 4M). Arterial tortuosity is often associated with arterial hypertension in addition to other factors such as female sex, older age, and other cardiovascular risk factors [73]. In a study population with a high percentage of patients with hypertension (72%), the prevalence of coronary artery tortuosity was 39%; moreover, hypertension was an independent predictor of coronary artery tortuosity in these patients and was associated with increased risk of lacunar infarction [74].
19. Nuclear Cardiology in HHD
The correlation between blood pressure and myocardial metabolism has been evaluated in 86 individuals undergoing fluorodeoxyglucose positron emission tomography (18-F-FDG-PET) PET [75]. This isotope is easily taken up by the (hypertrophied) heart, similar to cancer metabolism where 18-F-FDG shows increased uptake in areas of cell growth and proliferation. 18-F-FDG uptake correlated with patients’ systolic, diastolic, and mean blood pressure [75].
Apart from diagnosing macrovascular disease/ischaemia in patients with HHD, myocardial perfusion reserve (MPR) assessed by Rubidium (Rb)-82 PET can be used to risk stratify patients with hypertension. In a study of 517 hypertensive patients undergoing Rb-PET imaging, the 26% of patients who had resistant hypertension had more frequent LVH, lower myocardial blood flow rates, and lower MPR than subjects without resistant hypertension. Age, resistant hypertension, and impaired MPR were independent predictors of adverse events over a median follow-up of 38 months [76].
20. Extracardiac Imaging for Identification of Secondary Hypertension and Sequelae of Long-Standing Hypertension
Secondary hypertension affects approximately 5–10% of the general hypertensive population [77]. Imaging may play an important role in the detection and subsequent treatment of both endocrine (such as hyperaldosteronism, phaeochromocytoma, or hyperparathyroidism) and non-endocrine secondary hypertension [78].
The most common cause of primary hyperaldosteronism is an aldosterone-producing adrenal adenoma. Adrenal adenomas usually present as round or oval masses. Using a threshold value of ≤10 Hounsfield units (HU) at non-contrast CT leads to high sensitivity and specificity in their detection [79]. Given their fatty content, adrenal adenomas may also be detected using chemical shift imaging with characteristic signal drop-outs on in- and opposed-phase imaging [80].
Phaeochromocytomas are a rare cause of secondary hypertension and account for only <5% of patients with secondary hypertension. These tumours usually present as large, heterogeneous masses with both necrosis and cystic changes and avid contrast enhancement [81].
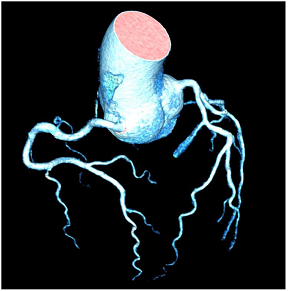
Common non-endocrine causes are aortic coarctation (Table 4N) and renovascular hypertension caused by diseases such as fibromuscular dysplasia (FMD) or renal artery stenosis.
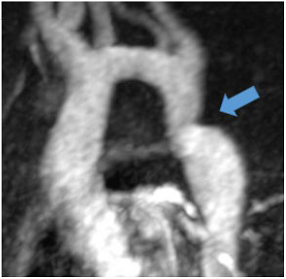
Aortic coarctation is defined as luminal narrowing near the origin of the left subclavian artery and ligamentum arteriosum and is a very rare cause of secondary hypertension with only 0.2% of patients with hypertension affected [82]. Increased afterload due to mechanical obstruction and potential renal ischaemia are regarded as underlying pathomechanisms. CT and, particularly for follow-up, MR angiography are the optimal imaging methods for the detection and quantification of coarctation.
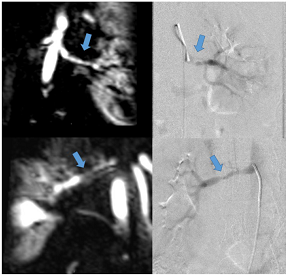
Regarding renovascular hypertension, FMD or renal artery stenosis are the most common causes (Table 4O). Both conditions lead to decreased renal perfusion and subsequent increased systemic blood pressure, but have a different appearance in terms of location: While (atherosclerotic) renal artery stenosis most commonly affects the proximal renal artery, FMD affects the middle segments with alternating strictures and dilatation [83].
Patients with long-standing hypertension often also develop increased arterial stiffness with accelerated aortic pulse wave velocity as measured by CMR velocity-encoded imaging. A stiff aorta may further increase LV afterload [31].
21. Limits and Caveats in Imaging
All markers of cardiac damage in HHD are influenced by age, gender, and ethnicity. Normalisation to body surface area may be misleading in obese individuals; normalisation for body height, a good surrogate of fat-free mass, appears to be more acceptable but used less frequently in clinical practice [25]. All imaging findings should always be interpreted in the broader clinical context of the patient including patient history, ECG, and blood biomarkers.
22. Conclusions
Due to the high prevalence of hypertension, HHD is one of the most common heart diseases and a frequent cause of diastolic heart failure and LV impairment. In clinical practice, comprehensive echocardiography may be sufficient to recognize most features of HHD. In patients with LV hypertrophy disproportionate to the degree of hypertension and patients where dual pathology is assumed, tissue characterization with CMR can be very helpful in the differential diagnosis, risk stratification, and also to detect extracardiac pathology associated with HHD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12093122/s1, Table S1: Electrocardiograms in various forms of LV hypertrophy.
Author Contributions
T.F.I., S.F., B.A.K., D.J.W., M.J.Z., D.T.B. and P.H. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable, the paper does not have information or images that can identify any patient.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CMR | Cardiovascular Magnetic Resonance |
| GLS | Global Longitudinal Strain |
| HFpEF | Heart Failure with preserved Ejection Fraction |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| HHD | Hypertensive Heart Disease |
| LA | Left Atrium/Atrial |
| LAVI | Left Atrial Volume Index |
| LGE | Late Gadolinium Enhancement |
| LV | Left Ventricle/Ventricular |
| LVDD | Left Ventricular Diastolic Dysfunction |
| LVEF | Left Ventricular Ejection Fraction |
| LVH | Left Ventricular Hypertrophy |
| LVM | Left Ventricular Mass |
| LVMI | Left Ventricular Mass Index |
| TTE | Transthoracic Echocardiography |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Schumann, C.L.; Jaeger, N.R.; Kramer, C.M. Recent Advances in Imaging of Hypertensive Heart Disease. Curr. Hypertens. Rep. 2019, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H. The progression of hypertensive heart disease. Circulation 2011, 123, 327–334. [Google Scholar] [CrossRef]
- Nadruz, W. Myocardial remodeling in hypertension. J. Hum. Hypertens. 2015, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition From Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Plein, S.; Milivojevic, I.G.; Wang, D.W.; Grassi, G.; Mancia, G. Comprehensive assessment of hypertensive heart disease: Cardiac magnetic resonance in focus. Heart Fail. Rev. 2021, 26, 1383–1390. [Google Scholar] [CrossRef]
- Mensah, G.A. Commentary: Hypertension Phenotypes: The Many Faces of a Silent Killer. Ethn. Dis. 2019, 29, 545–548. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef]
- Verdecchia, P.; Carini, G.; Circo, A.; Dovellini, E.; Giovannini, E.; Lombardo, M.; Solinas, P.; Gorini, M.; Maggioni, A.P. Left ventricular mass and cardiovascular morbidity in essential hypertension: The MAVI study. J. Am. Coll. Cardiol. 2001, 38, 1829–1835. [Google Scholar] [CrossRef]
- Kane, G.C.; Karon, B.L.; Mahoney, D.W.; Redfield, M.M.; Roger, V.L.; Burnett, J.C.; Jacobsen, S.J.; Rodeheffer, R.J. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011, 306, 856–863. [Google Scholar] [CrossRef]
- Saito, M.; Khan, F.; Stoklosa, T.; Iannaccone, A.; Negishi, K.; Marwick, T.H. Prognostic Implications of LV Strain Risk Score in Asymptomatic Patients with Hypertensive Heart Disease. JACC Cardiovasc. Imaging 2016, 9, 911–921. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Armstrong, A.C.; Gjesdal, O.; Almeida, A.; Nacif, M.; Wu, C.; Bluemke, D.A.; Brumback, L.; Lima, J.A.C. Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: The multi-ethnic study of atherosclerosis. Echocardiogr. Mt. Kisco. N. 2014, 31, 12–20. [Google Scholar] [CrossRef]
- Takeuchi, M.; Nishikage, T.; Mor-Avi, V.; Sugeng, L.; Weinert, L.; Nakai, H.; Salgo, I.S.; Gerard, O.; Lang, R.M. Measurement of left ventricular mass by real-time three-dimensional echocardiography: Validation against magnetic resonance and comparison with two-dimensional and m-mode measurements. J. Am. Soc. Echocardiogr. Off Publ. Am. Soc. Echocardiogr. 2008, 21, 1001–1005. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; De Buyzere, M.L.; Kronmal, R.A.; Raja, M.W.; De Bacquer, D.; Claessens, T.; Gillebert, T.C.; St John-Sutton, M.; Rietzschel, E.R.; et al. Left ventricular mass: Allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension 2010, 56, 91–98. [Google Scholar] [CrossRef]
- Tromp, J.; Bauer, D.; Claggett, B.L.; Frost, M.; Iversen, M.B.; Prasad, N.; Petrie, M.C.; Larson, M.G.; Ezekowitz, J.A.; Solomon, S.D.; et al. A formal validation of a deep learning-based automated workflow for the interpretation of the echocardiogram. Nat. Commun. 2022, 13, 6776. [Google Scholar] [CrossRef]
- Edwards, N.C.; Moody, W.E.; Yuan, M.; Weale, P.; Neal, D.; Townend, J.N.; Steeds, R.P. Quantification of left ventricular interstitial fibrosis in asymptomatic chronic primary degenerative mitral regurgitation. Circ. Cardiovasc. Imaging 2014, 7, 946–953. [Google Scholar] [CrossRef]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Righini, F.M.; Massoni, A.; Mondillo, S. Left ventricular remodeling and torsion dynamics in hypertensive patients. Int. J. Cardiovasc. Imaging 2013, 29, 79–86. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating Left Ventricular Filling Pressure by Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef]
- Grant, A.D.M.; Negishi, K.; Negishi, T.; Collier, P.; Kapadia, S.R.; Thomas, J.D.; Marwick, T.H.; Griffin, B.P.; Popović, Z.B. Grading diastolic function by echocardiography: Hemodynamic validation of existing guidelines. Cardiovasc. Ultrasound 2015, 13, 28. [Google Scholar] [CrossRef]
- Patel, D.A.; Lavie, C.J.; Milani, R.V.; Ventura, H.O. Left atrial volume index predictive of mortality independent of left ventricular geometry in a large clinical cohort with preserved ejection fraction. Mayo Clin. Proc. 2011, 86, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Pencic-Popovic, B.; Celic, V.; Mancia, G. The relationship between nighttime hypertension and left atrial function. J. Clin. Hypertens. 2017, 19, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Perrone-Filardi, P.; Coca, A.; Galderisi, M.; Paolillo, S.; Alpendurada, F.; de Simone, G.; Donal, E.; Kahan, T.; Mancia, G.; Redon, J.; et al. Non-invasive cardiovascular imaging for evaluating subclinical target organ damage in hypertensive patients: A consensus paper from the European Association of Cardiovascular Imaging (EACVI), the European Society of Cardiology Council on Hypertension, and the European Society of Hypertension (ESH). Eur. Heart J. Cardiovasc. Imaging 2017, 18, 945–960. [Google Scholar]
- Ismail, T.F.; Strugnell, W.; Coletti, C.; Božić-Iven, M.; Weingärtner, S.; Hammernik, K.; Correia, T.; Küstner, T. Cardiac MR: From Theory to Practice. Front. Cardiovasc. Med. 2022, 9, 826283. [Google Scholar] [CrossRef] [PubMed]
- Greupner, J.; Zimmermann, E.; Grohmann, A.; Dübel, H.P.; Althoff, T.F.; Borges, A.C.; Rutsch, W.; Schlattmann, P.; Hamm, B.; Dewey, M. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: Comparison with magnetic resonance imaging as the reference standard. J. Am. Coll. Cardiol. 2012, 59, 1897–1907. [Google Scholar]
- Parsai, C.; O’Hanlon, R.; Prasad, S.K.; Mohiaddin, R.H. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J. Cardiovasc. Magn. Reson. 2012, 14, 54. [Google Scholar] [CrossRef]
- Burnhope, E.; Polcaro, A.; Runge, J.H.; Granlund, I.; Bosio, F.; Troelstra, M.A.; Villa, A.D.M.; Chiribiri, A.; Carr-White, G.; Webb, J.; et al. Assessment of Myocardial Stiffness in Patients with Left Ventricular Hypertrophy: CMR Elastography Using Intrinsic Actuation. JACC Cardiovasc. Imaging 2022, 15, 1163–1165. [Google Scholar] [CrossRef]
- Zdravkovic, M.; Klasnja, S.; Popovic, M.; Djuran, P.; Mrda, D.; Ivankovic, T.; Manojlovic, A.; Koracevic, G.; Lovic, D.; Popadic, V. Cardiac Magnetic Resonance in Hypertensive Heart Disease: Time for a New Chapter. Diagnostics 2023, 13, 137. [Google Scholar] [CrossRef]
- Raman, S.V. The hypertensive heart. An integrated understanding informed by imaging. J. Am. Coll. Cardiol. 2010, 55, 91–96. [Google Scholar] [CrossRef]
- Pezel, T.; Venkatesh, B.A.; De Vasconcellos, H.D.; Kato, Y.; Shabani, M.; Xie, E.; Heckbert, S.R.; Post, W.S.; Shea, S.J.; Allen, N.B.; et al. Left Atrioventricular Coupling Index as a Prognostic Marker of Cardiovascular Events: The MESA Study. Hypertension 2021, 78, 661–671. [Google Scholar] [CrossRef]
- Rathod, R.H.; Powell, A.J.; Geva, T. Myocardial Fibrosis in Congenital Heart Disease. Circ. J. 2016, 80, 1300–1307. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Pan, Y.; Ge, Y.; Guo, Z.; Zhao, S. Early and Quantitative Assessment of Myocardial Deformation in Essential Hypertension Patients by Using Cardiovascular Magnetic Resonance Feature Tracking. Sci. Rep. 2020, 10, 3582. [Google Scholar] [CrossRef]
- Pichler, G.; Redon, J.; Martínez, F.; Solaz, E.; Calaforra, O.; Andrés, M.S.; Lopez, B.; Díez, J.; Oberbauer, R.; Adlbrecht, C.; et al. Cardiac magnetic resonance-derived fibrosis, strain and molecular biomarkers of fibrosis in hypertensive heart disease. J. Hypertens. 2020, 38, 2036–2042. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef]
- Heitner, J.F.; Kim, R.J.; Kim, H.W.; Klem, I.; Shah, D.J.; Debs, D.; Farzaneh-Far, A.; Polsani, V.; Kim, J.; Weinsaft, J.; et al. Prognostic Value of Vasodilator Stress Cardiac Magnetic Resonance Imaging: A Multicenter Study with 48,000 Patient-Years of Follow-up. JAMA Cardiol. 2019, 4, 256–264. [Google Scholar] [CrossRef]
- Sammut, E.C.; Villa, A.D.M.; Di Giovine, G.; Dancy, L.; Bosio, F.; Gibbs, T.; Jeyabraba, S.; Schwenke, S.; Williams, S.E.; Marber, M.; et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2018, 11, 686–694. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Ismail, T.F.; Hua, A.; Plein, S.; D’Cruz, D.P.; Fernando, M.M.A.; Friedrich, M.G.; Zellweger, M.J.; Giorgetti, A.; Caobelli, F.; Haaf, P. The role of cardiovascular magnetic resonance in the evaluation of acute myocarditis and inflammatory cardiomyopathies in clinical practice—A comprehensive review. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 450–464. [Google Scholar] [CrossRef]
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J. Am. Coll. Cardiol. 2009, 53, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; Flett, A.S.; Banypersad, S.M.; White, S.K.; Maestrini, V.; Quarta, G.; Lachmann, R.H.; Murphy, E.; Mehta, A.; Hughes, D.A.; et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012, 98, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Rosmini, S.; Bulluck, H.; Captur, G.; Treibel, T.A.; Abdel-Gadir, A.; Bhuva, A.N.; Culotta, V.; Merghani, A.; Fontana, M.; Maestrini, V.; et al. Myocardial native T1 and extracellular volume with healthy ageing and gender. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 615–621. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Winkler, J.; Ramos, I.; Do, Q.T.; Firat, H.; McDonald, K.; Gonzalez, A.; Thum, T.; Diez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail 2017, 19, 177–191. [Google Scholar] [CrossRef]
- Iyer, N.R.; Le, T.T.; Kui, M.S.L.; Tang, H.C.; Chin, C.T.; Phua, S.K.; Bryant, J.A.; Pua, C.J.; Ang, B.; Toh, D.F.; et al. Markers of Focal and Diffuse Nonischemic Myocardial Fibrosis Are Associated with Adverse Cardiac Remodeling and Prognosis in Patients with Hypertension: The REMODEL Study. Hypertension 2022, 79, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.M.; Mohiaddin, R.H. Cardiovascular magnetic resonance in systemic hypertension. J. Cardiovasc. Magn. Reson. 2012, 14, 28. [Google Scholar] [CrossRef]
- Scully, P.R.; Patel, K.P.; Treibel, T.A.; Thornton, G.D.; Hughes, R.K.; Chadalavada, S.; Katsoulis, M.; Hartman, N.; Fontana, M.; Pugliese, F.; et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.M.; Joshi, J.; Prasad, S.K.; Moon, J.C.; Perugini, E.; Harding, I.; Sheppard, M.N.; Poole-Wilson, P.A.; Hawkins, P.N.; Pennell, D.J.; et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005, 111, 186–193. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Zumbo, G.; Knight, D.S.; Kotecha, T.; Francis, R.; Hutt, D.F.; Rezk, T.; et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2017, 70, 466–477. [Google Scholar] [CrossRef]
- Dungu, J.N.; Valencia, O.; Pinney, J.H.; Gibbs, S.D.J.; Rowczenio, D.; Gilbertson, J.A.; Lachmann, H.J.; Wechalekar, A.; Gillmore, J.D.; Whelan, C.J.; et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 133–142. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Genovesi, D.; Vergaro, G.; Giorgetti, A.; Marzullo, P.; Scipioni, M.; Santarelli, M.F.; Pucci, A.; Buda, G.; Volpi, E.; Emdin, M. [18F]-Florbetaben PET/CT for Differential Diagnosis Among Cardiac Immunoglobulin Light Chain, Transthyretin Amyloidosis, and Mimicking Conditions. JACC Cardiovasc. Imaging 2021, 14, 246–255. [Google Scholar] [CrossRef]
- Maron, M.S. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2012, 14, 13. [Google Scholar] [CrossRef]
- Rodrigues, J.C.L.; Rohan, S.; Ghosh Dastidar, A.; Harries, I.; Lawton, C.B.; Ratcliffe, L.E.; Burchell, A.E.; Hart, E.C.; Hamilton, M.C.K.; Paton, J.F.R.; et al. Hypertensive heart disease versus hypertrophic cardiomyopathy: Multi-parametric cardiovascular magnetic resonance discriminators when end-diastolic wall thickness ≥ 15 mm. Eur. Radiol. 2017, 27, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Flett, A.S.; Maestrini, V.; Milliken, D.; Fontana, M.; Treibel, T.A.; Harb, R.; Sado, D.M.; Quarta, G.; Herrey, A.; Sneddon, J.; et al. Diagnosis of apical hypertrophic cardiomyopathy: T-wave inversion and relative but not absolute apical left ventricular hypertrophy. Int. J. Cardiol. 2015, 183, 143–148. [Google Scholar] [CrossRef]
- Reant, P.; Captur, G.; Mirabel, M.; Nasis, A.; Sado, M.D.; Maestrini, V.; Castelletti, S.; Manisty, C.; Herrey, A.S.; Syrris, P.; et al. Abnormal septal convexity into the left ventricle occurs in subclinical hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2015, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Captur, G.; Lopes, L.R.; Mohun, T.J.; Patel, V.; Li, C.; Bassett, P.; Finocchiaro, G.; Ferreira, V.M.; Esteban, M.T.; Muthurangu, V.; et al. Prediction of sarcomere mutations in subclinical hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Petryka, J.; Baksi, A.J.; Prasad, S.K.; Pennell, D.J.; Kilner, P.J. Prevalence of inferobasal myocardial crypts among patients referred for cardiovascular magnetic resonance. Circ. Cardiovasc. Imaging 2014, 7, 259–264. [Google Scholar] [CrossRef]
- Gruner, C.; Chan, R.H.; Crean, A.; Rakowski, H.; Rowin, E.J.; Care, M.; Deva, D.; Williams, L.; Appelbaum, E.; Gibson, C.M.; et al. Significance of left ventricular apical-basal muscle bundle identified by cardiovascular magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2014, 35, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Klues, H.G.; Maron, B.J.; Dollar, A.L.; Roberts, W.C. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation 1992, 85, 1651–1660. [Google Scholar] [CrossRef]
- Ranasinghe, I.; Ayoub, C.; Cheruvu, C.; Freedman, S.B.; Yiannikas, J. Isolated hypertrophy of the basal ventricular septum: Characteristics of patients with and without outflow tract obstruction. Int. J. Cardiol. 2014, 173, 487–493. [Google Scholar] [CrossRef]
- Ismail, T.F.; Jabbour, A.; Gulati, A.; Mallorie, A.; Raza, S.; Cowling, T.E.; Das, B.; Khwaja, J.; Alpendurada, F.D.; Wage, R.; et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014, 100, 1851–1858. [Google Scholar] [CrossRef]
- Ismail, T.F.; Prasad, S.K.; Pennell, D.J. Prognostic importance of late gadolinium enhancement cardiovascular magnetic resonance in cardiomyopathy. Heart Br. Card Soc. 2012, 98, 438–442. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Medina-Menacho, K.; Abdel-Gadir, A.; Baig, S.; Sado, D.M.; Lobascio, I.; Murphy, E.; Lachmann, R.H.; Mehta, A.; et al. Proposed Stages of Myocardial Phenotype Development in Fabry Disease. JACC Cardiovasc. Imaging 2019, 12, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Vardarli, I.; Rischpler, C.; Herrmann, K.; Weidemann, F. Diagnosis and Screening of Patients with Fabry Disease. Ther. Clin. Risk Manag. 2020, 16, 551–558. [Google Scholar] [CrossRef]
- Rigolli, M.; Kahn, A.M.; Brambatti, M.; Contijoch, F.J.; Adler, E.D. Cardiac Magnetic Resonance Imaging in Danon Disease Cardiomyopathy. JACC Cardiovasc. Imaging 2021, 14, 514–516. [Google Scholar] [CrossRef]
- Birnie, D.H.; Kandolin, R.; Nery, P.B.; Kupari, M. Cardiac manifestations of sarcoidosis: Diagnosis and management. Eur. Heart J. 2017, 38, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Angell, P.J.; Ismail, T.F.; Jabbour, A.; Smith, G.; Dahl, A.; Wage, R.; Whyte, G.; Green, D.J.; Prasad, S.; George, K. Ventricular structure, function, and focal fibrosis in anabolic steroid users: A CMR study. Eur. J. Appl. Physiol. 2014, 114, 921–928. [Google Scholar] [CrossRef]
- Swoboda, P.P.; McDiarmid, A.K.; Erhayiem, B.; Broadbent, D.A.; Dobson, L.E.; Garg, P.; Ferguson, C.; Page, S.P.; Greenwood, J.P.; Plein, S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy From Athlete’s Heart. J. Am. Coll. Cardiol. 2016, 67, 2189–2190. [Google Scholar] [CrossRef]
- Im, T.S.; Chun, E.J.; Lee, M.S.; Adla, T.; Kim, J.A.; Choi, S.I. Grade-response relationship between blood pressure and severity of coronary atherosclerosis in asymptomatic adults: Assessment with coronary CT angiography. Int. J. Cardiovasc. Imaging 2014, 30, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ciurică, S.; Lopez-Sublet, M.; Loeys, B.L.; Radhouani, I.; Natarajan, N.; Vikkula, M.; Maas, A.H.E.M.; Adlam, D.; Persu, A. Arterial Tortuosity. Hypertension 2019, 73, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, C.; Ji, Y.; Feng, Y.; Ma, G.; Liu, N. Clinical implication of coronary tortuosity in patients with coronary artery disease. PLoS ONE 2011, 6, e24232. [Google Scholar] [CrossRef]
- Rojulpote, C.; Mehdizadeh Seraj, S.; Zirakchian Zadeh, M.; Yadav, D.; Raynor, W.Y.; Kothekar, E.; Al-Zaghal, A.; Werner, T.J.; Gerke, O.; Høilund-Carlsen, P.F.; et al. Role of FDG-PET/CT in Assessing the Correlation between Blood Pressure and Myocardial Metabolic Uptake. Asia Ocean. J. Nucl. Med. Biol. 2020, 8, 36–45. [Google Scholar] [PubMed]
- Gaudieri, V.; Mannarino, T.; Zampella, E.; Assante, R.; D’Antonio, A.; Nappi, C.; Cantoni, V.; Green, R.; Petretta, M.; Arumugam, P.; et al. Prognostic value of coronary vascular dysfunction assessed by rubidium-82 PET/CT imaging in patients with resistant hypertension without overt coronary artery disease. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3162–3171. [Google Scholar] [CrossRef]
- Rimoldi, S.F.; Scherrer, U.; Messerli, F.H. Secondary arterial hypertension: When, who, and how to screen? Eur. Heart J. 2014, 35, 1245–1254. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Mittal, P.K.; Little, B.P.; Miller, F.H.; Akduman, E.I.; Ali, K.; Sartaj, S.; Moreno, C.C. Secondary Hypertension and Complications: Diagnosis and Role of Imaging. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2019, 39, 1036–1055. [Google Scholar] [CrossRef]
- Johnson, P.T.; Horton, K.M.; Fishman, E.K. Adrenal mass imaging with multidetector CT: Pathologic conditions, pearls, and pitfalls. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2009, 29, 1333–1351. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Mukundan, G.; Narra, V.R.; Lewis, J.S.; Shirkhoda, A.; Farooki, A.; Brown, J.J. Adrenal masses: MR imaging features with pathologic correlation. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2004, 24, S73–S86. [Google Scholar] [CrossRef]
- Blake, M.A.; Kalra, M.K.; Maher, M.M.; Sahani, D.V.; Sweeney, A.T.; Mueller, P.R.; Hahn, P.F.; Boland, G.W. Pheochromocytoma: An imaging chameleon. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2004, 24, S87–S99. [Google Scholar] [CrossRef] [PubMed]
- Prisant, L.M.; Mawulawde, K.; Kapoor, D.; Joe, C. Coarctation of the aorta: A secondary cause of hypertension. J. Clin. Hypertens. 2004, 6, 347–350+352. [Google Scholar] [CrossRef] [PubMed]
- Varennes, L.; Tahon, F.; Kastler, A.; Grand, S.; Thony, F.; Baguet, J.P.; Detante, O.; Touzé, E.; Krainik, A. Fibromuscular dysplasia: What the radiologist should know: A pictorial review. Insights Imaging 2015, 6, 295–307. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).