Effects of Angiotensin Receptor-Neprilysin Inhibitors (ARNIs) on the Glucose and Fat Metabolism Biomarkers Leptin and Fructosamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sampling
2.3. Biomarker Analysis
2.4. Ethics
2.5. Statistics
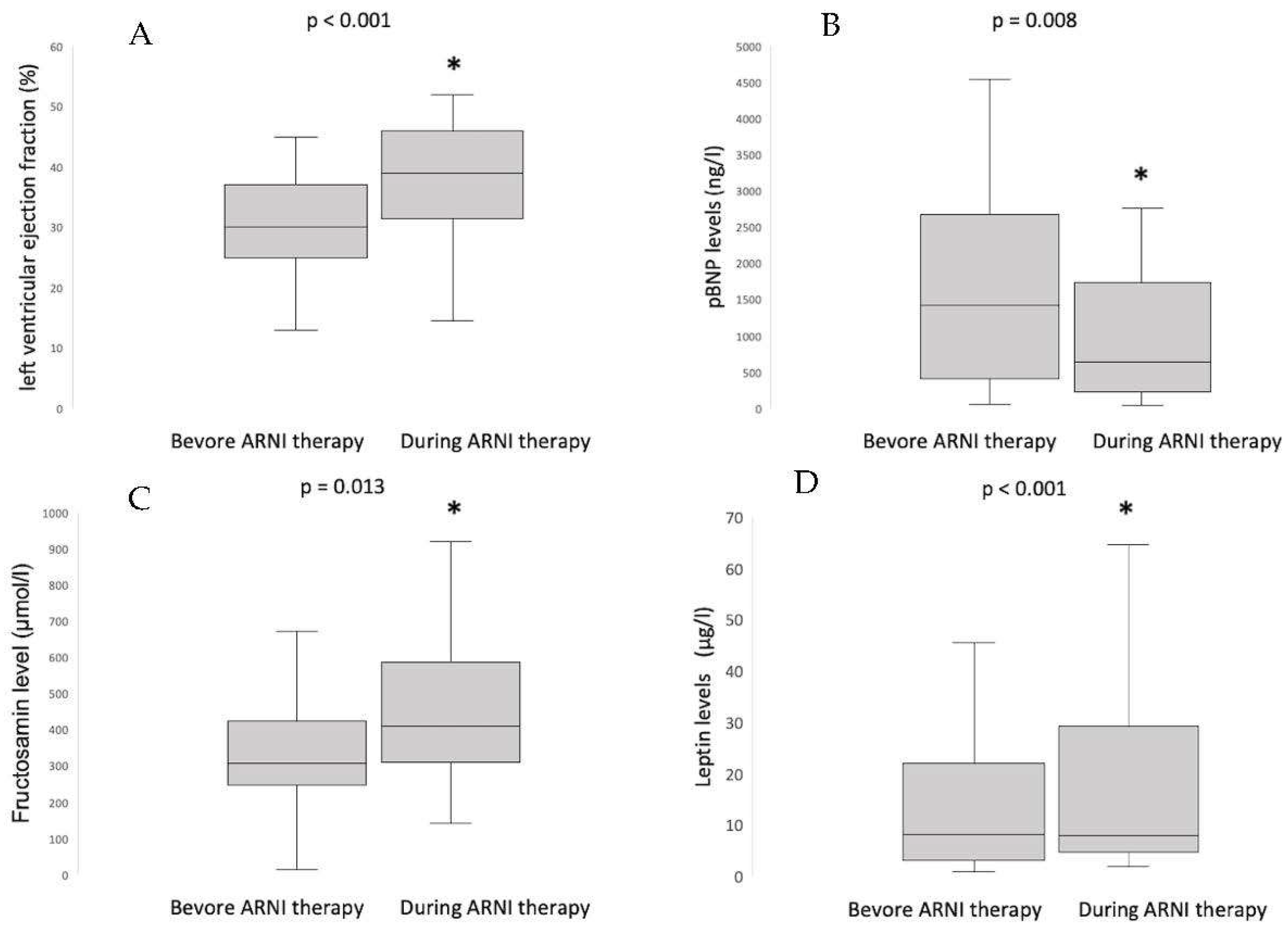
3. Results
3.1. Concentrations and Dynamics
3.2. Correlations
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Metabolism Parameter | Level Baseline Median | Level Follow Up Median | p-Value |
|---|---|---|---|
| LDL (mg/dL) | 85 (58;97) | 84 (60;103) | 0.814 |
| HDL (mg/dL) | 51 (37;58) | 49 (40;60) | 0.413 |
| Total cholesterol (mg/dL) | 156 (122;176) | 150 (132;169) | 0.575 |
| Triglycerides (mg/dL) | 130 (90;148) | 141 (92;178) | 0.009 * |
| Blood glucose (mg/dL) | 98 (90;115) | 99 (85;114) | 0.391 |
| hbA1c (%) | 5.9 (5.4;6.1) | 5.7 (5.4;5.9) | 0.521 |
| Renin (ng/L) | 28.9 (6.5;139.0) | 47.5 (6.2; 228.0) | 0.683 |
| Aldosterone (ng/L) | 81.5 (51.5;173.0) | 129.0 (77.1;179.0) | 0.208 |
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Larson, M.G.; Leip, E.P.; Beiser, A.; D’Agostino, R.B.; Kannel, W.B.; Murabito, J.M.; Vasan, R.S.; Benjamin, E.J.; Levy, D. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation 2002, 106, 3068–3072. [Google Scholar] [CrossRef]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail 2018, 6, 678–685. [Google Scholar] [CrossRef]
- Barasa, A.; Schaufelberger, M.; Lappas, G.; Swedberg, K.; Dellborg, M.; Rosengren, A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 25–32. [Google Scholar] [CrossRef]
- Hall, M.J.; DeFrances, C.J.; Williams, S.N.; Golosinskiy, A.; Schwartzman, A. National Hospital Discharge Survey: 2007 summary. Natl. Health Stat. Rep. 2010, 29, 1–20. [Google Scholar]
- Jhund, P.S.; Macintyre, K.; Simpson, C.R.; Lewsey, J.D.; Stewart, S.; Redpath, A.; Chalmers, J.W.; Capewell, S.; McMurray, J.J. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: A population study of 5.1 million people. Circulation 2009, 119, 515–523. [Google Scholar] [CrossRef]
- Lawson, C.A.; Zaccardi, F.; Squire, I.; Ling, S.; Davies, M.J.; Lam, C.S.P.; Mamas, M.A.; Khunti, K.; Kadam, U.T. 20-year trends in cause-specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: A population-based study. Lancet Public Health 2019, 4, e406–e420. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Atar, D.; Krum, H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ. Heart Fail 2015, 8, 71–78. [Google Scholar] [CrossRef]
- Pu, Q.; Amiri, F.; Gannon, P.; Schiffrin, E.L. Dual angiotensin-converting enzyme/neutral endopeptidase inhibition on cardiac and renal fibrosis and inflammation in DOCA-salt hypertensive rats. J. Hypertens 2005, 23, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Han, D.; Byun, S.H.; Kwon, M.; Cho, S.J.; Koh, Y.H.; Yoon, K. Neprilysin facilitates adipogenesis through potentiation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Mol. Cell. Biochem. 2017, 430, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sengenes, C.; Stich, V.; Berlan, M.; Hejnova, J.; Lafontan, M.; Pariskova, Z.; Galitzky, J. Increased lipolysis in adipose tissue and lipid mobilization to natriuretic peptides during low-calorie diet in obese women. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 24–32. [Google Scholar] [CrossRef]
- Bordicchia, M.; Liu, D.; Amri, E.Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Matsushita, K.; Lazo, M.; Bello, N.; Blumenthal, R.S.; Gerstenblith, G.; Nambi, V.; Ballantyne, C.M.; Solomon, S.D.; Selvin, E.; et al. Obesity and Subtypes of Incident Cardiovascular Disease. J. Am. Heart Assoc. 2016, 5, e003921. [Google Scholar] [CrossRef]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.R.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.C.; et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: A post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017, 5, 333–340. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Ndumele, C.E.; Zhang, S.; Florido, R.; Matsushita, K.; Coresh, J.; Skali, H.; Shah, A.M.; Selvin, E. Diabetes and Progression of Heart Failure: The Atherosclerosis Risk In Communities (ARIC) Study. J. Am. Coll. Cardiol. 2022, 79, 2285–2293. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Boschmann, M.; Moro, C.; Adams, F.; Heusser, K.; Franke, G.; Berlan, M.; Luft, F.C.; Lafontan, M.; Jordan, J. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J. Clin. Endocrinol. Metab. 2005, 90, 3622–3628. [Google Scholar] [CrossRef] [PubMed]
- Coué, M.; Badin, P.M.; Vila, I.K.; Laurens, C.; Louche, K.; Marquès, M.A.; Bourlier, V.; Mouisel, E.; Tavernier, G.; Rustan, A.C.; et al. Defective Natriuretic Peptide Receptor Signaling in Skeletal Muscle Links Obesity to Type 2 Diabetes. Diabetes 2015, 64, 4033–4045. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, A.L.; Boschmann, M.; Engeli, S.; Moro, C.; Arafat, A.M.; Luft, F.C.; Jordan, J. Atrial natriuretic peptide and adiponectin interactions in man. PLoS ONE 2012, 7, e43238. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Seferovic, J.P.; Solomon, S.D.; Seely, E.W. Potential mechanisms of beneficial effect of sacubitril/valsartan on glycemic control. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820970444. [Google Scholar] [CrossRef] [PubMed]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef]
- Wang, Y.; Asakawa, A.; Inui, A.; Kosai, K. Leptin gene therapy in the fight against diabetes. Expert. Opin. Biol. Ther. 2010, 10, 1405–1414. [Google Scholar] [CrossRef]
- Belke, D.D.; Larsen, T.S.; Gibbs, E.M.; Severson, D.L. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1104–E1113. [Google Scholar] [CrossRef]
- Schulze, P.C.; Kratzsch, J.; Linke, A.; Schoene, N.; Adams, V.; Gielen, S.; Erbs, S.; Moebius-Winkler, S.; Schuler, G. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur. J. Heart Fail 2003, 5, 33–40. [Google Scholar] [CrossRef]
- Packer, M. Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People With Obesity. Circulation 2018, 137, 1614–1631. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef] [PubMed]
- Na, T.; Dai, D.Z.; Tang, X.Y.; Dai, Y. Upregulation of leptin pathway correlates with abnormal expression of SERCA2a, phospholamban and the endothelin pathway in heart failure and reversal by CPU86017. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Miana, M.; Jurado-López, R.; Bartolomé, M.V.; Souza Neto, F.V.; Salaices, M.; López-Andrés, N.; Cachofeiro, V. The potential role of leptin in the vascular remodeling associated with obesity. Int. J. Obes. 2014, 38, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Jurado-López, R.; Valero-Muñoz, M.; Bartolomé, M.V.; Ballesteros, S.; Luaces, M.; Briones, A.M.; López-Andrés, N.; Miana, M.; Cachofeiro, V. Leptin induces cardiac fibrosis through galectin-3, mTOR and oxidative stress: Potential role in obesity. J. Hypertens. 2014, 32, 1104–1114, discussion 1114. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Farooqi, I.S.; Cole, T.J.; O’Rahilly, S.; Fewtrell, M.; Kattenhorn, M.; Lucas, A.; Deanfield, J. Influence of leptin on arterial distensibility: A novel link between obesity and cardiovascular disease? Circulation 2002, 106, 1919–1924. [Google Scholar] [CrossRef]
- Patel, V.B.; Mori, J.; McLean, B.A.; Basu, R.; Das, S.K.; Ramprasath, T.; Parajuli, N.; Penninger, J.M.; Grant, M.B.; Lopaschuk, G.D.; et al. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes 2016, 65, 85–95. [Google Scholar] [CrossRef]
- Ghantous, C.M.; Azrak, Z.; Hanache, S.; Abou-Kheir, W.; Zeidan, A. Differential Role of Leptin and Adiponectin in Cardiovascular System. Int. J. Endocrinol. 2015, 2015, 534320. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H.; Lennon, L.; Sattar, N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? J. Am. Coll. Cardiol. 2011, 58, 1870–1877. [Google Scholar] [CrossRef]
- Gounden, V.; Ngu, M.; Anastasopoulou, C.; Jialal, I. Fructosamine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Faxén, U.L.; Hage, C.; Andreasson, A.; Donal, E.; Daubert, J.C.; Linde, C.; Brismar, K.; Lund, L.H. HFpEF and HFrEF exhibit different phenotypes as assessed by leptin and adiponectin. Int. J. Cardiol. 2017, 228, 709–716. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Moravec, C.S.; McTiernan, C.F. Leptin signaling in the failing and mechanically unloaded human heart. Circ. Heart Fail 2009, 2, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O.; Bruthans, J.; Seidlerová, J.; Gelžinský, J.; Kučera, R.; Karnosová, P.; Mateřánková, M.; Wohlfahrt, P.; Cífková, R.; Filipovský, J. high leptin status indicates an increased risk of mortality and heart failure in stable coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2022, 2, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, P.; Jenke, A.; Bobbert, T.; Kühl, U.; Rauch, U.; Lassner, D.; Scheibenbogen, C.; Poller, W.; Schultheiss, H.P.; Skurk, C. High leptin and resistin expression in chronic heart failure: Adverse outcome in patients with dilated and inflammatory cardiomyopathy. Eur. J. Heart Fail 2012, 14, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Bakris, G.; Oparil, S.; Weber, M.A.; Li, H.; Mallick, M.; Bharucha, D.B.; Chen, C.; Ferguson, W.G.; Sorin, J.; et al. Correlations of plasma renin activity and aldosterone concentration with ambulatory blood pressure responses to nebivolol and valsartan, alone and in combination, in hypertension. J. Am. Soc. Hypertens 2015, 9, 845–854. [Google Scholar] [CrossRef]
- Huby, A.C.; Antonova, G.; Groenendyk, J.; Gomez-Sanchez, C.E.; Bollag, W.B.; Filosa, J.A.; Belin de Chantemèle, E.J. Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation 2015, 132, 2134–2145. [Google Scholar] [CrossRef]
- Pocock, S.J.; Wang, D.; Pfeffer, M.A.; Yusuf, S.; McMurray, J.J.; Swedberg, K.B.; Ostergren, J.; Michelson, E.L.; Pieper, K.S.; Granger, C.B. Predictors of mortality and morbidity in patients with chronic heart failure. Eur. Heart J. 2006, 27, 65–75. [Google Scholar] [CrossRef]
- Mizuno, A.; Murakami, T.; Otani, S.; Kuwajima, M.; Shima, K. Leptin affects pancreatic endocrine functions through the sympathetic nervous system. Endocrinology 1998, 139, 3863–3870. [Google Scholar] [CrossRef]
- Seufert, J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes 2004, 53 (Suppl. S1), S152–S158. [Google Scholar] [CrossRef]
- Seufert, J.; Kieffer, T.J.; Habener, J.F. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc. Natl. Acad. Sci. USA 1999, 96, 674–679. [Google Scholar] [CrossRef]
- Marroquí, L.; Gonzalez, A.; Ñeco, P.; Caballero-Garrido, E.; Vieira, E.; Ripoll, C.; Nadal, A.; Quesada, I. Role of leptin in the pancreatic β-cell: Effects and signaling pathways. J. Mol. Endocrinol. 2012, 49, R9–R17. [Google Scholar] [CrossRef]
- Park, J.Y.; Chong, A.Y.; Cochran, E.K.; Kleiner, D.E.; Haller, M.J.; Schatz, D.A.; Gorden, P. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: The effect of long-term leptin therapy. J. Clin. Endocrinol. Metab. 2008, 93, 26–31. [Google Scholar] [CrossRef]
- Ebihara, K.; Masuzaki, H.; Nakao, K. Long-term leptin-replacement therapy for lipoatrophic diabetes. N. Engl. J. Med. 2004, 351, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, Y.; Rong, X.; Zheng, C.; Guo, J. Anti-Lipolysis Induced by Insulin in Diverse Pathophysiologic Conditions of Adipose Tissue. Diabetes Metab. Syndr. Obes. 2020, 13, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; McLean, B.; Badiwala, M.; Billia, F. Heart Failure and Drug Therapies: A Metabolic Review. Int. J. Mol. Sci. 2022, 23, 2960. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Rauchhaus, M.; Godsland, I.F.; Egerer, K.; Niebauer, J.; Sharma, R.; Cicoira, M.; Florea, V.G.; Coats, A.J.; Anker, S.D. Insulin resistance in moderate chronic heart failure is related to hyperleptinaemia, but not to norepinephrine or TNF-alpha. Int. J. Cardiol. 2002, 83, 73–81. [Google Scholar] [CrossRef]
- Riehle, C.; Abel, E.D. Insulin Signaling and Heart Failure. Circ. Res. 2016, 118, 1151–1169. [Google Scholar] [CrossRef]
- Moon, H.S.; Matarese, G.; Brennan, A.M.; Chamberland, J.P.; Liu, X.; Fiorenza, C.G.; Mylvaganam, G.H.; Abanni, L.; Carbone, F.; Williams, C.J.; et al. Efficacy of metreleptin in obese patients with type 2 diabetes: Cellular and molecular pathways underlying leptin tolerance. Diabetes 2011, 60, 1647–1656. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Dahlström, U.; Filippatos, G.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Metra, M.; et al. EURObservational Research Programme: The Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail 2010, 12, 1076–1084. [Google Scholar] [CrossRef]
- Jordan, J.; Stinkens, R.; Jax, T.; Engeli, S.; Blaak, E.E.; May, M.; Havekes, B.; Schindler, C.; Albrecht, D.; Pal, P.; et al. Improved Insulin Sensitivity With Angiotensin Receptor Neprilysin Inhibition in Individuals with Obesity and Hypertension. Clin. Pharmacol. Ther. 2017, 101, 254–263. [Google Scholar] [CrossRef]
- Wang, M.Y.; Chen, L.; Clark, G.O.; Lee, Y.; Stevens, R.D.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Charron, M.J.; Newgard, C.B.; et al. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Pflaum, C.D.; Rauchhaus, M.; Godsland, I.F.; Egerer, K.; Cicoira, M.; Florea, V.G.; Sharma, R.; Bolger, A.P.; Coats, A.J.; et al. Leptin, insulin sensitivity and growth hormone binding protein in chronic heart failure with and without cardiac cachexia. Eur. J. Endocrinol. 2001, 145, 727–735. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Specification | Results | Q1; Q3/SD or % |
|---|---|---|---|
| Demographics | Age (years) | 62.0 | (±12.7) |
| Gender (male/female) | 54/20 | (73;27) | |
| Medical history | Ischemic Cardiomyopathy | 36 | (50.0) |
| Non-ischemic Cardiomyopathy | 36 | (50.0) | |
| Atrial fibrillation * | 20 | (26.8) | |
| Dyslipidemia | 42 | (56.3) | |
| Diabetes Mellitus | 24 | (32.4) | |
| Hypertension | 39 | (54.9) | |
| Chronic kidney disease ** | 22 | (30.6) | |
| History of smoking | 36 | (51.4) | |
| Clinical Measurement | BMI | 28.1 | (23.4;30.9) |
| SBP (mmHg) | 125 | (113,137) | |
| Heart rate (bpm) | 72 | (60;81) | |
| LVEF (%) | 30.2 | (25.0;37.0) | |
| LVEDD (mm) | 60.4 | (±8.8) | |
| Treatment | ACE/ARB (before ARNI) | 64 | (88.9) |
| BB | 63 | (87.5) | |
| MRA | 49 | (68.1) | |
| Statin | 44 | (61.1) | |
| Ezetimib | 7 | (9.3) | |
| Loop Diuretics | 40 | (55.6) | |
| Thiazides | 3 | (4.2) | |
| Metformin | 20 | (27.7) | |
| GLP-1 Inhibitors | 7 | (9.2) | |
| Insulin | 5 | (6.7) | |
| Laboratory | NT-proBNP (ng/L) | 2251 | (415;2679) |
| Hemoglobin g/L | 13.9 | (±1.8) | |
| eGFR (mL/min/1.73 m2) | 69.7 | (56.0;87.0) | |
| Total cholesterol (mg/dL) | 156.3 | (122;176) | |
| Triglyceride (mg/dL) | 130.7 | (90;148) | |
| LDL (mg/dL) | 85.6 | (58;97) | |
| HDL (mg/dL) | 51.5 | (37;58) | |
| HbA1c (%) | 5.9 | (5.4;6.1) | |
| CRP (mg/dL) | 0.7 | (0.38;0.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohnewein, B.; Shomanova, Z.; Paar, V.; Topf, A.; Jirak, P.; Fiedler, L.; Granitz, C.; Van Almsick, V.; Semo, D.; Zagidullin, N.; et al. Effects of Angiotensin Receptor-Neprilysin Inhibitors (ARNIs) on the Glucose and Fat Metabolism Biomarkers Leptin and Fructosamine. J. Clin. Med. 2023, 12, 3083. https://doi.org/10.3390/jcm12093083
Ohnewein B, Shomanova Z, Paar V, Topf A, Jirak P, Fiedler L, Granitz C, Van Almsick V, Semo D, Zagidullin N, et al. Effects of Angiotensin Receptor-Neprilysin Inhibitors (ARNIs) on the Glucose and Fat Metabolism Biomarkers Leptin and Fructosamine. Journal of Clinical Medicine. 2023; 12(9):3083. https://doi.org/10.3390/jcm12093083
Chicago/Turabian StyleOhnewein, Bernhard, Zornitsa Shomanova, Vera Paar, Albert Topf, Peter Jirak, Lukas Fiedler, Christina Granitz, Vincent Van Almsick, Dilvin Semo, Naufal Zagidullin, and et al. 2023. "Effects of Angiotensin Receptor-Neprilysin Inhibitors (ARNIs) on the Glucose and Fat Metabolism Biomarkers Leptin and Fructosamine" Journal of Clinical Medicine 12, no. 9: 3083. https://doi.org/10.3390/jcm12093083
APA StyleOhnewein, B., Shomanova, Z., Paar, V., Topf, A., Jirak, P., Fiedler, L., Granitz, C., Van Almsick, V., Semo, D., Zagidullin, N., Dieplinger, A.-M., Sindermann, J., Reinecke, H., Hoppe, U. C., Pistulli, R., & Motloch, L. J. (2023). Effects of Angiotensin Receptor-Neprilysin Inhibitors (ARNIs) on the Glucose and Fat Metabolism Biomarkers Leptin and Fructosamine. Journal of Clinical Medicine, 12(9), 3083. https://doi.org/10.3390/jcm12093083






