Lung Ultrasound in the Evaluation of Lung Disease Severity in Children with Clinically Stable Cystic Fibrosis: A Prospective Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study, Control and Interobserver Group
2.2. Study Design
2.3. Diagnostic Methods
- -
- -
- -
- -
- -
- -
- -
- -
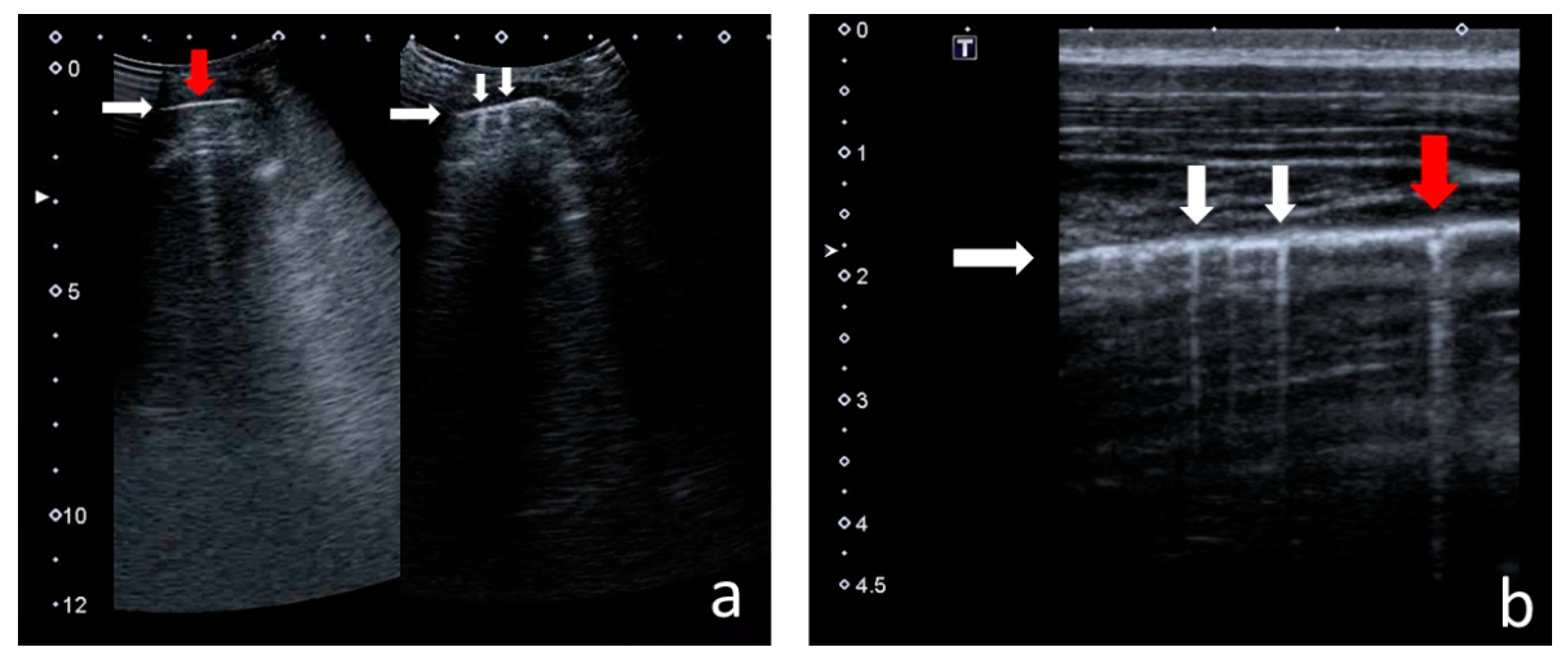
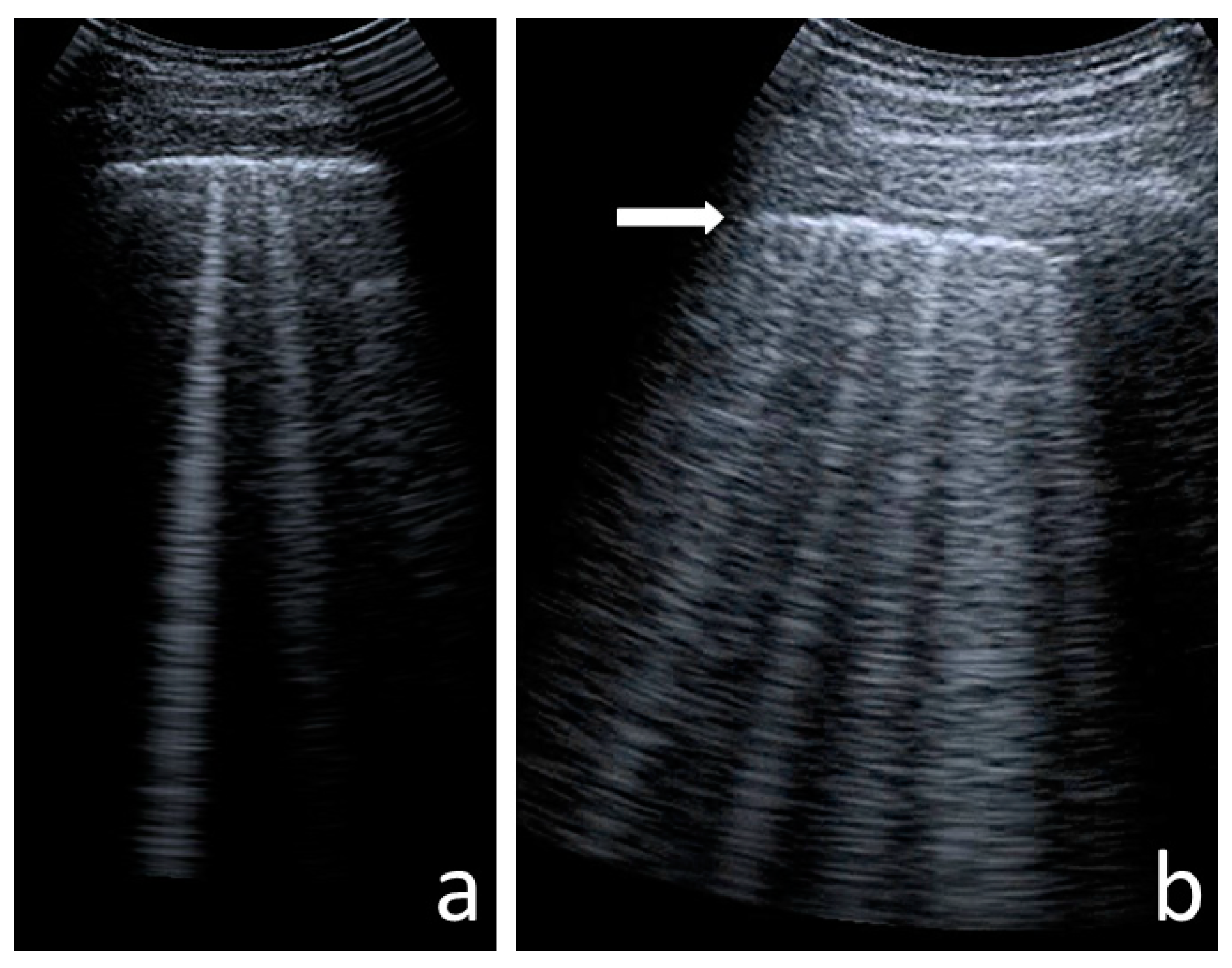
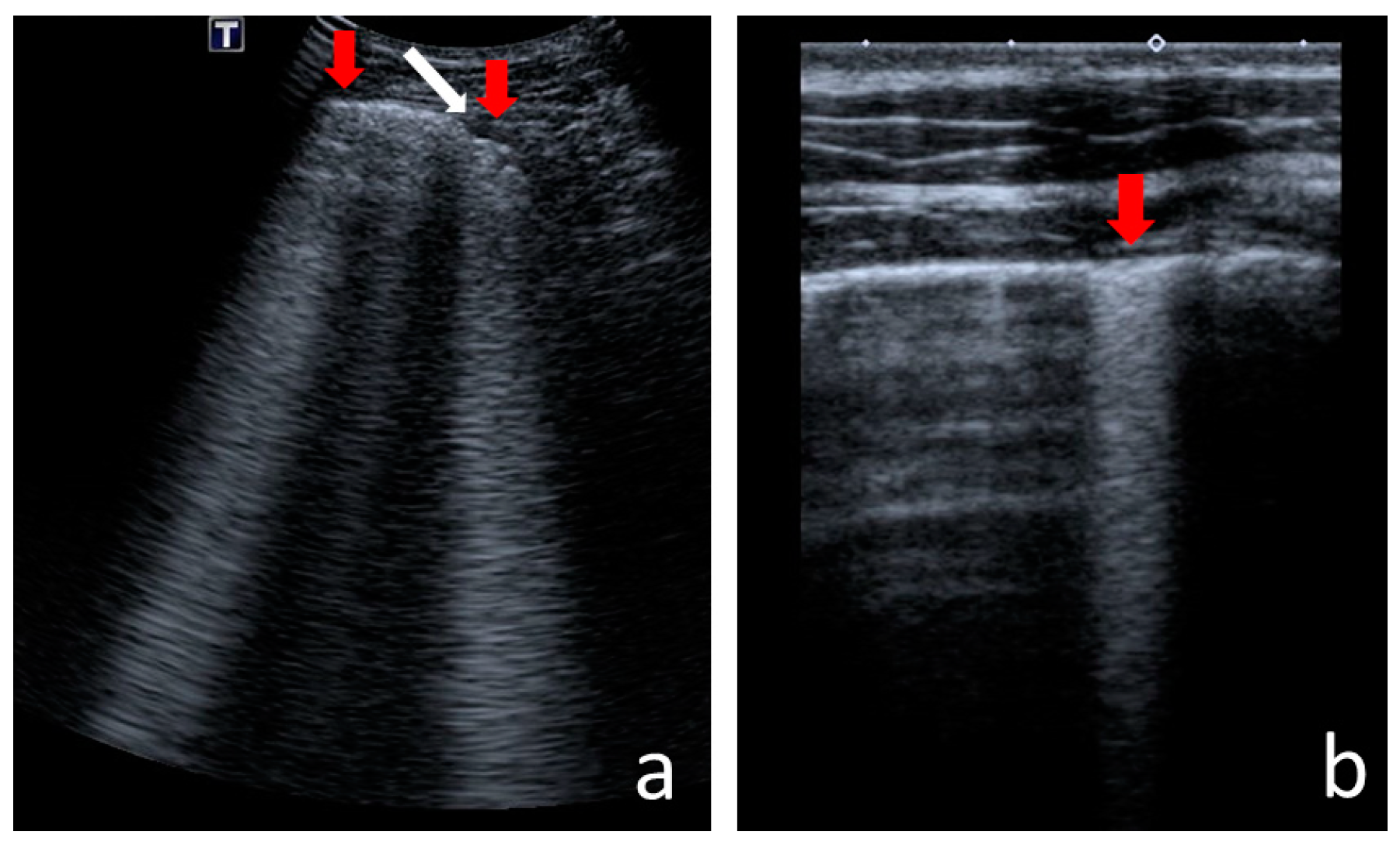
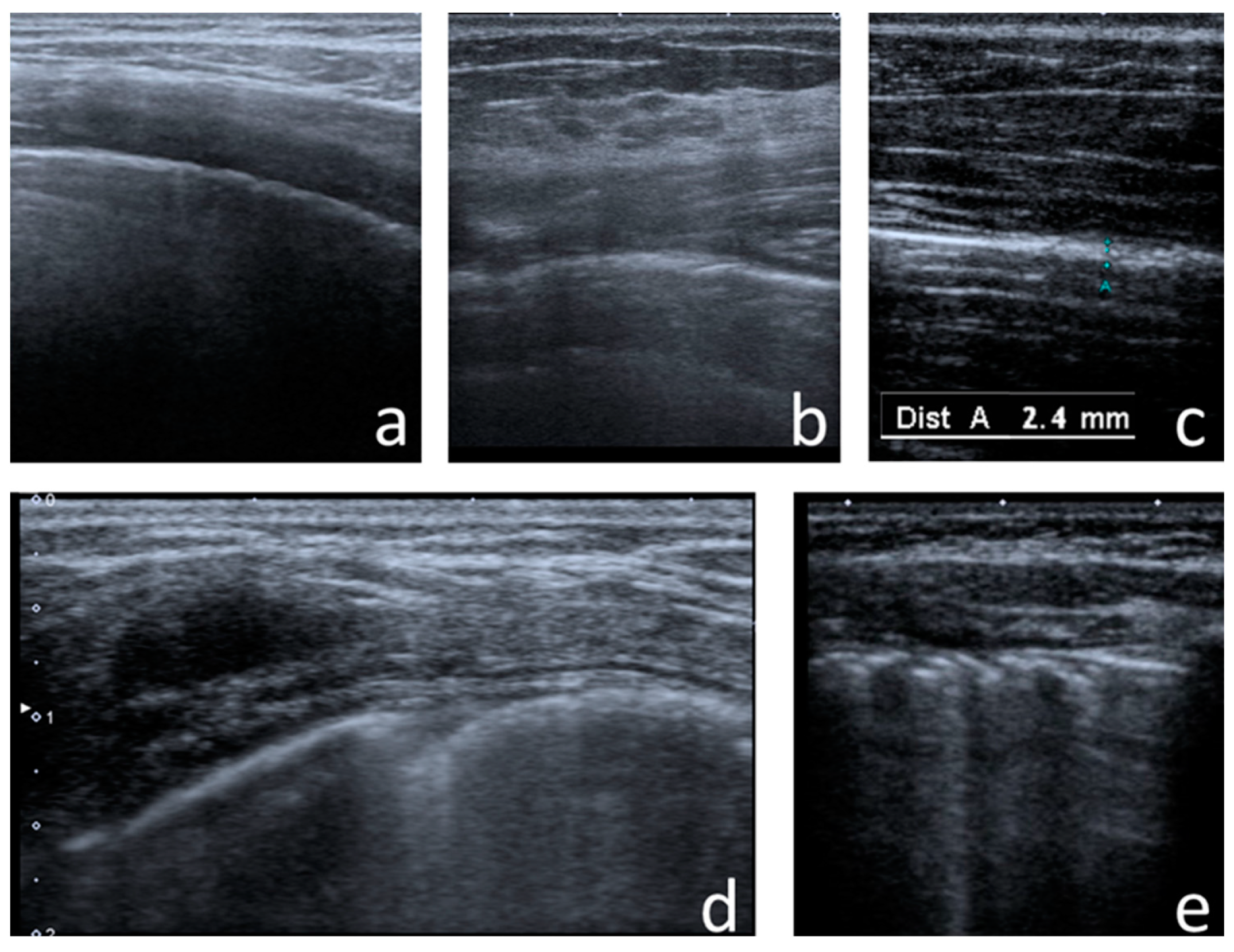
- Consolidations—hypoechoic, tissue-like areas: (a) small (≤10 mm) (Figure 6), and (b) major (>10 mm) (Figure 7); usually, major consolidations have the following associated features: the loss of pleural line echogenicity over the area of consolidation, absence of A-lines, presence of dynamic or static air bronchogram/air trapping and vascular pattern (in CD option) within the area, C-lines below the area, B-lines surrounding it [17,31];
- -
2.4. Statistical Analysis
2.5. Ethical Aspects
3. Results
3.1. US Signs Found in CF Patients vs. Healthy Children
3.1.1. Artefacts
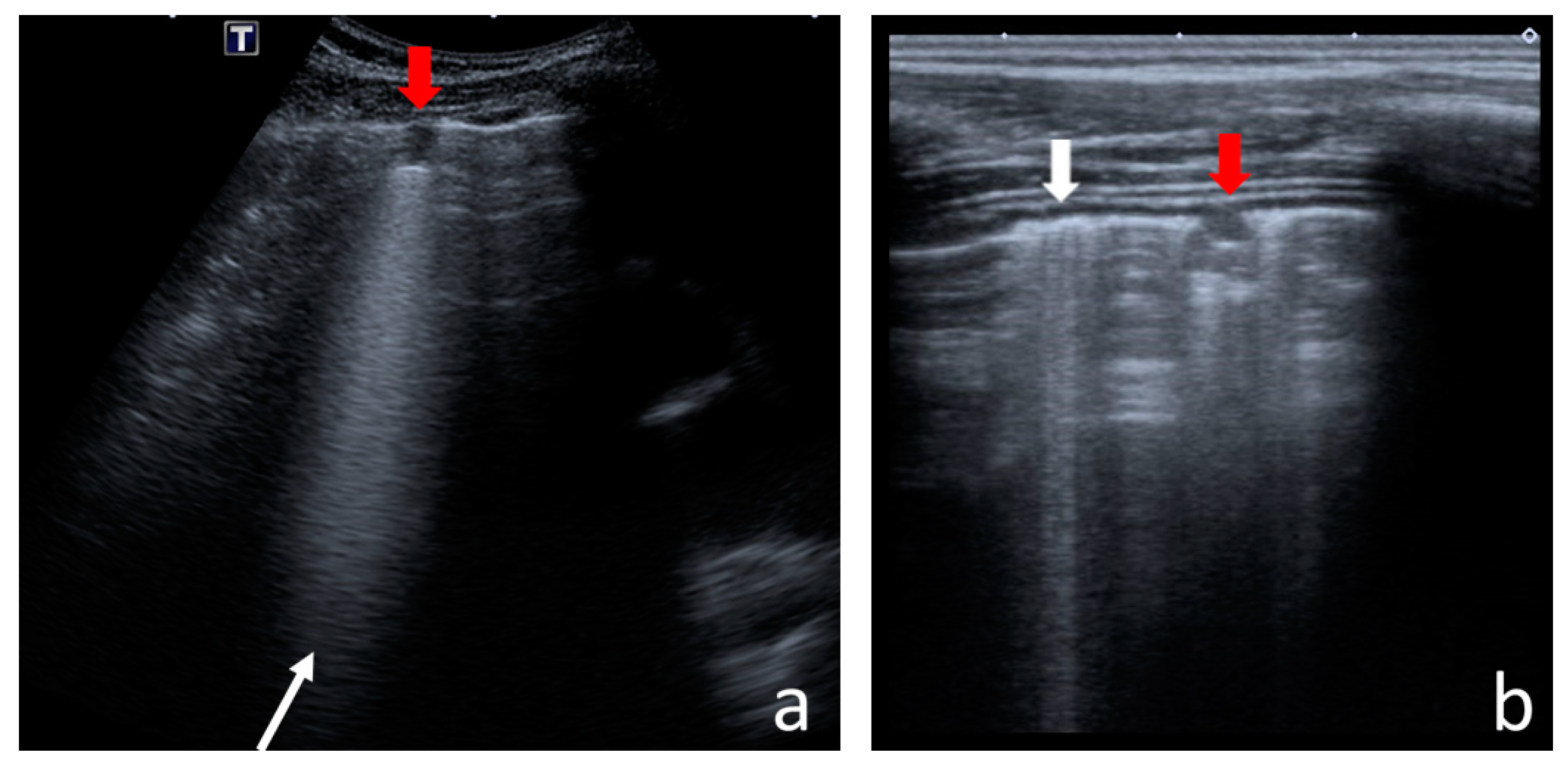
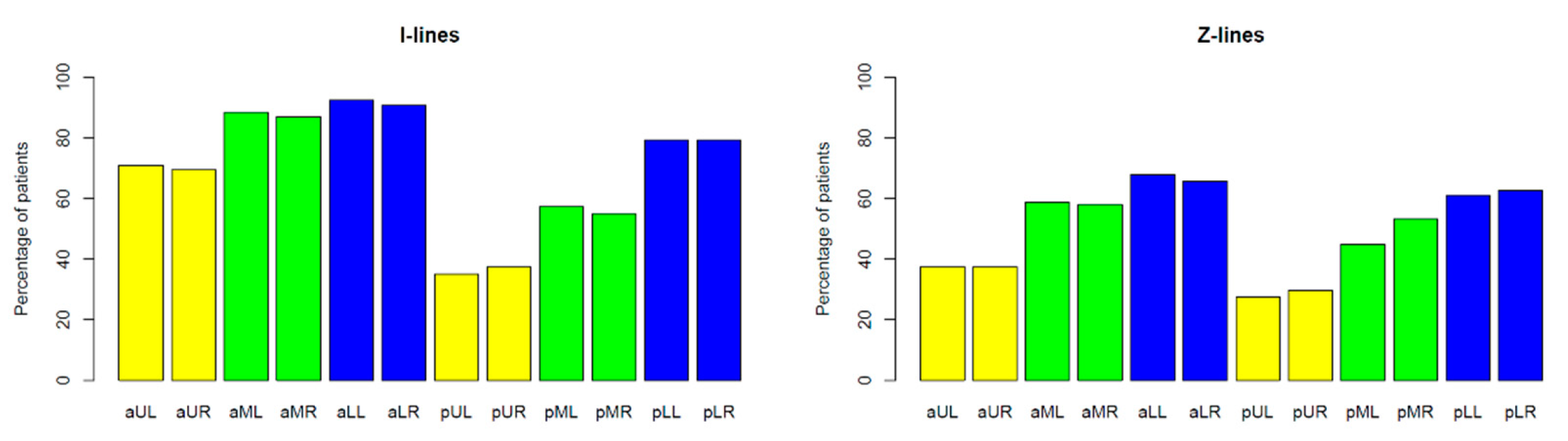
I-Line and Z-Line Artefacts
B-Line Artefacts
Am-Line Artefacts
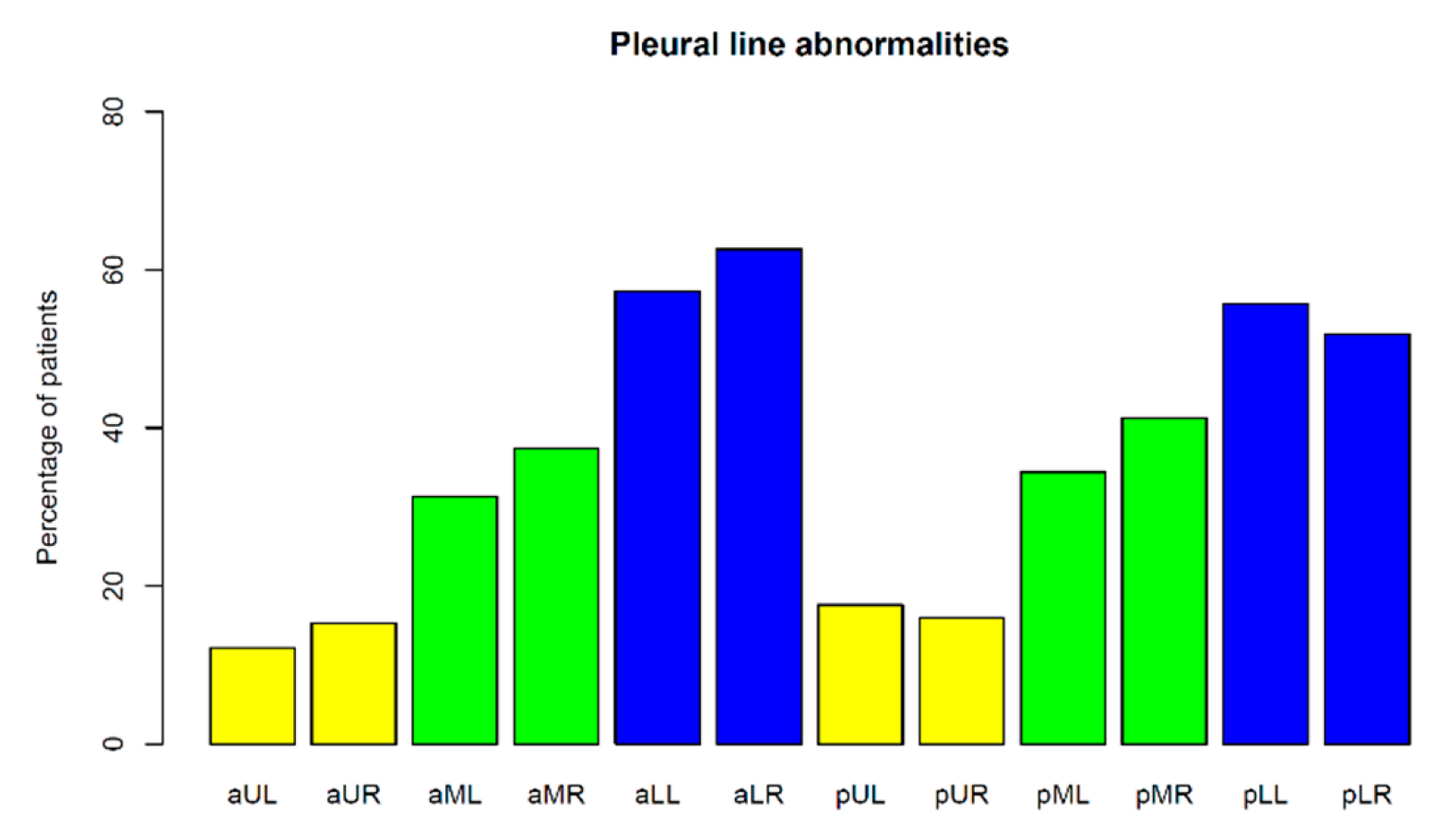
3.1.2. Pleural Line Abnormalities
3.1.3. Consolidations
3.1.4. Pleural Fluid
3.2. CF LUS Score Results
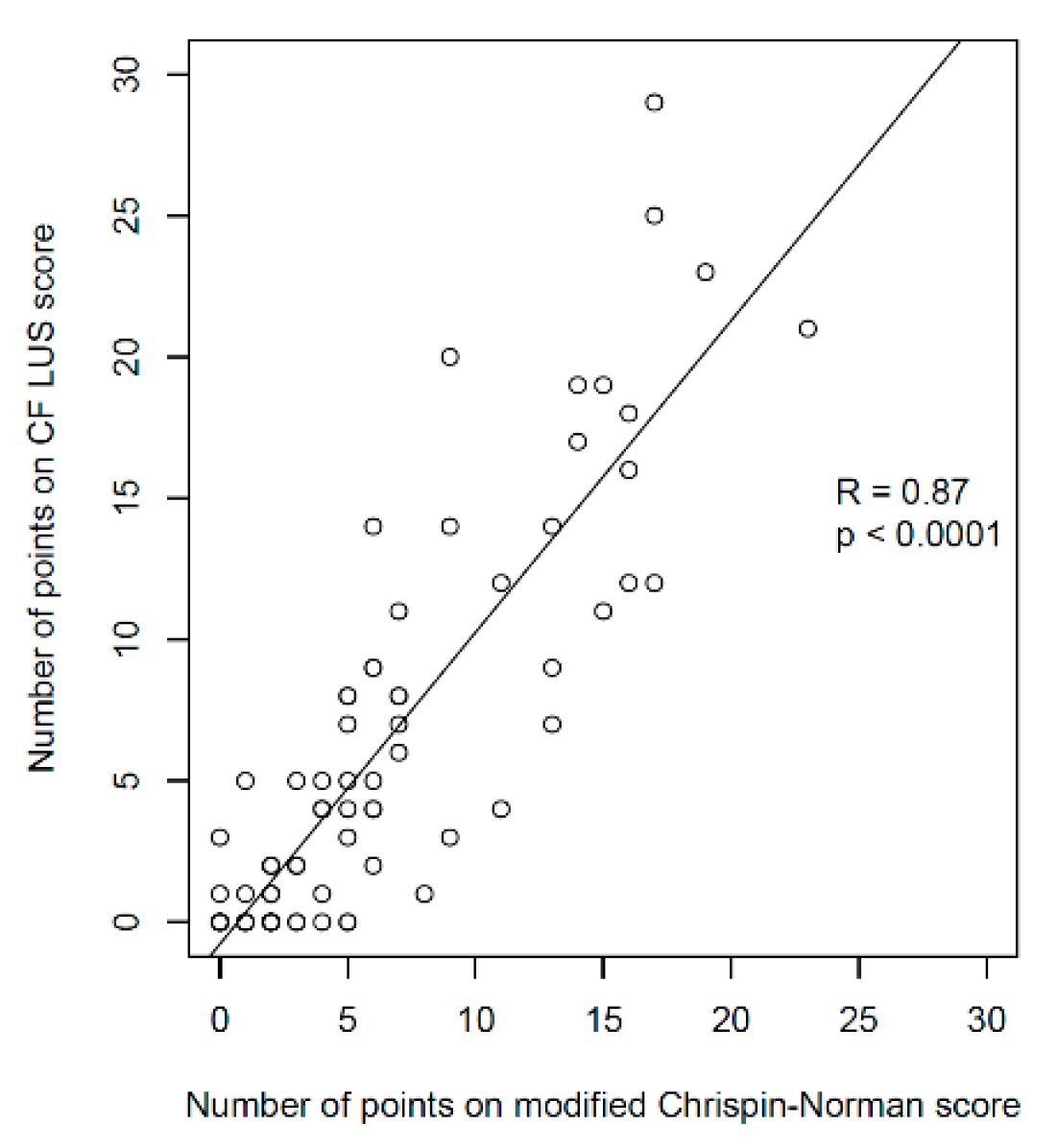
3.2.1. Ultrasound and Radiographic Score Comparison
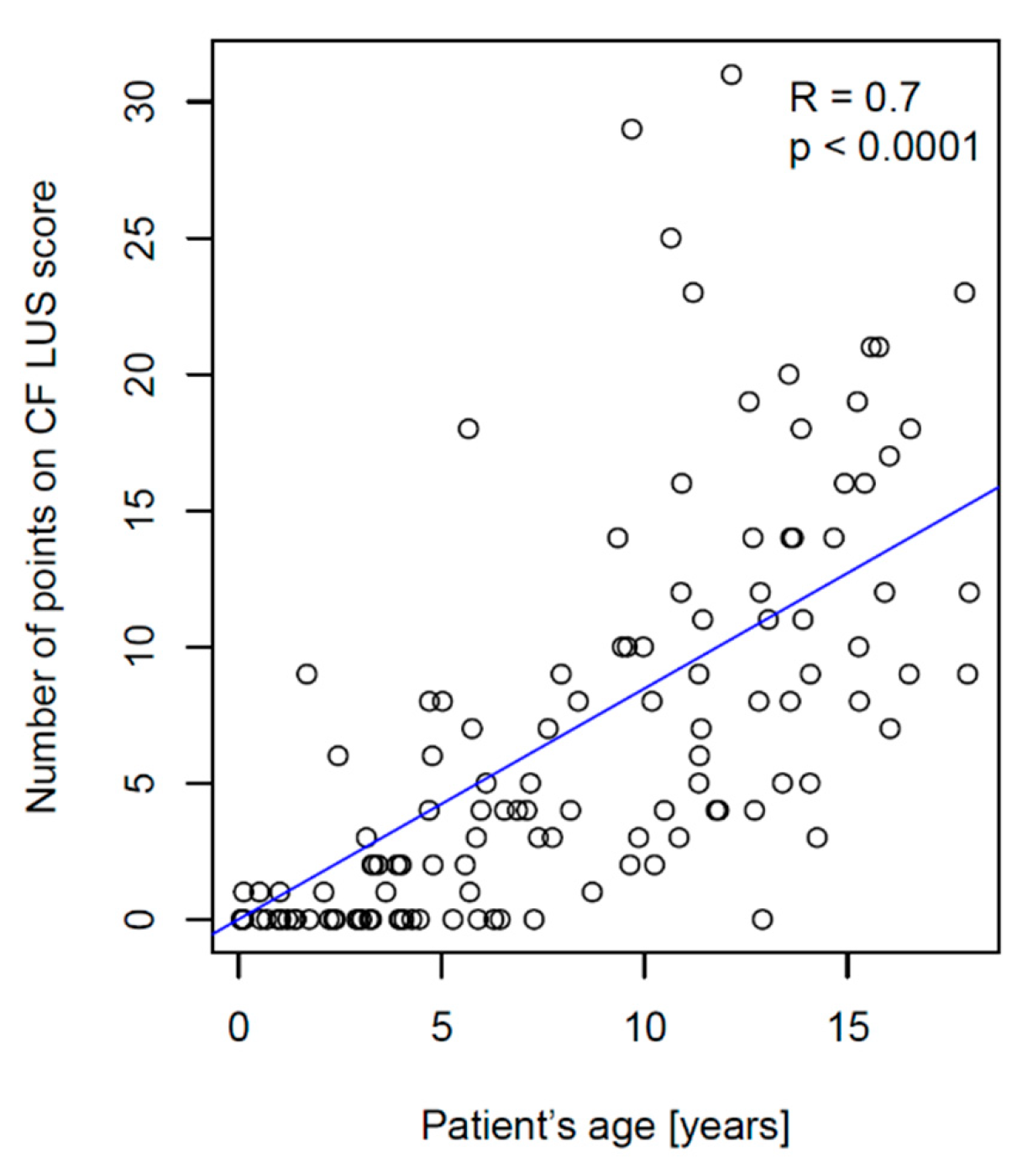
3.2.2. Correlation between CF LUS Score and PFTs Results
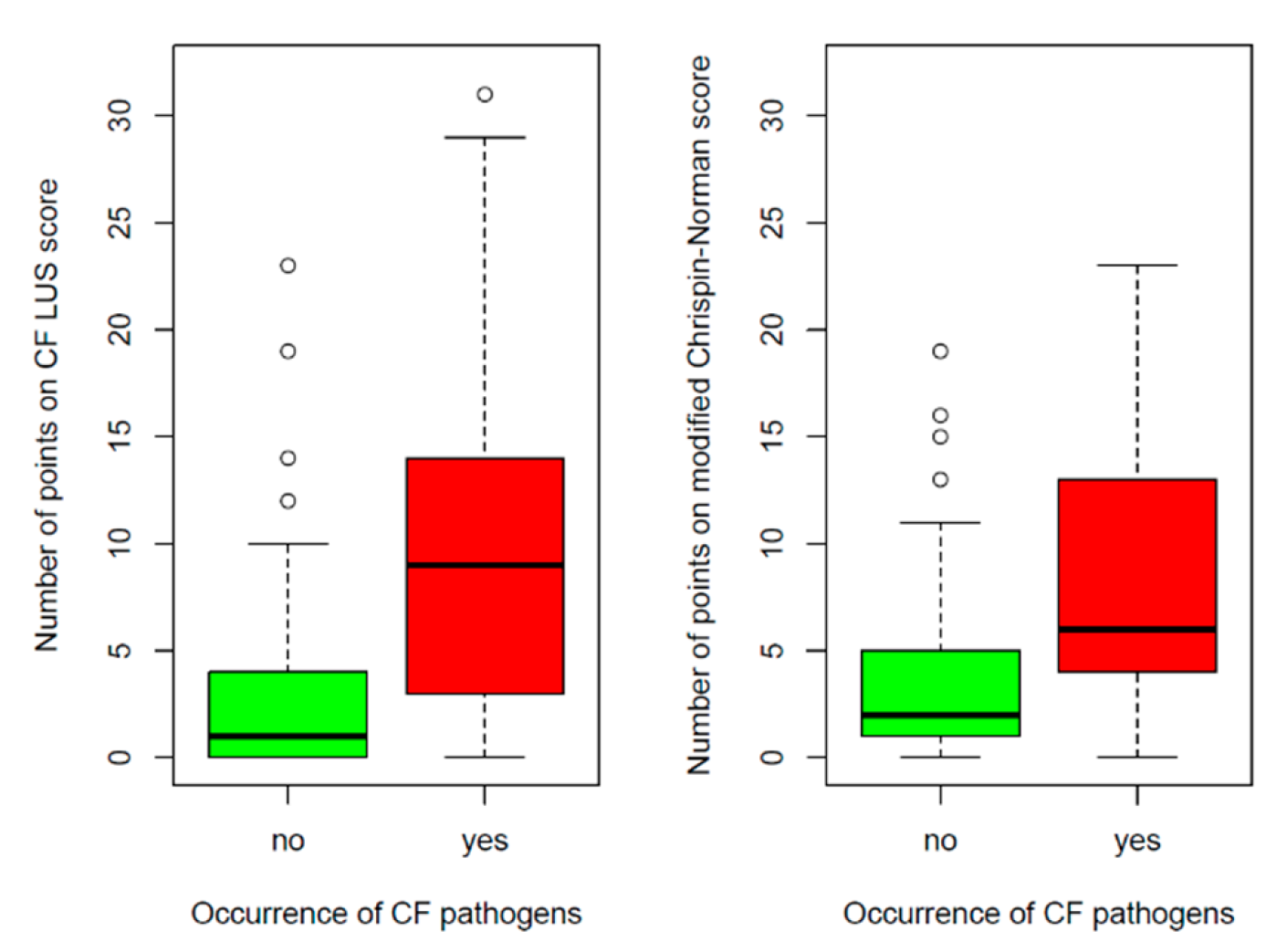
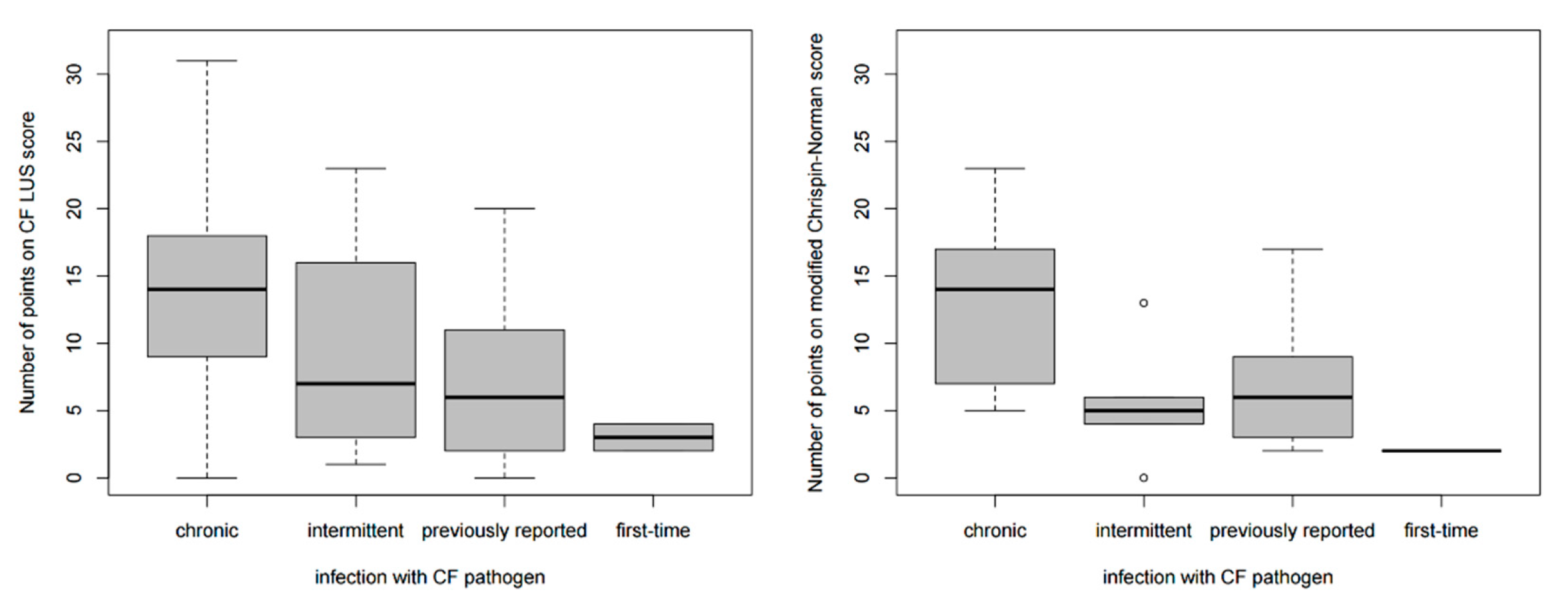
3.2.3. Correlation between CF LUS Score and Microbiological Status
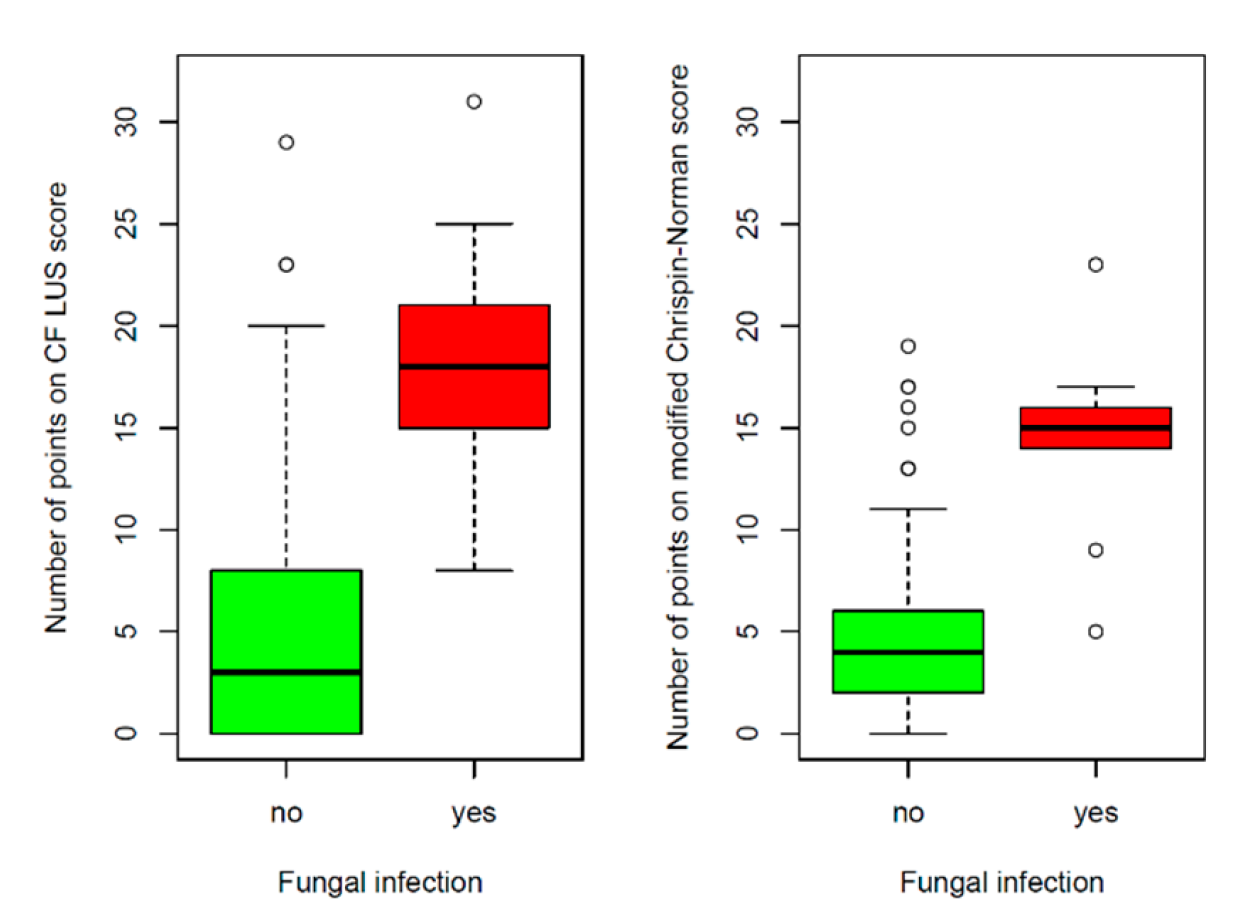
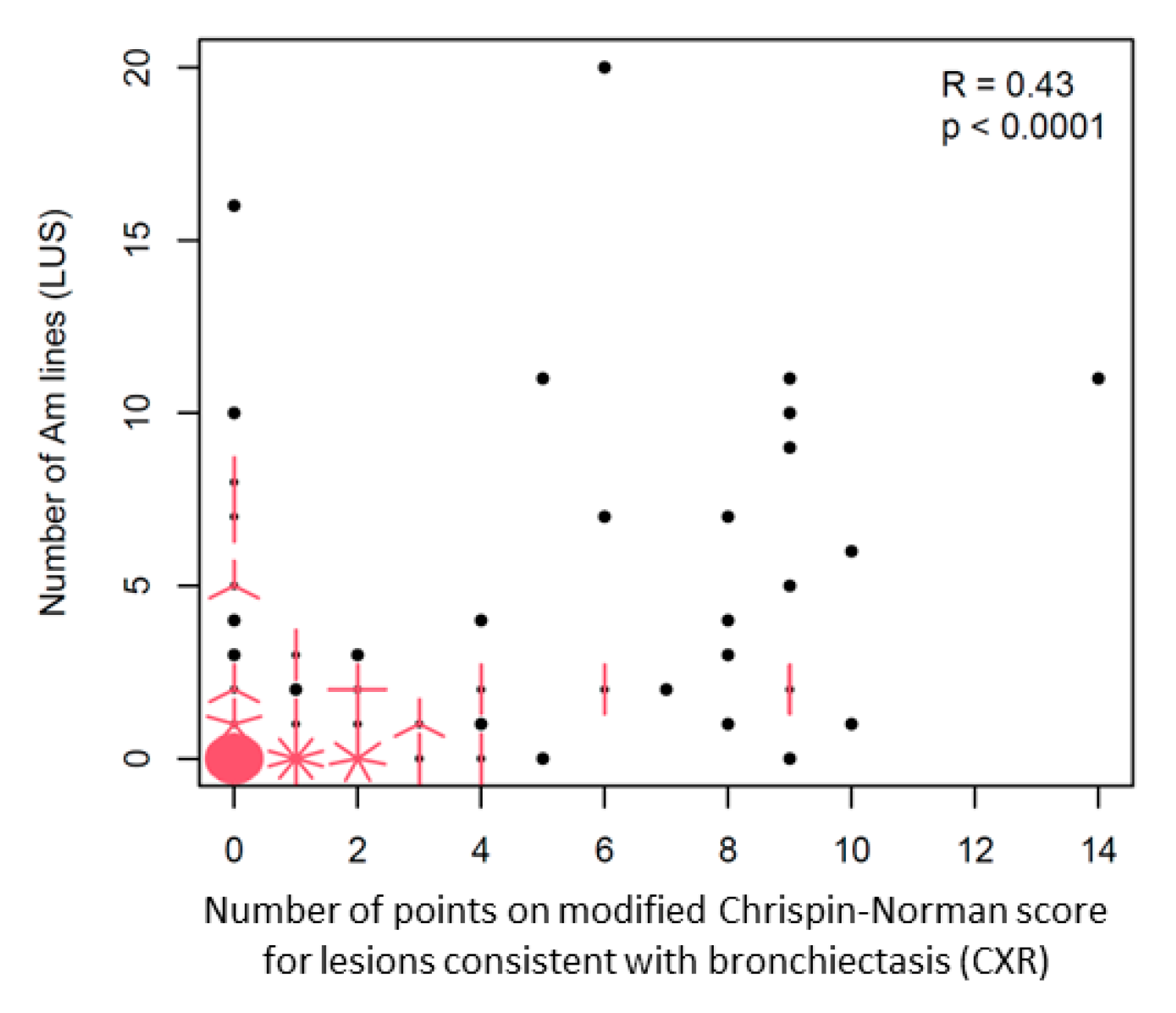
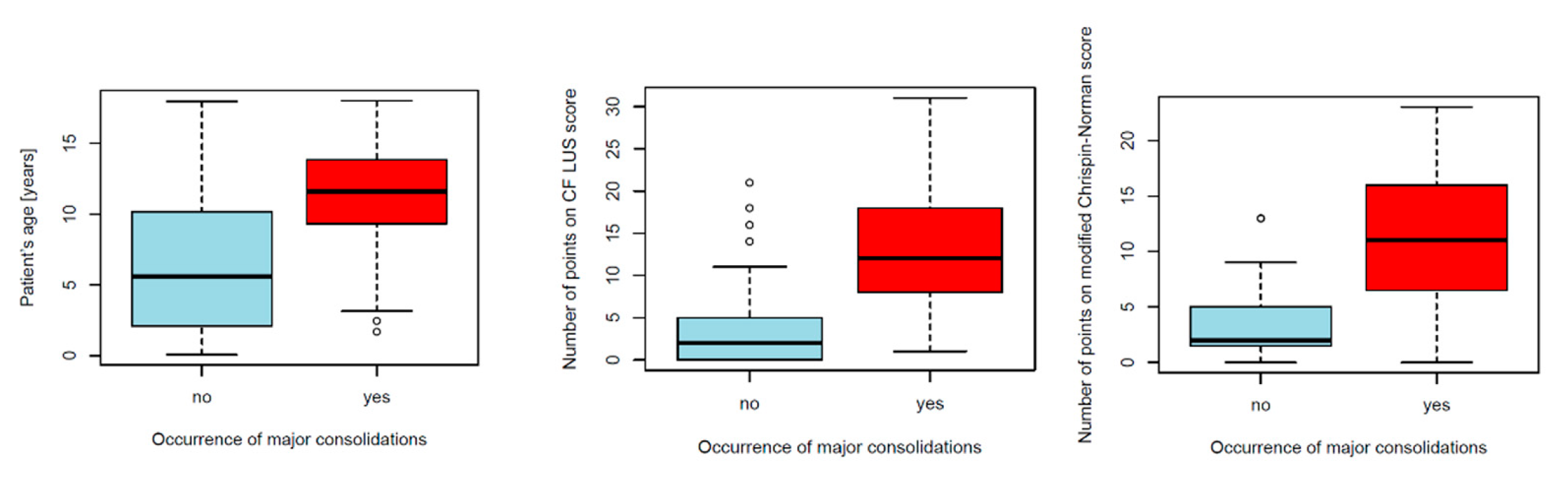
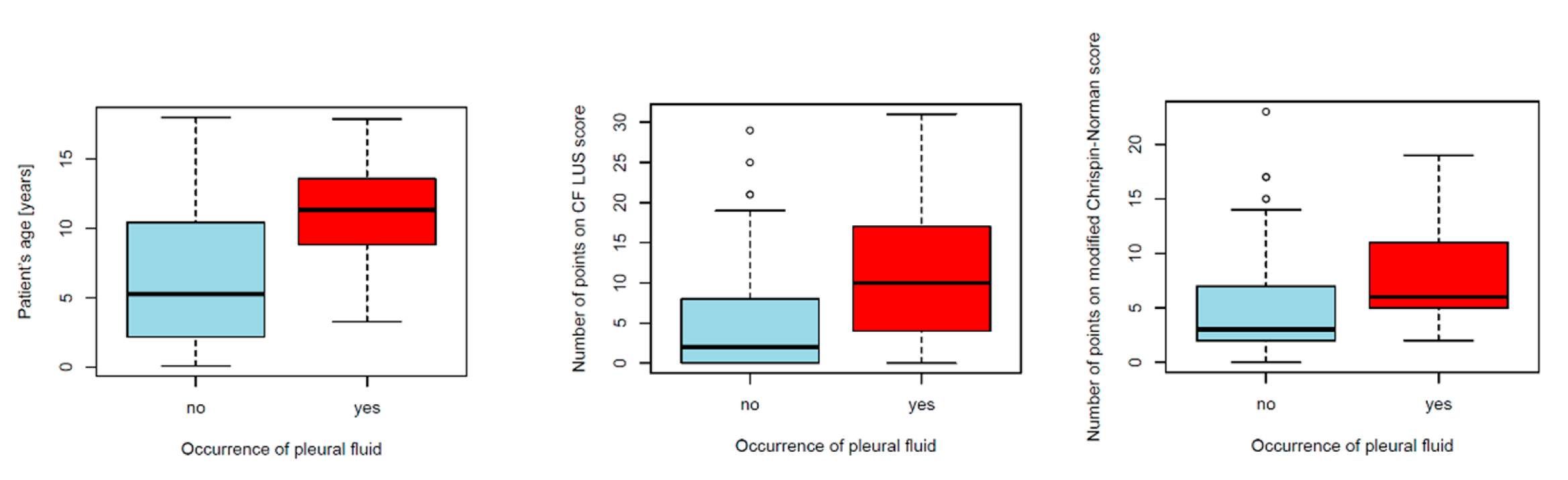
3.2.4. Reference of Selected US Signs to the Results of Other Diagnostic Tests
Numerous B-Line Artefacts
Am-Line Artefacts
Small Consolidations
Major Consolidations
Pleural Fluid
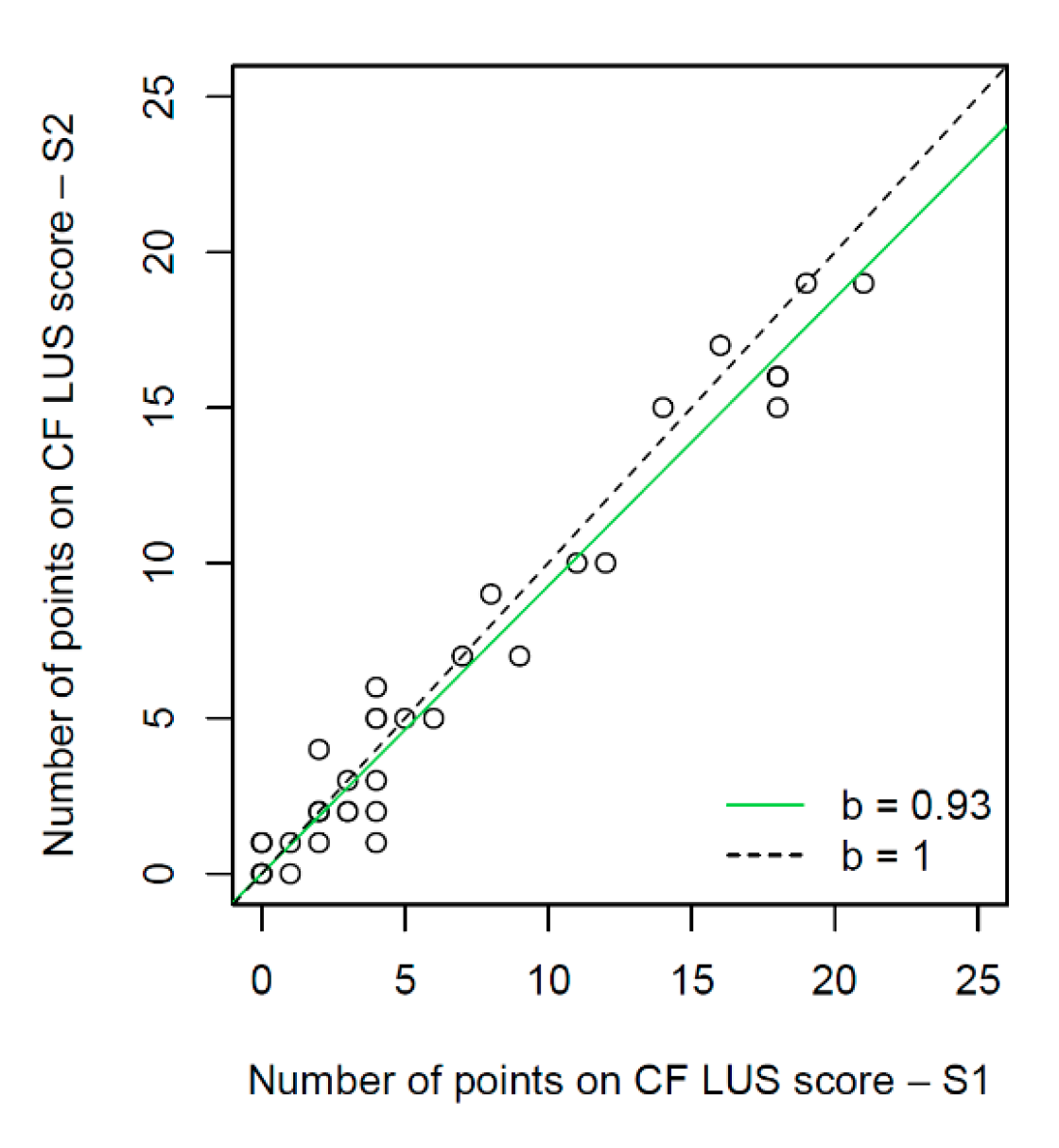
3.3. Interobserver Study
3.3.1. Interobserver Agreement for the Assessment of Individual US Signs
3.3.2. Interobserver Agreement for the Evaluation of CF Lung Disease Severity
4. Discussion
4.1. Specificity of the Study Group
4.2. The Meaning of US Signs Found in Children with CF
4.2.1. I-Line and Z-Line Artefacts
4.2.2. B-Line Artefacts
4.2.3. Am-Line Artefacts
4.2.4. Small Consolidations
4.2.5. Major Consolidations
4.2.6. Pleural Line Abnormalities
4.2.7. Pleural Fluid
4.3. Ultrasound Scores
- Significantly higher correlation with the radiographic score (R = 0.87 vs. 0.52);
- Significantly higher correlation with patient’s age (R = 0.7 vs. 0.12);
- Greater diversification of the number of points depending on the patient’s age;
- Higher maximum number of points obtained in the study group (31 vs. 16).
4.4. Interobserver Study
4.5. Strengths and Limitations of the Study
- Comparison of LUS results in CF patients with those of healthy children (although the control group could have been larger);
- Large study group (over two times larger than the biggest group among the compared studies), also including the youngest children—patients diagnosed with CF in the second month of life;
- Inclusion of Am-lines in assessment, artefacts which may be consistent with bronchiectasis [34];
- Conducting an interobserver study on a large proportion of the study group (29%), which enabled us to check the reproducibility of LUS in terms of individual US signs and in terms of the evaluation of the lung disease severity using the ultrasound score.
4.6. Summary
5. Conclusions
- Lung ultrasound is a valuable test in the evaluation of CF lung disease severity. It is comparable with methods routinely used during check-ups of patients with clinically stable CF, i.e., chest radiograph, pulmonary function tests and microbiological tests of the respiratory system.
- Lung ultrasound and chest radiograph should be treated as complementary diagnostic procedures as each of these imaging methods has different advantages and limitations.
- Lung ultrasound is a method of high interobserver reproducibility in the evaluation of lung disease severity in children with CF, when appropriately standardised.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Boeck, K. From the discovery of the CFTR gene in 1989 through to 2014. In Hoddson and Geddes’ Cystic Fibrosis, 4th ed.; Bush, A., Bilton, D., Hodson, M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 3–17. [Google Scholar]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [PubMed]
- Kerem, E.; Conway, S.; Elborn, S.; Heijerman, H.; Committee, C. Standards of care for patients with cystic fibrosis: A European consensus. J. Cyst. Fibros 2005, 4, 7–26. [Google Scholar] [CrossRef]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Sly, P.D.; Brennan, S.; Gangell, C.; de Klerk, N.; Murray, C.; Mott, L.; Stick, S.M.; Robinson, P.J.; Robertson, C.F.; Ranganathan, S.C.; et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am. J. Respir. Crit. Care Med. 2009, 180, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.; Kemner-van de Corput, M.P.; Perez-Rovira, A.; de Bruijne, M.; Fajac, I.; Tiddens, H.A.; van Straten, M. Multicentre chest computed tomography standardisation in children and adolescents with cystic fibrosis: The way forward. Eur. Respir. J. 2016, 47, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.M.; Aurora, P.; Stanojevic, S.; Bush, A.; Wade, A.; Oliver, C.; Calder, A.; Price, J.; Carr, S.B.; Shankar, A.; et al. Lung Clearance Index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax 2011, 66, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.F.; Pontello, E.; Pintani, E.; de Winter-de Groot, K.M.; D’Orazio, C.; Assael, B.M.; Hunink, M.G.M.; Tiddens, H.A.W.M.; Caudri, D. The impact of chest computed tomography and chest radiography on clinical management of cystic fibrosis lung disease. J. Cyst. Fibros 2020, 19, 641–646. [Google Scholar] [CrossRef]
- Joyce, S.; Carey, B.W.; Moore, N.; Mullane, D.; Moore, M.; McEntee, M.F.; Plant, B.J.; Maher, M.M.; O’Connor, O.J. Computed tomography in cystic fibrosis lung disease: A focus on radiation exposure. Pediatr. Radiol. 2021, 51, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, K.P.; Glynn, D.; Joyce, S.; Maher, M.M.; Boland, F.; O’Connor, O.J. Best Practices: Imaging Strategies for Reduced-Dose Chest CT in the Management of Cystic Fibrosis-Related Lung Disease. AJR Am. J. Roentgenol. 2021, 217, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Dillman, J.R.; Goodsitt, M.M.; Christodoulou, E.G.; Keshavarzi, N.; Strouse, P.J. Model-based iterative reconstruction: Effect on patient radiation dose and image quality in pediatric body CT. Radiology 2014, 270, 526–534. [Google Scholar] [CrossRef]
- de Jong, P.A.; Mayo, J.R.; Golmohammadi, K.; Nakano, Y.; Lequin, M.H.; Tiddens, H.A.; Aldrich, J.; Coxson, H.O.; Sin, D.D. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am. J. Respir. Crit. Care Med. 2006, 173, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Wielpütz, M.O.; von Stackelberg, O.; Stahl, M.; Jobst, B.J.; Eichinger, M.; Puderbach, M.U.; Nährlich, L.; Barth, S.; Schneider, C.; Kopp, M.V.; et al. Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J. Cyst. Fibros 2018, 17, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Leutz-Schmidt, P.; Eichinger, M.; Stahl, M.; Sommerburg, O.; Biederer, J.; Kauczor, H.U.; Puderbach, M.U.; Mall, M.A.; Wielpütz, M.O. Ten years of chest MRI for patients with cystic fibrosis: Translation from the bench to clinical routine. Radiologe 2019, 59, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Steinke, E.; Graeber, S.Y.; Joachim, C.; Seitz, C.; Kauczor, H.U.; Eichinger, M.; Hämmerling, S.; Sommerburg, O.; Wielpütz, M.O.; et al. Magnetic Resonance Imaging Detects Progression of Lung Disease and Impact of Newborn Screening in Preschool Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 943–953. [Google Scholar] [CrossRef]
- Zar, H.J.; Andronikou, S.; Nicol, M.P. Advances in the diagnosis of pneumonia in children. BMJ 2017, 358, j2739. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2022, 42, 309–344. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, I.; Pop, L. Lung ultrasound in CF children’s exacerbation—One center experience. J. Cyst. Fibros. 2015, 14, S95. [Google Scholar] [CrossRef]
- Ciuca, I.; Pop, L.; Marc, M.; Oancea, C. How useful is the lung ultrasound in cystic fibrosis? Eur. Respir. J. 2016, 48, PA1261. [Google Scholar] [CrossRef]
- Ciuca, I.; Dediu, M.; Pop, L. Lung clearance index and lung ultrasound in cystic fibrosis children. Eur. Respir. J. 2018, 52, OA4988. [Google Scholar] [CrossRef]
- Ciuca, I.; Dediu, M.; Tomas, L.; Pop, L. Lung ultrasound score and the relation with lung clearance index. J. Cyst. Fibros. 2018, 17, 11–12. [Google Scholar] [CrossRef]
- Peixoto, A.O.; Marson, F.A.L.; Souza, T.H.; Fraga, A.M.A.; Ribeiro, J.D. Lung ultrasound assessment of response to antibiotic therapy in cystic fibrosis exacerbations: A study of two cases. J. Bras. Pneumol. 2019, 45, e20190128. [Google Scholar] [CrossRef] [PubMed]
- Strzelczuk-Judka, L.; Wojsyk-Banaszak, I.; Zakrzewska, A.; Jończyk-Potoczna, K. Diagnostic value of chest ultrasound in children with cystic fibrosis—Pilot study. PLoS ONE 2019, 14, e0215786. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.O.; Marson, F.A.; Dertkigil, S.S.; Dertkigil, R.P.; Souza, T.H.; Fraga, A.M.; Ribeiro, A.F.; Toro, A.A.; Ribeiro, J.D. The Use of Ultrasound as a Tool to Evaluate Pulmonary Disease in Cystic Fibrosis. Respir. Care 2020, 65, 293–303. [Google Scholar] [CrossRef]
- Hassanzad, M.; Kiani, A.; Abedini, A.; Ghaffaripour, H.; Emami, H.; Alizadeh, N.; Zoghi, G.; Hashemi, S.; Velayati, A.A. Lung ultrasound for the diagnosis of cystic fibrosis pulmonary exacerbation. BMC Pulm. Med. 2021, 21, 353. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, I.M.; Pop, L.L.; Dediu, M.; Stoicescu, E.R.; Marc, M.S.; Manea, A.M.; Manolescu, D.L. Lung Ultrasound in Children with Cystic Fibrosis in Comparison with Chest Computed Tomography: A Feasibility Study. Diagnostics 2022, 12, 376. [Google Scholar] [CrossRef]
- Ter Haar, G. Ultrasonic imaging: Safety considerations. Interface Focus 2011, 1, 686–697. [Google Scholar] [CrossRef] [PubMed]
- ter Haar, G. Ultrasound bio-effects and safety considerations. Front. Neurol. Neurosci. 2015, 36, 23–30. [Google Scholar] [CrossRef]
- Fowlkes, J.B. American Institute of Ultrasound in Medicine consensus report on potential bioeffects of diagnostic ultrasound: Executive summary. J. Ultrasound Med. 2008, 27, 503–515. [Google Scholar]
- Buda, N. Ocena Przydatnosci Przezklatkowej Ultrasonografii Pluc w Diagnostyce Wloknienia w Przebiegu Srodmiazszowych Chorob Pluc; Medical University of Gdansk: Gdansk, Poland, 2014. [Google Scholar]
- Lichtenstein, D. Novel approaches to ultrasonography of the lung and pleural space: Where are we now? Breathe 2017, 13, 100–111. [Google Scholar] [CrossRef]
- Lichtenstein, D. General Ultrasound in the Critically Ill; Springer: Berlin/Heidelberg, Germany, 2010; pp. 117–127. [Google Scholar]
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 4, 1. [Google Scholar] [CrossRef]
- Buda, N.; Piskunowicz, M.; Porzezińska, M.; Kosiak, W.; Zdrojewski, Z. Lung Ultrasonography in the Evaluation of Interstitial Lung Disease in Systemic Connective Tissue Diseases: Criteria and Severity of Pulmonary Fibrosis—Analysis of 52 Patients. Ultraschall Med. 2016, 37, 379–385. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.A.; Achterberg, J.A.; Kessels, O.A.; van Ginneken, B.; Hogeweg, L.; Beek, F.J.; Terheggen-Lagro, S.W. Modified Chrispin-Norman chest radiography score for cystic fibrosis: Observer agreement and correlation with lung function. Eur. Radiol. 2011, 21, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Benden, C.; Wallis, C.; Owens, C.M.; Ridout, D.A.; Dinwiddie, R. The Chrispin-Norman score in cystic fibrosis: Doing away with the lateral view. Eur. Respir. J. 2005, 26, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Beydon, N.; Davis, S.D.; Lombardi, E.; Allen, J.L.; Arets, H.G.; Aurora, P.; Bisgaard, H.; Davis, G.M.; Ducharme, F.M.; Eigen, H.; et al. An official American Thoracic Society/European Respiratory Society statement: Pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 2007, 175, 1304–1345. [Google Scholar] [CrossRef]
- Robinson, P.D.; Latzin, P.; Verbanck, S.; Hall, G.L.; Horsley, A.; Gappa, M.; Thamrin, C.; Arets, H.G.; Aurora, P.; Fuchs, S.I.; et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur. Respir. J. 2013, 41, 507–522. [Google Scholar] [CrossRef]
- Walicka-Serzysko, K.; Postek, M.; Milczewska, J.; Sands, D. Lung Clearance Index in Children with Cystic Fibrosis during Pulmonary Exacerbation. J. Clin. Med. 2021, 10, 4884. [Google Scholar] [CrossRef]
- Parkins, M.D.; Floto, R.A. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J. Cyst. Fibros 2015, 14, 293–304. [Google Scholar] [CrossRef]
- Gilligan, P.H.; Downey, D.G.; Elborn, J.S.; Flume, P.A.; Funk, S.; Gilpin, D.; Kidd, T.J.; McCaughan, J.; Millar, B.C.; Murphy, P.G.; et al. “Pathogen Eradication” and “Emerging Pathogens”: Difficult Definitions in Cystic Fibrosis. J. Clin. Microbiol. 2018, 56, e00193-18. [Google Scholar] [CrossRef]
- Mahenthiralingam, E. Emerging cystic fibrosis pathogens and the microbiome. Paediatr. Respir. Rev. 2014, 15 (Suppl. S1), 13–15. [Google Scholar] [CrossRef]
- Altman, G.D. Practical Statistics for Medical Research 1999; CRC Press: Boca Raton, FL, USA, 1999; p. 624. [Google Scholar]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Guilford, J.P. Fundamental Statistics in Psychology and Education; McGraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Lichtenstein, D.A.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Buda, N.; Skoczylas, A.; Demi, M.; Wojteczek, A.; Cylwik, J.; Soldati, G. Clinical Impact of Vertical Artifacts Changing with Frequency in Lung Ultrasound. Diagnostics 2021, 11, 401. [Google Scholar] [CrossRef]
- Reissig, A.; Kroegel, C. Transthoracic sonography of diffuse parenchymal lung disease: The role of comet tail artifacts. J. Ultrasound Med. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Gravel, C.A.; Neuman, M.I.; Monuteaux, M.C.; Neal, J.T.; Miller, A.F.; Bachur, R.G. Significance of Sonographic Subcentimeter, Subpleural Consolidations in Pediatric Patients Evaluated for Pneumonia. J. Pediatr. 2022, 243, 193–199.e192. [Google Scholar] [CrossRef]
- Jones, B.P.; Tay, E.T.; Elikashvili, I.; Sanders, J.E.; Paul, A.Z.; Nelson, B.P.; Spina, L.A.; Tsung, J.W. Feasibility and Safety of Substituting Lung Ultrasonography for Chest Radiography When Diagnosing Pneumonia in Children: A Randomized Controlled Trial. Chest 2016, 150, 131–138. [Google Scholar] [CrossRef]
- Claes, A.S.; Clapuyt, P.; Menten, R.; Michoux, N.; Dumitriu, D. Performance of chest ultrasound in pediatric pneumonia. Eur. J. Radiol. 2017, 88, 82–87. [Google Scholar] [CrossRef]
- Iorio, G.; Capasso, M.; Prisco, S.; De Luca, G.; Mancusi, C.; Laganà, B.; Piscopo, M.A.; Comune, V. Lung Ultrasound Findings Undetectable by Chest Radiography in Children with Community-Acquired Pneumonia. Ultrasound Med. Biol. 2018, 44, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Milliner, B.H.A.; Tsung, J.W. Lung Consolidation Locations for Optimal Lung Ultrasound Scanning in Diagnosing Pediatric Pneumonia. J. Ultrasound Med. 2017, 36, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Di Mauro, A.; Scalini, E.; Comes, P.; Lofù, I.; Mostert, M.; Tafuri, S.; Manzionna, M.M. Lung ultrasound: A useful tool in diagnosis and management of bronchiolitis. BMC Pediatr. 2015, 15, 63. [Google Scholar] [CrossRef]
- Bueno-Campaña, M.; Sainz, T.; Alba, M.; Del Rosal, T.; Mendez-Echevarría, A.; Echevarria, R.; Tagarro, A.; Ruperez-Lucas, M.; Herrreros, M.L.; Latorre, L.; et al. Lung ultrasound for prediction of respiratory support in infants with acute bronchiolitis: A cohort study. Pediatr. Pulmonol. 2019, 54, 873–880. [Google Scholar] [CrossRef]
- Özkaya, A.K.; Yilmaz, H.L.; Kendir, Ö.; Gökay, S.S.; Eyüboğlu, İ. Lung Ultrasound Findings and Bronchiolitis Ultrasound Score for Predicting Hospital Admission in Children With Acute Bronchiolitis. Pediatr. Emerg. Care 2020, 36, e135–e142. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Musolino, A.M.; Gatto, A.; Lazzareschi, I.; Curatola, A.; Valentini, P. Lung ultrasound in infants with bronchiolitis. BMC Pulm. Med. 2019, 19, 159. [Google Scholar] [CrossRef]
- Nazerian, P.; Vanni, S.; Volpicelli, G.; Gigli, C.; Zanobetti, M.; Bartolucci, M.; Ciavattone, A.; Lamorte, A.; Veltri, A.; Fabbri, A.; et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Chest 2014, 145, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Kosiak, M.; Korbus-Kosiak, A.; Kosiak, W.; Potaz, P. Is chest sonography a breakthrough in diagnosis of pulmonary thromboembolism in children? Pediatr. Pulmonol. 2008, 43, 1183–1187. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Shah, V.P.; Tunik, M.G.; Tsung, J.W. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013, 167, 119–125. [Google Scholar] [CrossRef]
- Esposito, S.; Papa, S.S.; Borzani, I.; Pinzani, R.; Giannitto, C.; Consonni, D.; Principi, N. Performance of lung ultrasonography in children with community-acquired pneumonia. Ital. J. Pediatr. 2014, 40, 37. [Google Scholar] [CrossRef]
- Harel-Sterling, M.; Diallo, M.; Santhirakumaran, S.; Maxim, T.; Tessaro, M. Emergency Department Resource Use in Pediatric Pneumonia: Point-of-Care Lung Ultrasonography versus Chest Radiography. J. Ultrasound Med. 2019, 38, 407–414. [Google Scholar] [CrossRef]
- Tsung, J.W.; Kessler, D.O.; Shah, V.P. Prospective application of clinician-performed lung ultrasonography during the 2009 H1N1 influenza A pandemic: Distinguishing viral from bacterial pneumonia. Crit. Ultrasound J. 2012, 4, 16. [Google Scholar] [CrossRef]
- Lissaman, C.; Kanjanauptom, P.; Ong, C.; Tessaro, M.; Long, E.; O’Brien, A. Prospective observational study of point-of-care ultrasound for diagnosing pneumonia. Arch. Dis. Child. 2019, 104, 12–18. [Google Scholar] [CrossRef]
- Iwanowska, B. New method of scoring lung changes using computed tomography in patients with cystic fibrosis. Med. Wieku Rozw. 2012, 16, 290–302. [Google Scholar]
- Tiddens, H.A. Detecting early structural lung damage in cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 228–231. [Google Scholar] [CrossRef]
- de Jong, P.A.; Nakano, Y.; Lequin, M.H.; Mayo, J.R.; Woods, R.; Paré, P.D.; Tiddens, H.A. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur. Respir. J. 2004, 23, 93–97. [Google Scholar] [CrossRef]
- Kumar, A.; Weng, Y.; Graglia, S.; Chung, S.; Duanmu, Y.; Lalani, F.; Gandhi, K.; Lobo, V.; Jensen, T.; Nahn, J.; et al. Interobserver Agreement of Lung Ultrasound Findings of COVID-19. J. Ultrasound Med. 2021, 40, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Gravel, C.A.; Monuteaux, M.C.; Levy, J.A.; Miller, A.F.; Vieira, R.L.; Bachur, R.G. Interrater reliability of pediatric point-of-care lung ultrasound findings. Am. J. Emerg. Med. 2020, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.R.; Castro, M.R.; Guimarães, T.P.; Pinheiro, A.J.T.; Figueiredo, A.C.T.C.; Martins, B.J.; Carmo, D.R.D.; Oliveira, W.A. Evaluation of pulmonary B lines by different intensive care physicians using bedside ultrasonography: A reliability study. Rev. Bras. Ter. Intensiv. 2019, 31, 354–360. [Google Scholar] [CrossRef]
- Lerchbaumer, M.H.; Lauryn, J.H.; Bachmann, U.; Enghard, P.; Fischer, T.; Grune, J.; Hegemann, N.; Khadzhynov, D.; Kruse, J.M.; Lehner, L.J.; et al. Point-of-care lung ultrasound in COVID-19 patients: Inter- and intra-observer agreement in a prospective observational study. Sci. Rep. 2021, 11, 10678. [Google Scholar] [CrossRef] [PubMed]
- Ambroggio, L.; Sucharew, H.; Rattan, M.S.; O’Hara, S.M.; Babcock, D.S.; Clohessy, C.; Steinhoff, M.C.; Macaluso, M.; Shah, S.S.; Coley, B.D. Lung Ultrasonography: A Viable Alternative to Chest Radiography in Children with Suspected Pneumonia? J. Pediatr. 2016, 176, 93–98.e97. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.; Khera, D.; Toteja, N.; Sureka, B.; Choudhary, B.; Ganakumar, V.M.; Singh, K. Point-of-Care Thoracic Ultrasound in Children with Bronchiolitis. Indian J. Pediatr. 2022, 89, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Fochi, O.; Bronco, A.; Nacoti, M.; Signori, D.; Gatti, S.; Sala, F.; Rozen, T.; Bonanomi, E.; Bellani, G. Modified pediatric lung ultrasound score compared with computed tomography for assessment of lung aeration in children. Minerva Anestesiol. 2021, 87, 675–683. [Google Scholar] [CrossRef]
- Gomond-Le Goff, C.; Vivalda, L.; Foligno, S.; Loi, B.; Yousef, N.; De Luca, D. Effect of Different Probes and Expertise on the Interpretation Reliability of Point-of-Care Lung Ultrasound. Chest 2020, 157, 924–931. [Google Scholar] [CrossRef]
- Ciet, P.; Bertolo, S.; Ros, M.; Casciaro, R.; Cipolli, M.; Colagrande, S.; Costa, S.; Galici, V.; Gramegna, A.; Lanza, C.; et al. State-of-the-art review of lung imaging in cystic fibrosis with recommendations for pulmonologists and radiologists from the “iMAging managEment of cySTic fibROsis” (MAESTRO) consortium. Eur. Respir. Rev. 2022, 31, 210173. [Google Scholar] [CrossRef]
- Diaz-Escobar, J.; Ordóñez-Guillén, N.E.; Villarreal-Reyes, S.; Galaviz-Mosqueda, A.; Kober, V.; Rivera-Rodriguez, R.; Lozano Rizk, J.E. Deep-learning based detection of COVID-19 using lung ultrasound imagery. PLoS ONE 2021, 16, e0255886. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Zhou, B.; Sohn, J.J.; Zhou, J.; Jacob, J.T.; Higgins, K.A.; Bradley, J.D.; Liu, T. Review of Machine Learning in Lung Ultrasound in COVID-19 Pandemic. J. Imaging 2022, 8, 65. [Google Scholar] [CrossRef]
- Arntfield, R.; VanBerlo, B.; Alaifan, T.; Phelps, N.; White, M.; Chaudhary, R.; Ho, J.; Wu, D. Development of a convolutional neural network to differentiate among the etiology of similar appearing pathological B lines on lung ultrasound: A deep learning study. BMJ Open 2021, 11, e045120. [Google Scholar] [CrossRef]




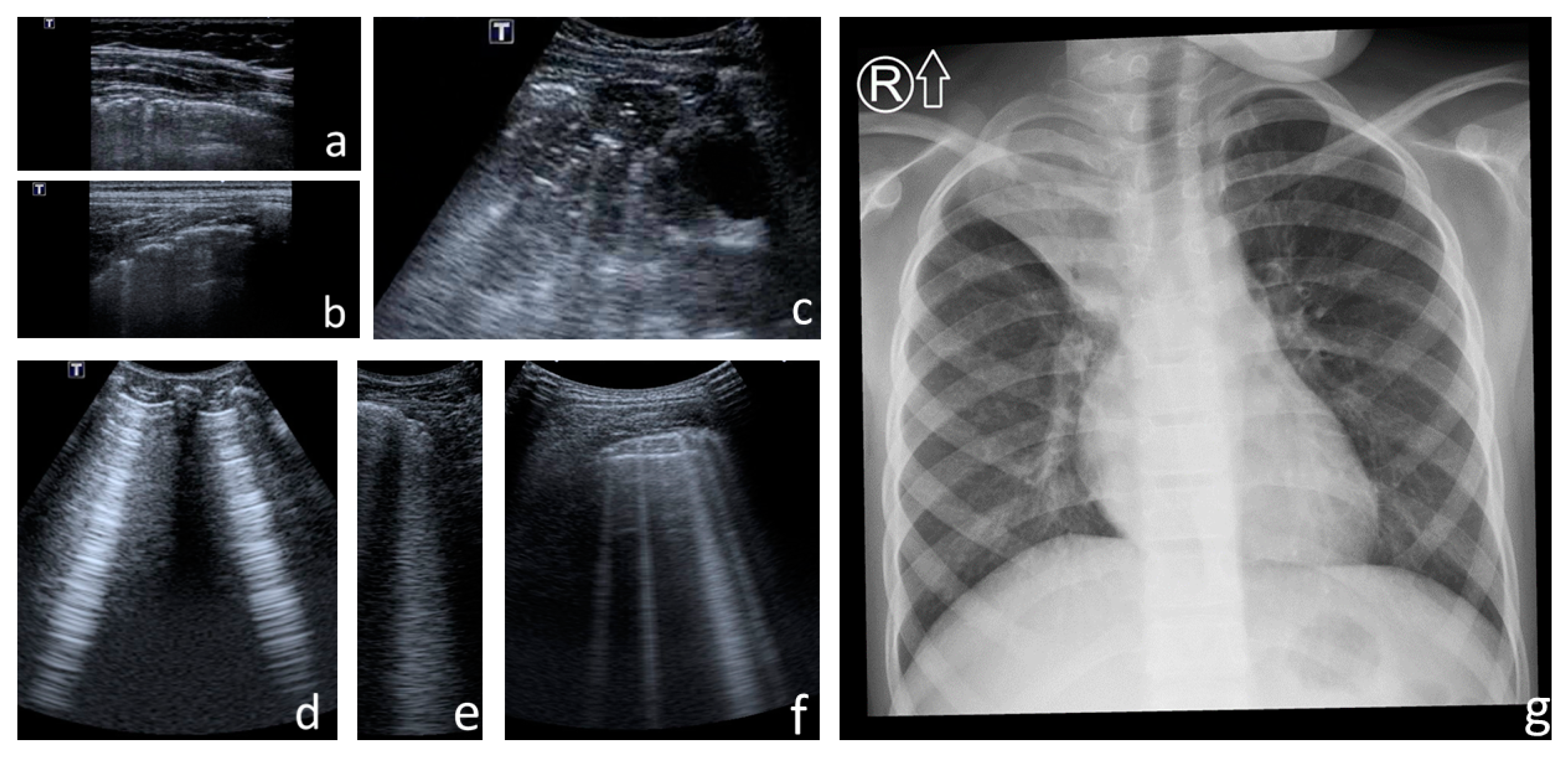

| Finding | Degree of Intensity | ||
|---|---|---|---|
| small consolidations (≤10 mm) | ≤1 | 2–3 | ≥4 |
| Right Lung—Anterior Area (RL-A) | 0 | 1 | 2 |
| Right Lung—Posterior Area (RL-P) | 0 | 1 | 2 |
| Left Lung—Anterior Area (LL-A) | 0 | 1 | 2 |
| Left Lung—Posterior Area (LL-P) | 0 | 1 | 2 |
| major consolidations (>10 mm) | absent | single ≤ 40 mm | ≥2 or 1 > 40 mm |
| RL-A | 0 | 1 | 2 |
| RL-P | 0 | 1 | 2 |
| LL-A | 0 | 1 | 2 |
| LL-P | 0 | 1 | 2 |
| pleural line abnormalities | absent or minimal | present, not marked | marked or widespread |
| RL-A | 0 | 1 | 2 |
| RL-P | 0 | 1 | 2 |
| LL-A | 0 | 1 | 2 |
| LL-P | 0 | 1 | 2 |
| Am-line artefacts | absent | ≤2 | ≥3 |
| RL-A | 0 | 1 | 2 |
| RL-P | 0 | 1 | 2 |
| LL-A | 0 | 1 | 2 |
| LL-P | 0 | 1 | 2 |
| B-line artefacts * | ≤3 | confluent or ≥4 in 1 of 3 lung fields | ≥4 in most of the area (in at least 2 of 3 lung fields) |
| RL-A | 0 * | 1 | 2 |
| RL-P | 0 * | 1 | 2 |
| LL-A | 0 * | 1 | 2 |
| LL-P | 0 * | 1 | 2 |
| * Additional 1 point is to be given if 1-3 B-line artefacts are present in all 3 lung fields in the area (max. 4 additional points). | |||
| US signs | Study Group (n = 131) | Control Group (n = 32) | ||
|---|---|---|---|---|
| Number of Children | Percentage | Number of Children | Percentage | |
| I-lines | 123 | 94% | 17 | 53% |
| Z-lines | 108 | 82% | 15 | 47% |
| single B-lines | 130 | 99% | 23 | 72% |
| numerous B-lines | 36 | 27% | 0 | 0% |
| confluent B-lines | 7 | 5% | 0 | 0% |
| Am-lines | 57 | 44% | 0 | 0% |
| pleural line abnormalities | 95 | 73% | 5 | 16% |
| small consolidations | 84 | 64% | 2 | 6% |
| major consolidations | 38 | 29% | 0 | 0% |
| pleural fluid | 32 | 24% | 3 | 9% |
| US Signs | ĸ Coefficient Values | ||
|---|---|---|---|
| Min. | Max. | Mean | |
| I-lines | 0.26 | 1.00 | 0.60 |
| Z-lines | 0.05 | 0.53 | 0.34 |
| single B-lines | 0.37 | 0.89 | 0.61 |
| numerous B-lines | 0.84 | 1.00 | 0.95 |
| Am-lines | 0.63 | 1.00 | 0.84 |
| pleural line abnormalities | 0.26 | 0.84 | 0.57 |
| small consolidations | 0.68 | 1.00 | 0.84 |
| major consolidations | 0.79 | 1.00 | 0.94 |
| pleural fluid * | 0.63 | 1.00 | 0.81 |
| Peixoto 2019 [22] | Strzelczuk-Judka 2019 [23] | Peixoto 2020 [24] | Hassanzad 2021 [25] | Ciuca 2022 [26] | Jaworska 2023 | ||
|---|---|---|---|---|---|---|---|
| study type | case study | cross-sectional (observational in 9 cases) | cross-sectional | cross-sectional | cross-sectional | cross-sectional | |
| number of patients | 2 | 48 | 18 | 30 | 57 | 131 | |
| patients’ age | 18 and 21 years | 5–18 years | 9–22 years | 2–>29 years | 0.5–18 years | 5 weeks –18 years | |
| patients’ clinical condition | PEx | stable | stable | PEx | stable | stable | |
| control group | — | — | — | — | — | + | |
| interobserver group | — | — | — | — | — | + | |
| LUS score | + | + | + | — | + | + | |
| correlation between LUS score and | CXR | — | + | — | [+] | — | + |
| chest CT | (+) | — | + | [+] | + | — | |
| PFTs | (+) | — | + | — | + | + | |
| microbiological status | (+) | — | — | — | — | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworska, J.; Buda, N.; Kwaśniewicz, P.; Komorowska-Piotrowska, A.; Sands, D. Lung Ultrasound in the Evaluation of Lung Disease Severity in Children with Clinically Stable Cystic Fibrosis: A Prospective Cross-Sectional Study. J. Clin. Med. 2023, 12, 3086. https://doi.org/10.3390/jcm12093086
Jaworska J, Buda N, Kwaśniewicz P, Komorowska-Piotrowska A, Sands D. Lung Ultrasound in the Evaluation of Lung Disease Severity in Children with Clinically Stable Cystic Fibrosis: A Prospective Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(9):3086. https://doi.org/10.3390/jcm12093086
Chicago/Turabian StyleJaworska, Joanna, Natalia Buda, Piotr Kwaśniewicz, Anna Komorowska-Piotrowska, and Dorota Sands. 2023. "Lung Ultrasound in the Evaluation of Lung Disease Severity in Children with Clinically Stable Cystic Fibrosis: A Prospective Cross-Sectional Study" Journal of Clinical Medicine 12, no. 9: 3086. https://doi.org/10.3390/jcm12093086
APA StyleJaworska, J., Buda, N., Kwaśniewicz, P., Komorowska-Piotrowska, A., & Sands, D. (2023). Lung Ultrasound in the Evaluation of Lung Disease Severity in Children with Clinically Stable Cystic Fibrosis: A Prospective Cross-Sectional Study. Journal of Clinical Medicine, 12(9), 3086. https://doi.org/10.3390/jcm12093086








