Post-Stroke Rehabilitation of Distal Upper Limb with New Perspective Technologies: Virtual Reality and Repetitive Transcranial Magnetic Stimulation—A Mini Review
Abstract
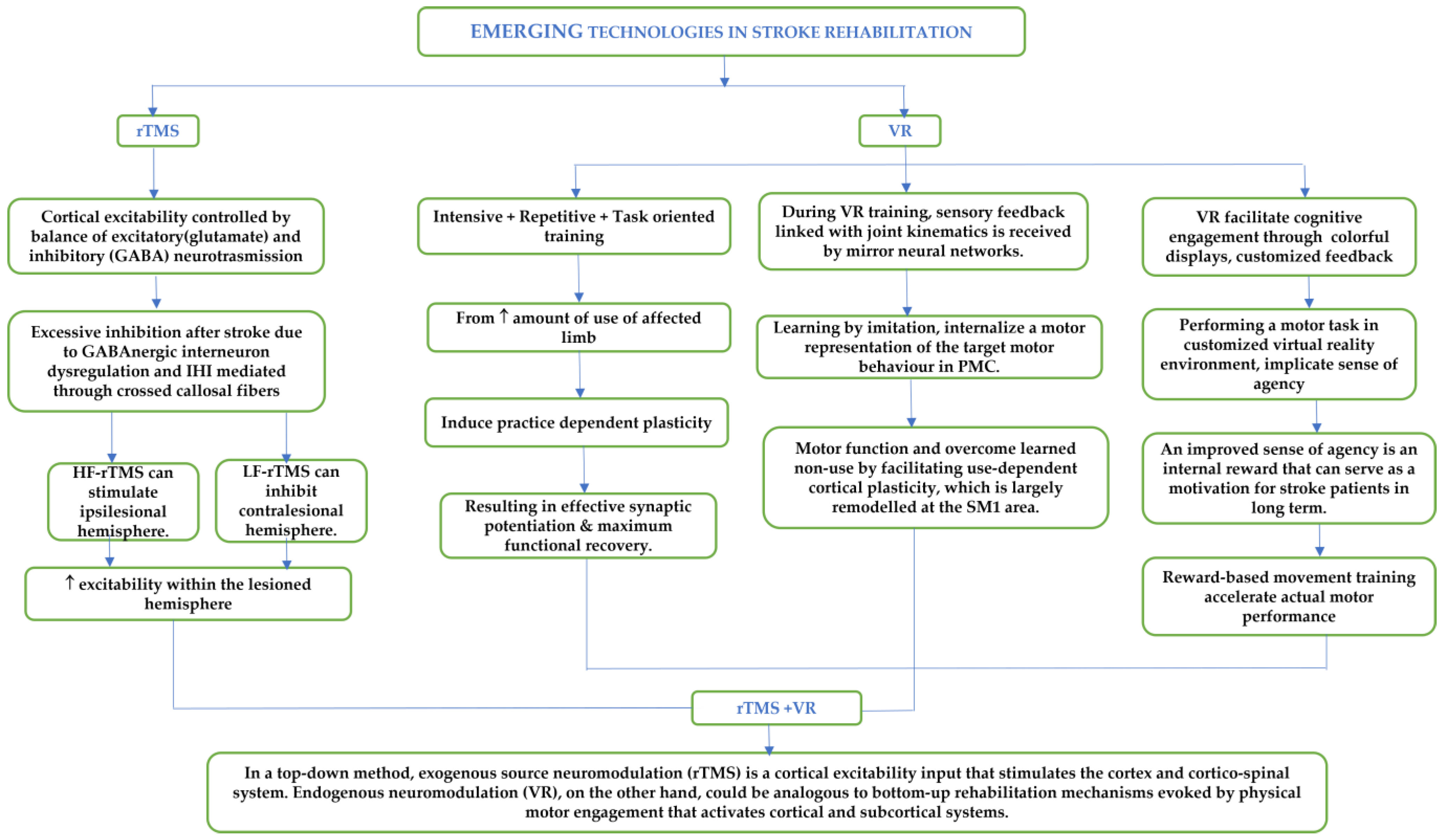
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Study Result
3. Virtual Reality Therapy System
3.1. Types of VR Therapy
3.2. Mechanisms of VR Training
3.3. VR-Based Rehabilitation for Distal Upper Extremity
| Sl No. and Studies | Subjects | Device Used | Intervention | Outcome Measures | Joints Involved | Conclusion |
|---|---|---|---|---|---|---|
| 1. Nath et al. [53] | 1 chronic stroke survivor | Extreme 3D Pro Joystick (Logitech, Lausanne, Switzerland) (Non-immersive) | 45 min/session, 5 sessions per week for 4 weeks | Clinical Scales (FMA, MAS, MBI, SIS, BS, MRS), Neurophysiological measures (fMRI, DTI, MEP), task-specific metrics | Wrist and fingers | The pilot study exhibited preliminary clinical potential of the customized VR tasks specific for distal upper limb in chronic phase of recovery |
| 2. Fong et al. [54] | 20 chronic stroke survivors | Leap Motion (LMC®; Leap Motion, Inc, San Francisco, CA, USA) (Non-immersive) | 30 min/session, 5 sessions per week for 2 weeks | FMA-UE, WMFT, MAL | Wrist and fingers | Task-specific VR training was helpful in upper-extremity recovery in patients with chronic stroke |
| 3. Miclaus et al. [55] | 52 Stroke survivors Experimental group [6 = subacute group, 20 = chronic] Control Group [5 = subacute group, 21 = chronic control group] | MIRA software (MIRA Rehab Ltd., London, UK) for Non-immersive virtual reality (NIVR) therapy | 2 weeks | FMA-UE, MRS, FIM, AROM, MMT, MAS, FRT | Wrist and Fingers | The results suggest that NIVR rehabilitation is efficient to be administered to post-stroke patients, and the study design can be used for a further trial, in the perspective that NIVR therapy can be more efficient than standard physiotherapy within the first six months post-stroke |
| 4. Qiu et al. [56] | 15 chronic Stroke survivors | Home-Based Virtual Rehabilitation (HoVRS) | 15 min every weekday for 3 months | Hand Opening Range (HOR), Hand Opening Accuracy (HOA), Wrist Pitch Range (WPR), Wrist Pitch Accuracy (WPA), Hand Roll Range (HRR), Hand Roll Accuracy (HRA), FMA-UE | Shoulder, Elbow, Wrist, Hand, Whole arm | Persons with chronic stroke were able to use the system safely and productively with minimal supervision resulting in measurable improvements in upper extremity function |
| 5. Ögün et al. [57] | 33 chronic Stroke survivors | Leap Motion (LMC®; Leap Motion, Inc, San Francisco, CA, USA) (Non-immersive) | 60 min, 3 days/week, 6 weeks (18 sessions) | ARAT, FIM, FMA-UE | All finger gestures | Immersive VR rehabilitation appeared to be effective in improving upper extremity function and self-care skills, but it did not improve functional independence |
| 6. Ahmadi et al. [58] | 30 chronic stroke survivors | VR E-Link (Biometrics Ltd., Gwent, UK) | 1 h (3× per week) | FMA-UE, SIS, CAHAI, MI, MAS, MMSE and goniometer | Forearm and wrist | VR-based computer games in combination with routine occupational therapy interventions could improve upper extremities functional impairments in chronic stroke patients. |
| 7. Kim et al. [59] | 23 sub-acute stroke survivors | Kinect (Microsoft Corp., Redmond, WA, USA) (Non-immersive) | 30 min/day for 10 days | BBT, FMA, BS, K-MBI, total activity count | Wrist angle, grasp | Kinect-based upper limb rehabilitation system was not more efficacious compared with sham VR. However, the compliance in VR was good, and VR system induced more arm motion than control and similar activity compared with the conventional therapy, which suggests its utility as an adjuvant additional therapy during inpatient stroke rehabilitation |
| 8. Wang et al. [27] | 26 subacute stroke survivors | Leap Motion based VR system (LMC®; Leap Motion, Inc, San Francisco, CA, USA) (Non-immersive) | EG were given VR training for (5× a week for 4 weeks), + OT for 45 min, (5× a week for 4 weeks). CG received conventional OT twice a day, each for 45 min, (5× a week for 4 weeks) | Primary Outcome-WMFT Secondary Outcome-fMRI | Hands and fingers | ↓ Action performance time in WFMT in EG. ↑↑ Activation intensity and laterality index of contralateral primary sensorimotor cortex (both in EG and CG) |
| 9. Standen et al. [60] | 18 stroke survivors | Virtual glove (with 4 IR LEDs on finger tips), fingers tracked using Nintendo Wiimote (Non-immersive) | 20 min (thrice in a day) for 8 weeks | WMFT, 9-HPT, MAL, NE-ADL | Movements of reach to grasp, grasp and release, pronation and supination | Significantly greater change from baseline in the intervention group on midpoint Wolf Grip strength and two subscales of the final MAL |
| 10. Brunner et al. [61] | 120 sub-acute stroke survivors | YouGrabber system (Non-immersive) | 60 min sessions, 4 weeks | ARAT, BBT, FIM | Fingers and arm | Additional upper extremity VR training was not superior but equally as effective as additional CT in the subacute phase after stroke. VR may constitute a motivating training alternative as a supplement to standard rehabilitation |
| 11. Shin et al. [5] | 46 stroke survivors | RAPAEL Smart Glove (Neofect, Yong-in, Korea) (Non-immersive) | 4 weeks (SG or CON groups) (20 sessions × 30 min/day) along with standard OT daily for 30 min | Primary outcome—FM scores, and the Secondary outcomes—JTHFT, PPT, and SIS version 3.0 | Forearm, Wrist and fingers | VR-based rehabilitation combined with standard occupational therapy might be more effective than amount-matched conventional rehabilitation for improving distal upper extremity function and HRQoL |
| 12. Tsoupikova et al. [50] | 6 chronic stroke survivors | VR system with PneuGlove [62] (Immersive) | 18 (1 h training sessions) with the VR system over a 6-week period | FMA-UE, FMWH, CMSA_A and CMSA_H, ARAT, BBT, Grip and palmar and lateral pinch strengths | Arm, wrist, hand | ↑ lateral pinch strength |
| 13. Brown et al. [63] | 9 chronic stroke survivors | Neuro game therapy video game [64] (Non-immersive) | 45 min, 5 times a week, 4 weeks | WMFT, CAHAI, pre-post EMG measures | Wrist ROM | use of the electromyography-controlled video game impacts muscle activation. Limited changes in kinematic and activity level outcomes |
| 14. Schuster-Amft et al. [65] | 60 chronic stroke survivors | YouGrabber system, G*Power (Non-immersive) | 16 sessions (45 min each), 4 weeks | BBT, CMSA, SIS, MBI, MMSE | Finger and wrist | Study Ongoing |
| 15. Merians et al. [66] | 12 stroke survivors | CyberGlove (Immersion Corporation, San Jose, CA, USA) and CyberGrasp (Immersion Corporation, San Jose, CA, USA) (Immersive) | 4 UE gaming simulations (4×/day,2weeks) Training on day 1 (2–3 h) along with 15 min increments during 1st Week, up to 3 h in Week 2 | Primary outcome—WMFT and JTHFT Secondary outcome—kinematic measures obtained from the Hammer task and the Virtual Piano | Hand and fingers | Complex gaming simulations interfaced with adaptive robots requiring integrated control of shoulder, elbow, forearm, wrist, and finger movements appear to have a substantial effect on improving hemiparetic hand function |
| 16. Proffitt et al. [67] | 1 chronic stroke survivor | Nintendo wii remotes (Non-immersive) | 5 days/week for 6 weeks, 60–75 min each day | ARAT, ACS, RPS | Shoulder, elbow and wrist flexion and extension | Results indicate that computer games have the potential to be a useful intervention for people with stroke |
| 17. Yavuzer et al. [68] | 10 sub-acute stroke survivors | eyetoy playstation (Sony, Tokyo, Japan) game (Non-immersive) | 5 days/week, 4 weeks, 2–5 h/day | BS, FIM | Flexion and extension of paretic shoulder, elbow, wrist, abduction of shoulders | “PlayStation EyeToy” Games combined with a CT have a potential to enhance upper-extremity-related motor functioning in sub-acute stroke patients |
| 18. Merians et al. [69] | 8 chronic stroke survivors | Cyberglove (Position), RMII Force feedback glove [70] (Non-immersive) | 3 weeks, 2–2.5 h/day | JTHFT | Fingers | Transfer of the improvements was demonstrated through changes in the JTHFT and a decrease after the therapy in the overall time from hand peak velocity to the moment when an object was lifted from the table |
| 19. Adamovich et al. [71] | 8 stroke survivors | Cyberglove, RMII glove, EM position trackers (Non-immersive) | 2–2.5 h/day, 13 days | JTHFT | Finger | Improved JTT on transfer of motor learning to real world tasks |
| 20.Boian et al. [72] | 4 stroke survivors | Cyberglove, RMII glove (Non-immersive) | 2 h/day, 5 days/week for 3 weeks | JTHFT | Thumb and finger | Gain in thumb range, finger speed, fractionation, good retention, improved JTT, faster grasping |
| 21. Jack et al. [73] | 3 stroke survivors | Cyberglove and (RMII) force feedback glove (Non-immersive) | Conventional rehab+ VR, 9 daily sessions (5hr each) | Hand movement, Range, Speed, Fractionation, Strength | Hand | Thumb ROM, angular speed (improved), fractionation improved, approx. session’s mechanical work capacity improved, improved grasping force, +changes in Jebsen hand score |
4. Transcranial Magnetic Stimulation Therapy
4.1. Mechanism of Modulation of Cortical Excitability with Repetitive Transcranial Magnetic Stimulation (rTMS)
| Sl No. and Studies | Participants | Muscle Involved (MEP) | Intervention | Outcome Measure | Findings |
|---|---|---|---|---|---|
| 1. Askin et al. [84] | 40 chronic stroke survivors | Index Finger Flexion | 2 groups: rTMS Group- LFrTMS-1Hz, 1200 pulses with an intensity of 90% of RMT were delivered to the unaffected hemisphere for 20 min. Each patient received a total of 10 sessions in 2 weeks (5 days/week) before PT sessions; CON group: 20 session of PT (5 days/week × 4 weeks) | BRS, UE-FMA, BBT, MAS, FIM scale, MMSE, and FAS. | ↓ Distal and Hand MAS score significantly ↑ FMA-UL, BBT, FIM, FAS, FIM cognitive score, MMSE score in both LF-rTMS and CON groups; these changes were significantly greater in the rTMS group. |
| 2. Saadati et al. [17] | 24 sub-acute stroke survivors | Thenar muscle | 3 groups: HF-rTMs (10 Hz); LF-rTMS (1 Hz); Routine Rehab (3× a week for 10 sessions) | WFMT and Hand Grip | ↓ active MEP within the group ↑ WFMT and grip test in the HF group |
| 3. Wang et al. [85] | 44 stroke survivors (3 to 12 months following stroke) | FDI muscle | 3 groups: cPMD(dorsal premotor cortex); cM1(primary motor cortex); Sham Each received 10 session of 1-Hz rTMS | MRC, FMA, WFMT | cPMd modulation yielded significant improvements in MRC, FMA, and WMFT scores compared with sham stimulation and a significant effect on cortical excitability suppression equivalent to that of cM1 modulation, but engendered effects on motor improvement inferior to those of cM1 modulation |
| 4. Galvão et al. [86] | 10 in rTMS group, 10 in sham stroke survivors | FDI muscle | 10 sessions of rTMS | MAS, FMA-UE, maximum PROM of the paretic wrist joint, FIM | MAS decreased with rTMS |
| 5. Sung et al. [87] | 54 sub-acute stroke survivors (15 group A: 1-Hz + iTBS, 12 group B: sham 1-Hz + iTBS, 13 group C: 1-Hz + sham iTBS, 14 group D: sham 1-Hz + sham iTBS) | FDI muscle | 20 sessions | WMFT, FMA—UE, finger flexor MRC, index FTT | MRC, FMA, WMFT, FTT, and RT showed significantly greater improvement in patients who experienced real stimulation |
| 6. Kakuda et al. [88] | 39 chronic stroke survivors | FDI muscle | 22 sessions of LF-rTMS applied to the non-lesional hemisphere and OT (one-to-one training and self-training) | MAS, WMFT, FMA-UE | Decrease in MAS for wrist and finger, increase in FMA-UE and lesser WMFT performance time |
| 7. KoganEmaru et al. [89] | 9 chronic stroke survivors | EDC muscle | 1 exercise + rTMS (Eex-TMS) + 1 exercise + sham (Eex) + 1 rest + rTMS (TMS) (each session on separate days) at 5 Hz, 15 cycles (15 min), each cycle: 50 s exercises/rest + 1 s rest + 8 s rTMS (40 pulses)/sham + 1 s rest, (SM) sham coil | AROM and PROM, pinch force, grip power and MAS | Active range of movement was significantly increased in extension for the wrist joint, thumb, index, and middle finger MCP joint by “TMS” session |
| 8. Takeuchi et al. [83] | 20 chronic stroke survivors | First Dorsal Interosseous (FDI) | 2 groups: Sham vs. Real rTMS (10 in each group) and received rTMS at contralesional M1(1 Hz, 25 min) | Pinch force and acceleration, RMT, MEP amplitude, and TCI duration | ↓ amplitude of MEP in contralesional M1 and TCI duration (rTMS group) rTMS induced improvement in pinch acceleration of the affected hand |
| 9. Boggio et al. [23] | 1 chronic stroke survivor | Abductor pollicis brevis muscle | A sham stimulation for 2 months and active stimulation after 2 months LF- rTMS) of on unaffected hemisphere at intensity of 100% of MT in a continuous train of 20 min, 1200 pulses. After 4 mos, the patient returned for a new session of active rTMS using the same parameters of stimulation | Thumb flexion, extension, abduction, and adduction and wrist flexion and extension were assessed before and after the treatment. | Significant improvement in motor function after active, but not after sham stimulation of the unaffected primary motor cortex |
4.2. rTMS Studies on Distal Upper Extremities
5. Combined rTMS and VR Training
| Sl No. and Studies | Participants | Device Used | Intervention | Outcome measures | Joints Involved | Findings |
|---|---|---|---|---|---|---|
| 1. Chen et al. [93] | 23 stroke survivors | VCT (Virtual Reality-based cycling training) | 2 groups:
| ARAT, FMA-UE, SIS, MAS-UE, MAL, 9HPT, BBT | Fingers, Wrist, and Elbow | ↑ ARAT, FMA-UE in both groups ↑ SIS, MAS-UE, MAL, in iTBS+VCT |
| 2. Sánchez-Cuesta et al. [12] | 42 sub-acute stroke survivors | “NewROW” BCI-VR [94] | 10 sessions rTMS | MI, FMA-UE, SIS, MAS, BI, FTT, 9HPT, RMT | Results to be published | Trial showed the additive value of VR immersive motor imagery as an adjuvant therapy combined with a known effective neuromodulation approach opening new perspectives for clinical rehabilitation protocols |
| 3. Johnson et al. [11] | 3 chronic stroke survivors | rTMS and BCI training was conducted using 64 channel tms compatible EEG caps along with BrainAmp MR Amplifier | 3x/week, 3 weeks of combined real rTMS + BCI to one participant, Sham rTMS + BCI to another participant followed by BCI alone to third participant. [rTMS applied immediately prior to BCI training] | Outcome measure— Beck depression inventory, MMSE, FMA UL, MAS, EHI | Hand | ↑↑ ipsilesional motor activity and improvement in behavioral function for the real rTMS + BCI group. Behavioral improvement demonstrated by for the sham rTMS + BCI condition |
| 4. Zheng et al. [24] | 112 stroke survivors | The BioMaste system (Jumho Electric Co., China). (Non-immersive) | 2 groups (real LF-rTMS + VE, sham rTMS + VE); (30 min/session, 6 times/wk, total 24 sessions) VE started within 10 min of LF-rTMS; all participants provided with 30 min of PT, 30 min of OT, and 30 min of task practice in VE | Primary outcome—U-FMA, WMFT Secondary Outcome—MBI and SF-36 | Shoulder, Elbow and Wrist | Significant ↑ in U-FMA, WMFT, MBI scores suggested the combined use of LF rTMS with VR training could effectively improve the upper limb function, the living activity, and the quality of life in patients with hemiplegia following subacute stroke |
6. Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, H.; Zhang, H.; Liang, H.; Fan, H.; Zhou, J.; Ambrose Lo, W.L.; Li, L. A Novel Glasses-Free Virtual Reality Rehabilitation System on Improving Upper Limb Motor Function among Patients with Stroke: A Feasibility Pilot Study. Med. Nov. Technol. Devices 2021, 11, 100069. [Google Scholar] [CrossRef]
- Domínguez-Téllez, P.; Moral-Muñoz, J.A.; Salazar, A.; Casado-Fernández, E.; Lucena-Antón, D. Game-Based Virtual Reality Interventions to Improve Upper Limb Motor Function and Quality of Life after Stroke: Systematic Review and Meta-Analysis. Games Health J. 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Baniña, M.C.; Frenkel-Toledo, S.; Berman, S.; Soroker, N.; Solomon, J.M.; Liebermann, D.G. Personalized Upper Limb Training Combined with Anodal-TDCS for Sensorimotor Recovery in Spastic Hemiparesis: Study Protocol for a Randomized Controlled Trial. Trials 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Peng, Y.; Xu, G.; Li, L.; Wang, J. Using Corticomuscular Coherence to Reflect Function Recovery of Paretic Upper Limb after Stroke: A Case Study. Front. Neurol. 2018, 8, 728. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kim, M.-Y.; Lee, J.-Y.; Jeon, Y.-J.; Kim, S.; Lee, S.; Seo, B.; Choi, Y. Effects of Virtual Reality-Based Rehabilitation on Distal Upper Extremity Function and Health-Related Quality of Life: A Single-Blinded, Randomized Controlled Trial. J. Neuroeng. Rehabil. 2016, 13, 17. [Google Scholar] [CrossRef]
- Bui, J.; Luauté, J.; Farnè, A. Enhancing Upper Limb Rehabilitation of Stroke Patients with Virtual Reality: A Mini Review. Front. Virtual Real. 2021, 2, 595771. [Google Scholar] [CrossRef]
- Housman, S.J.; Scott, K.M.; Reinkensmeyer, D.J. A Randomized Controlled Trial of Gravity-Supported, Computer-Enhanced Arm Exercise for Individuals with Severe Hemiparesis. Neurorehabil. Neural Repair 2009, 23, 505–514. [Google Scholar] [CrossRef]
- Kiper, P.; Piron, L.; Turolla, A.; Stożek, J.; Tonin, P. The Effectiveness of Reinforced Feedback in Virtual Environment in the First 12 Months after Stroke. Neurol. Neurochir. Pol. 2011, 45, 436–444. [Google Scholar] [CrossRef]
- Levin, M.F.; Snir, O.; Liebermann, D.G.; Weingarden, H.; Weiss, P.L. Virtual Reality versus Conventional Treatment of Reaching Ability in Chronic Stroke: Clinical Feasibility Study. Neurol. Ther. 2012, 1, 3. [Google Scholar] [CrossRef]
- Huo, C.-C.; Zheng, Y.; Lu, W.-W.; Zhang, T.-Y.; Wang, D.-F.; Xu, D.-S.; Li, Z.-Y. Prospects for Intelligent Rehabilitation Techniques to Treat Motor Dysfunction. Neural Regen. Res. 2021, 16, 264. [Google Scholar]
- Johnson, N.N.; Carey, J.; Edelman, B.J.; Doud, A.; Grande, A.; Lakshminarayan, K.; He, B. Combined RTMS and Virtual Reality Brain–Computer Interface Training for Motor Recovery after Stroke. J. Neural Eng. 2018, 15, 16009. [Google Scholar] [CrossRef]
- Sánchez-Cuesta, F.J.; Arroyo-Ferrer, A.; González-Zamorano, Y.; Vourvopoulos, A.; Badia, S.B.I.; Figuereido, P.; Serrano, J.I.; Romero, J.P. Clinical Effects of Immersive Multimodal BCI-VR Training after Bilateral Neuromodulation with RTMS on Upper Limb Motor Recovery after Stroke. a Study Protocol for a Randomized Controlled Trial. Medicina 2021, 57, 736. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.A.; Borrego, A.; Latorre, J.; Colomer, C.; Alcañiz, M.; Sánchez-Ledesma, M.J.; Noé, E.; Llorens, R. Combined Transcranial Direct Current Stimulation and Virtual Reality-Based Paradigm for Upper Limb Rehabilitation in Individuals with Restricted Movements. A Feasibility Study with a Chronic Stroke Survivor with Severe Hemiparesis. J. Med. Syst. 2018, 42, 87. [Google Scholar] [CrossRef] [PubMed]
- Mekbib, D.B.; Han, J.; Zhang, L.; Fang, S.; Jiang, H.; Zhu, J.; Roe, A.W.; Xu, D. Virtual Reality Therapy for Upper Limb Rehabilitation in Patients with Stroke: A Meta-Analysis of Randomized Clinical Trials. Brain Inj. 2020, 34, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Ahmed, M.A.; Fathy, N.; Rothwell, J.C. Therapeutic Trial of Repetitive Transcranial Magnetic Stimulation after Acute Ischemic Stroke. Neurology 2005, 65, 466–468. [Google Scholar] [CrossRef]
- Kim, Y.-H.; You, S.H.; Ko, M.-H.; Park, J.-W.; Lee, K.H.; Jang, S.H.; Yoo, W.-K.; Hallett, M. Repetitive Transcranial Magnetic Stimulation–Induced Corticomotor Excitability and Associated Motor Skill Acquisition in Chronic Stroke. Stroke 2006, 37, 1471–1476. [Google Scholar] [CrossRef]
- Saadati, H.; Abdollahi, I.; Mohseni, B.M.A.L.I.; Azimian, M.; Motamedi, H.; Biglarian, A.; Motamed, M.; Rezvani, A. The Effect of RTMS with Rehabilitation on Hand Function and Corticomotor Excitability in Sub-Acute Stroke. Iran. Rehabil. J. 2015, 13, 46–52. [Google Scholar]
- Sasaki, N.; Mizutani, S.; Kakuda, W.; Abo, M. Comparison of the Effects of High-and Low-Frequency Repetitive Transcranial Magnetic Stimulation on Upper Limb Hemiparesis in the Early Phase of Stroke. J. stroke Cerebrovasc. Dis. 2013, 22, 413–418. [Google Scholar] [CrossRef]
- Avenanti, A.; Coccia, M.; Ladavas, E.; Provinciali, L.; Ceravolo, M.G. Low-Frequency RTMS Promotes Use-Dependent Motor Plasticity in Chronic Stroke: A Randomized Trial. Neurology 2012, 78, 256–264. [Google Scholar] [CrossRef]
- Gillick, B.T.; Krach, L.E.; Feyma, T.; Rich, T.L.; Moberg, K.; Thomas, W.; Cassidy, J.M.; Menk, J.; Carey, J.R. Primed Low-frequency Repetitive Transcranial Magnetic Stimulation and Constraint-induced Movement Therapy in Pediatric Hemiparesis: A Randomized Controlled Trial. Dev. Med. Child Neurol. 2014, 56, 44–52. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Valle, A.C.; Rocha, R.R.; Duarte, J.; Ferreira, M.J.L.; Wagner, T.; Fecteau, S.; Rigonatti, S.P.; Riberto, M. A Sham-Controlled Trial of a 5-Day Course of Repetitive Transcranial Magnetic Stimulation of the Unaffected Hemisphere in Stroke Patients. Stroke 2006, 37, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Tretriluxana, J.; Gordon, J.; Fisher, B.E.; Winstein, C.J. Hemisphere Specific Impairments in Reach-to-Grasp Control after Stroke: Effects of Object Size. Neurorehabil. Neural Repair 2009, 23, 679–691. [Google Scholar] [CrossRef]
- Boggio, P.S.; Alonso-Alonso, M.; Mansur, C.G.; Rigonatti, S.P.; Schlaug, G.; Pascual-Leone, A.; Fregni, F. Hand Function Improvement with Low-Frequency Repetitive Transcranial Magnetic Stimulation of the Unaffected Hemisphere in a Severe Case of Stroke. Am. J. Phys. Med. Rehabil. 2006, 85, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Liao, W.J.; Xia, W.G. Effect of Combined Low-Frequency Repetitive Transcranial Magnetic Stimulation and Virtual Reality Training on Upper Limb Function in Subacute Stroke: A Double-Blind Randomized Controlled Trail. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Nowak, D.A.; Wang, L.E.; Dafotakis, M.; Eickhoff, S.B.; Fink, G.R. Modulating Cortical Connectivity in Stroke Patients by RTMS Assessed with FMRI and Dynamic Causal Modeling. Neuroimage 2010, 50, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.; George, S.; Thomas, S.; Deutsch, J.E.; Crotty, M. Virtual Reality for Stroke Rehabilitation. Stroke 2012, 43, 20–21. [Google Scholar] [CrossRef]
- Wang, Z.R.; Wang, P.; Xing, L.; Mei, L.P.; Zhao, J.; Zhang, T. Leap Motion-Based Virtual Reality Training for Improving Motor Functional Recovery of Upper Limbs and Neural Reorganization in Subacute Stroke Patients. Neural Regen. Res. 2017, 12, 1823. [Google Scholar] [CrossRef]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 834–842.e4. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, É.; Mercier, L. Validation of the Box and Block Test as a Measure of Dexterity of Elderly People: Reliability, Validity, and Norms Studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar] [CrossRef]
- Barreca, S.R.; Stratford, P.W.; Lambert, C.L.; Masters, L.M.; Streiner, D.L. Test-Retest Reliability, Validity, and Sensitivity of the Chedoke Arm and Hand Activity Inventory: A New Measure of Upper-Limb Function for Survivors of Stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1616–1622. [Google Scholar] [CrossRef]
- Schuster-Amft, C.; Eng, K.; Suica, Z.; Thaler, I.; Signer, S.; Lehmann, I.; Schmid, L.; McCaskey, M.A.; Hawkins, M.; Verra, M.L. Effect of a Four-Week Virtual Reality-Based Training versus Conventional Therapy on Upper Limb Motor Function after Stroke: A Multicenter Parallel Group Randomized Trial. PLoS ONE 2018, 13, e0204455. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.L.; Andrade, A.; Soares, L.; Bermúdez, S. Benefits of Virtual Reality Based Cognitive Rehabilitation through Simulated Activities of Daily Living: A Randomized Controlled Trial with Stroke Patients. J. Neuroeng. Rehabil. 2016, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Gamito, P.; Oliveira, J.; Santos, N.; Pacheco, J.; Morais, D.; Saraiva, T.; Soares, F.; Mayor, C.S.; Barata, A.F. Virtual Exercises to Promote Cognitive Recovery in Stroke Patients: The Comparison between Head Mounted Displays versus Screen Exposure Methods. Int. J. Disabil. Hum. Dev. 2014, 13, 337–342. [Google Scholar] [CrossRef]
- Shahrbanian, S.; Ma, X.; Aghaei, N.; Korner-Bitensky, N.; Moshiri, K.; Simmonds, M.J. Use of Virtual Reality (Immersive vs. Non Immersive) for Pain Management in Children and Adults: A Systematic Review of Evidence from Randomized Controlled Trials. Eur. J. Exp. Biol. 2012, 2, 1408–1422. [Google Scholar]
- Arcuri, F.; Porcaro, C.; Ciancarelli, I.; Tonin, P.; Cerasa, A. Electrophysiological Correlates of Virtual-Reality Applications in the Rehabilitation Setting: New Perspectives for Stroke Patients. Electronics 2021, 10, 836. [Google Scholar] [CrossRef]
- Liepert, J.; Bauder, H.; Miltner, W.H.R.; Taub, E.; Weiller, C. Treatment-Induced Cortical Reorganization After Stroke in Humans. Stroke 2000, 31, 1210–1216. [Google Scholar] [CrossRef]
- Kim, W.-S.; Cho, S.; Ku, J.; Kim, Y.; Lee, K.; Hwang, H.-J.; Paik, N.-J. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J. Clin. Med. 2020, 9, 3369. [Google Scholar] [CrossRef]
- Kilbride, C.; Scott, D.J.M.; Butcher, T.; Norris, M.; Ryan, J.M.; Anokye, N.; Warland, A.; Baker, K.; Athanasiou, D.A.; Singla-Buxarrais, G. Rehabilitation via HOMe Based Gaming Exercise for the Upper-Limb Post Stroke (RHOMBUS): Protocol of an Intervention Feasibility Trial. BMJ Open 2018, 8, e026620. [Google Scholar] [CrossRef]
- Sveistrup, H. Motor Rehabilitation Using Virtual Reality. J. Neuroeng. Rehabil. 2004, 1, 10. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Lourenço, C.B.; Chilingaryan, G.; Sveistrup, H.; Levin, M.F. Arm Motor Recovery Using a Virtual Reality Intervention in Chronic Stroke: Randomized Control Trial. Neurorehabil. Neural Repair 2013, 27, 13–23. [Google Scholar] [CrossRef]
- Ballester, B.R.; Nirme, J.; Camacho, I.; Duarte, E.; Rodríguez, S.; Cuxart, A.; Duff, A.; Verschure, P.F.M.J. Domiciliary VR-Based Therapy for Functional Recovery and Cortical Reorganization: Randomized Controlled Trial in Participants at the Chronic Stage Post Stroke. JMIR Serious Games 2017, 5, e6773. [Google Scholar] [CrossRef] [PubMed]
- Cauraugh, J.H.; Summers, J.J. Neural Plasticity and Bilateral Movements: A Rehabilitation Approach for Chronic Stroke. Prog. Neurobiol. 2005, 75, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; You, S.H.; Hallett, M.; Cho, Y.W.; Park, C.-M.; Cho, S.-H.; Lee, H.-Y.; Kim, T.-H. Cortical Reorganization and Associated Functional Motor Recovery after Virtual Reality in Patients with Chronic Stroke: An Experimenter-Blind Preliminary Study. Arch. Phys. Med. Rehabil. 2005, 86, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Molier, B.I.; Van Asseldonk, E.H.F.; Hermens, H.J.; Jannink, M.J.A. Nature, Timing, Frequency and Type of Augmented Feedback; Does It Influence Motor Relearning of the Hemiparetic Arm after Stroke? A Systematic Review. Disabil. Rehabil. 2010, 32, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, P.M.; Wulf, G. Extrinsic Feedback for Motor Learning after Stroke: What Is the Evidence? Disabil. Rehabil. 2006, 28, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K.; Wen, W.; An, Q.; Hamasaki, S.; Yamakawa, H.; Tamura, Y.; Yamashita, A.; Asama, H. Modified Sensory Feedback Enhances the Sense of Agency during Continuous Body Movements in Virtual Reality. Sci. Rep. 2021, 11, 2553. [Google Scholar] [CrossRef]
- Moore, J.W. What Is the Sense of Agency and Why Does It Matter? Front. Psychol. 2016, 7, 1272. [Google Scholar] [CrossRef]
- Nataraj, R.; Hollinger, D.; Liu, M.; Shah, A. Disproportionate Positive Feedback Facilitates Sense of Agency and Performance for a Reaching Movement Task with a Virtual Hand. PLoS ONE 2020, 15, e0233175. [Google Scholar] [CrossRef]
- Sakabe, N.; Altukhaim, S.; Hayashi, Y.; Sakurada, T.; Yano, S.; Kondo, T. Enhanced Visual Feedback Using Immersive VR Affects Decision Making Regarding Hand Use with a Simulated Impaired Limb. Front. Hum. Neurosci. 2021, 15, 677578. [Google Scholar] [CrossRef]
- Tsoupikova, D.; Stoykov, N.S.; Corrigan, M.; Thielbar, K.; Vick, R.; Li, Y.; Triandafilou, K.; Preuss, F.; Kamper, D. Virtual Immersion for Post-Stroke Hand Rehabilitation Therapy. Ann. Biomed. Eng. 2015, 43, 467–477. [Google Scholar] [CrossRef]
- Sears, E.D.; Chung, K.C. Validity and Responsiveness of the Jebsen–Taylor Hand Function Test. J. Hand Surg. Am. 2010, 35, 30–37. [Google Scholar] [CrossRef]
- Nath, D.; Singh, N.; Saini, M.; Srivastava, M.V.P.; Mehndiratta, A. Design and Validation of Virtual Reality Task for Neuro-Rehabilitation of Distal Upper Extremities. Int. J. Environ. Res. Public Heal. 2022, 19, 1442. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Singh, N.; Saini, M.; Banduni, O.; Kumar, N.; Vasantha, M.; Srivastava, P.; Kumaran, S.S.; Mehndiratta, A. Clinical Effectiveness of Non-Immersive Virtual Reality Tasks for Post-Stroke Neuro-Rehabilitation of Distal Upper-Extremities: A Case Report. J. Clin. Med. 2023, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.N.K.; Tang, Y.M.; Sie, K.; Yu, A.K.H.; Lo, C.C.W.; Ma, Y.W.T. Task-Specific Virtual Reality Training on Hemiparetic Upper Extremity in Patients with Stroke. Virtual Real. 2022, 26, 453–464. [Google Scholar] [CrossRef]
- Miclaus, R.; Roman, N.; Caloian, S.; Mitoiu, B.; Suciu, O.; Onofrei, R.R.; Pavel, E.; Neculau, A. Non-Immersive Virtual Reality for Post-Stroke Upper Extremity Rehabilitation: A Small Cohort Randomized Trial. Brain Sci. 2020, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Cronce, A.; Patel, J.; Fluet, G.G.; Mont, A.J.; Merians, A.S.; Adamovich, S.V. Development of the Home Based Virtual Rehabilitation System (HoVRS) to Remotely Deliver an Intense and Customized Upper Extremity Training. J. Neuroeng. Rehabil. 2020, 17, 155. [Google Scholar] [CrossRef]
- Ögün, M.N.; Kurul, R.; Yaşar, M.F.; Turkoglu, S.A.; Avci, Ş.; Yildiz, N. Effect of Leap Motion-Based 3D Immersive Virtual Reality Usage on Upper Extremity Function in Ischemic Stroke Patients. Arq. Neuropsiquiatr. 2019, 77, 681–688. [Google Scholar] [CrossRef]
- Ahmadi, H.S.; Mehraban, A.H.; Amini, M.; Sheikhi, M. The Effects of Virtual Reality on Upper Limb Function in Chronic Stroke Patients: A Clinical Trial. Iran. Rehabil. J. 2019, 17, 81–89. [Google Scholar] [CrossRef]
- Kim, W.S.; Cho, S.; Park, S.H.; Lee, J.Y.; Kwon, S.; Paik, N.J. A Low Cost Kinect-Based Virtual Rehabilitation System for Inpatient Rehabilitation of the Upper Limb in Patients with Subacute Stroke. Medicine 2018, 97, e11173. [Google Scholar] [CrossRef]
- Standen, P.J.; Threapleton, K.; Richardson, A.; Connell, L.; Brown, D.J.; Battersby, S.; Platts, F.; Burton, A. A Low Cost Virtual Reality System for Home Based Rehabilitation of the Arm Following Stroke: A Randomised Controlled Feasibility Trial. Clin. Rehabil. 2017, 31, 340–350. [Google Scholar] [CrossRef]
- Brunner, I.; Skouen, J.S.; Hofstad, H.; Aßmuss, J.; Becker, F.; Pallesen, H. Is Upper Limb Virtual Reality Training More Intensive than Conventional Training for Patients in the Subacute Phase after Stroke? An Analysis of Treatment Intensity and Content. BMC Neurol. 2016, 16, 219. [Google Scholar] [CrossRef]
- Connelly, L.; Jia, Y.; Toro, M.L.; Stoykov, M.E.; Kenyon, R.V.; Kamper, D.G. A Pneumatic Glove and Immersive Virtual Reality Environment for Hand Rehabilitative Training after Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 551–559. [Google Scholar] [CrossRef]
- Donoso Brown, E.V.; McCoy, S.W.; Fechko, A.S.; Price, R.; Gilbertson, T.; Moritz, C.T. Preliminary Investigation of an Electromyography-Controlled Video Game as a Home Program for Persons in the Chronic Phase of Stroke Recovery. Arch. Phys. Med. Rehabil. 2014, 95, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Zanos, S.; Richardson, A.G.; Shupe, L.; Miles, F.P.; Fetz, E.E. The Neurochip-2: An Autonomous Head-Fixed Computer for Recording and Stimulating in Freely Behaving Monkeys. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 427–435. [Google Scholar] [CrossRef]
- Schuster-Amft, C.; Eng, K.; Lehmann, I.; Schmid, L.; Kobashi, N.; Thaler, I.; Verra, M.L.; Henneke, A.; Signer, S.; McCaskey, M.; et al. Using Mixed Methods to Evaluate Efficacy and User Expectations of a Virtual Reality-Based Training System for Upper-Limb Recovery in Patients after Stroke: A Study Protocol for a Randomised Controlled Trial. Trials 2014, 15, 350. [Google Scholar] [CrossRef]
- Merians, A.S.; Fluet, G.G.; Qiu, Q.; Saleh, S.; Lafond, I.; Davidow, A.; Adamovich, S.V. Robotically Facilitated Virtual Rehabilitation of Arm Transport Integrated with Finger Movement in Persons with Hemiparesis. J. Neuroeng. Rehabil. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, R.; Alankus, G.; Kelleher, C.; Engsberg, J. Use of Computer Games as an Intervention for Stroke. Top. Stroke Rehabil. 2011, 18, 417–427. [Google Scholar] [CrossRef]
- Yavuzer, G.; Senel, A.; Atay, M.B.; Stam, H.J. Playstation Eyetoy Games” Improve Upper Extremity-Related Motor Functioning in Subacute Stroke: A Randomized Controlled Clinical Trial. Eur. J. Phys. Rehabil. Med. 2008, 44, 237–244. [Google Scholar]
- Merians, A.S.; Poizner, H.; Boian, R.; Burdea, G.; Adamovich, S. Sensorimotor Training in a Virtual Reality Environment: Does It Improve Functional Recovery Poststroke? Neurorehabil. Neural Repair 2006, 20, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Bouzit, M.; Burdea, G.; Popescu, G.; Boian, R. The Rutgers Master II-New Design Force-Feedback Glove. IEEE/ASME Trans. Mechatron. 2002, 7, 256–263. [Google Scholar] [CrossRef]
- Adamovich, S.V.; Merians, A.S.; Boian, R.; Tremaine, M.; Burdea, G.S.; Recce, M.; Poizner, H. A Virtual Reality Based Exercise System for Hand Rehabilitation Post-Stroke: Transfer to Function. Annu. Int. Conf. IEEE Eng. Med. Biol. Proc. 2004, 26 VII, 4936–4939. [Google Scholar] [CrossRef]
- Boian, R.; Sharma, A.; Han, C.; Merians, A.; Burdea, G.; Adamovich, S.; Recce, M.; Tremaine, M.; Poizner, H. Virtual Reality-Based Post-Stroke Hand Rehabilitation. Stud. Health Technol. Inform. 2002, 85, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Jack, D.; Boian, R.; Member, S.; Merians, A.S.; Tremaine, M.; Burdea, G.C.; Member, S.; Adamovich, S.V.; Recce, M.; Poizner, H. Virtual Reality-Enhanced Stroke Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 308–318. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Debeli, D.K.; Zhang, L.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; Jiang, H.; Zhu, J.; Zhao, Z. A Novel Fully Immersive Virtual Reality Environment for Upper Extremity Rehabilitation in Patients with Stroke. Ann. N. Y. Acad. Sci. 2021, 1493, 75–89. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, H.; Yun, S.J.; Oh, B.; Seo, H.G. Upper Extremity Rehabilitation Using Fully Immersive Virtual Reality Games with a Head Mount Display: A Feasibility Study. PM&R 2020, 12, 257–262. [Google Scholar]
- Voinescu, A.; Sui, J.; Stanton Fraser, D. Virtual Reality in Neurorehabilitation: An Umbrella Review of Meta-Analyses. J. Clin. Med. 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Hernández, M.; Jacinto-Villegas, J.M.; Portillo-Rodríguez, O.; Vilchis-González, A.H. User-Centered Design and Evaluation of an Upper Limb Rehabilitation System with a Virtual Environment. Appl. Sci. 2021, 11, 9500. [Google Scholar] [CrossRef]
- Chervyakov, A.V.; Chernyavsky, A.Y.; Sinitsyn, D.O.; Piradov, M.A. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front. Hum. Neurosci. 2015, 9, 303. [Google Scholar] [CrossRef]
- Du, J.; Tian, L.; Liu, W.; Hu, J.; Xu, G.; Ma, M.; Fan, X.; Ye, R.; Jiang, Y.; Yin, Q. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Recovery and Motor Cortex Excitability in Patients with Stroke: A Randomized Controlled Trial. Eur. J. Neurol. 2016, 23, 1666–1672. [Google Scholar] [CrossRef]
- Bashir, S.; Mizrahi, I.; Weaver, K.; Fregni, F.; Pascual-Leone, A. Assessment and Modulation of Neural Plasticity in Rehabilitation with Transcranial Magnetic Stimulation. PM&R 2010, 2, S253–S268. [Google Scholar]
- Cassani, R.; Novak, G.S.; Falk, T.H.; Oliveira, A.A. Virtual Reality and Non-Invasive Brain Stimulation for Rehabilitation Applications: A Systematic Review. J. Neuroeng. Rehabil. 2020, 17, 147. [Google Scholar] [CrossRef]
- Dionisio, A.; Duarte, I.C.; Patricio, M.; Castelo-Branco, M. The Use of Repetitive Transcranial Magnetic Stimulation for Stroke Rehabilitation: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2018, 27, 1–31. [Google Scholar] [CrossRef]
- Takeuchi, N.; Chuma, T.; Matsuo, Y.; Watanabe, I.; Ikoma, K. Repetitive Transcranial Magnetic Stimulation of Contralesional Primary Motor Cortex Improves Hand Function after Stroke. Stroke 2005, 36, 2681–2686. [Google Scholar] [CrossRef]
- Aşkın, A.; Tosun, A.; Demirdal, Ü.S. Effects of Low-Frequency Repetitive Transcranial Magnetic Stimulation on Upper Extremity Motor Recovery and Functional Outcomes in Chronic Stroke Patients: A Randomized Controlled Trial. Somatosens. Mot. Res. 2017, 34, 102–107. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, C.-P.; Tsai, P.-Y.; Hsieh, C.-Y.; Chan, R.-C.; Yeh, S.-C. Inhibitory Repetitive Transcranial Magnetic Stimulation of the Contralesional Premotor and Primary Motor Cortices Facilitate Poststroke Motor Recovery. Restor. Neurol. Neurosci. 2014, 32, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Galvão, S.C.B.; Dos Santos, R.B.C.; Dos Santos, P.B.; Cabral, M.E.; Monte-Silva, K. Efficacy of Coupling Repetitive Transcranial Magnetic Stimulation and Physical Therapy to Reduce Upper-Limb Spasticity in Patients with Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2014, 95, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.H.; Wang, C.P.; Chou, C.L.; Chen, Y.C.; Chang, Y.C.; Tsai, P.Y. Efficacy of Coupling Inhibitory and Facilitatory Repetitive Transcranial Magnetic Stimulation to Enhance Motor Recovery in Hemiplegic Stroke Patients. Stroke 2013, 44, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, W.; Abo, M.; Kobayashi, K.; Momosaki, R.; Yokoi, A.; Fukuda, A.; Ito, H.; Tominaga, A.; Umemori, T.; Kameda, Y. Anti-Spastic Effect of Low-Frequency RTMS Applied with Occupational Therapy in Post-Stroke Patients with Upper Limb Hemiparesis. Brain Inj. 2011, 25, 496–502. [Google Scholar] [CrossRef]
- Koganemaru, S.; Mima, T.; Thabit, M.N.; Ikkaku, T.; Shimada, K.; Kanematsu, M.; Takahashi, K.; Fawi, G.; Takahashi, R.; Fukuyama, H. Recovery of Upper-Limb Function Due to Enhanced Use-Dependent Plasticity in Chronic Stroke Patients. Brain 2010, 133, 3373–3384. [Google Scholar] [CrossRef]
- Le, Q.; Qu, Y.; Tao, Y.; Zhu, S. Effects of Repetitive Transcranial Magnetic Stimulation on Hand Function Recovery and Excitability of the Motor Cortex after Stroke: A Meta-Analysis. Am. J. Phys. Med. Rehabil. 2014, 93, 422–430. [Google Scholar] [CrossRef]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of Interhemispheric Interactions on Motor Function in Chronic Stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hutchinson, S.; Theoret, H.; Schlaug, G.; Pascual-Leone, A. Repetitive TMS of the Motor Cortex Improves Ipsilateral Sequential Simple Finger Movements. Neurology 2004, 62, 91–98. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chen, C.-L.; Huang, Y.-Z.; Chen, H.-C.; Chen, C.-Y.; Wu, C.-Y.; Lin, K. Augmented Efficacy of Intermittent Theta Burst Stimulation on the Virtual Reality-Based Cycling Training for Upper Limb Function in Patients with Stroke: A Double-Blinded, Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Ferreira, A.; i Badia, S.B. NeuRow: An Immersive VR Environment for Motor-Imagery Training with the Use of Brain-Computer Interfaces and Vibrotactile Feedback. In Proceedings of the 3rd International Conference on Physiological Computing Systems–PhyCS, Lisbon, Portugal, 27–28 July 2016; pp. 43–53. [Google Scholar]
- Pallesen, H.; Andersen, M.B.; Hansen, G.M.; Lundquist, C.B.; Brunner, I. Patients’ and Health Professionals’ Experiences of Using Virtual Reality Technology for Upper Limb Training after Stroke: A Qualitative Substudy. Rehabil. Res. Pract. 2018, 2018, 4318678. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, E.C.C.; Jacobs, L.D.; Pelsma, M.; Dijkhuizen, R.M.; Visser-Meily, J.M.A. Exploring the Experiences of Stroke Patients Treated with Transcranial Magnetic Stimulation for Upper Limb Recovery: A Qualitative Study. BMC Neurol. 2020, 20, 365. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banduni, O.; Saini, M.; Singh, N.; Nath, D.; Kumaran, S.S.; Kumar, N.; Srivastava, M.V.P.; Mehndiratta, A. Post-Stroke Rehabilitation of Distal Upper Limb with New Perspective Technologies: Virtual Reality and Repetitive Transcranial Magnetic Stimulation—A Mini Review. J. Clin. Med. 2023, 12, 2944. https://doi.org/10.3390/jcm12082944
Banduni O, Saini M, Singh N, Nath D, Kumaran SS, Kumar N, Srivastava MVP, Mehndiratta A. Post-Stroke Rehabilitation of Distal Upper Limb with New Perspective Technologies: Virtual Reality and Repetitive Transcranial Magnetic Stimulation—A Mini Review. Journal of Clinical Medicine. 2023; 12(8):2944. https://doi.org/10.3390/jcm12082944
Chicago/Turabian StyleBanduni, Onika, Megha Saini, Neha Singh, Debasish Nath, S. Senthil Kumaran, Nand Kumar, M. V. Padma Srivastava, and Amit Mehndiratta. 2023. "Post-Stroke Rehabilitation of Distal Upper Limb with New Perspective Technologies: Virtual Reality and Repetitive Transcranial Magnetic Stimulation—A Mini Review" Journal of Clinical Medicine 12, no. 8: 2944. https://doi.org/10.3390/jcm12082944
APA StyleBanduni, O., Saini, M., Singh, N., Nath, D., Kumaran, S. S., Kumar, N., Srivastava, M. V. P., & Mehndiratta, A. (2023). Post-Stroke Rehabilitation of Distal Upper Limb with New Perspective Technologies: Virtual Reality and Repetitive Transcranial Magnetic Stimulation—A Mini Review. Journal of Clinical Medicine, 12(8), 2944. https://doi.org/10.3390/jcm12082944









