Impact of Age and Sex Interaction on Post-Acute Sequelae of COVID-19: An Italian Cohort Study on Adults and Children
Abstract
1. Introduction
2. Materials and Methods
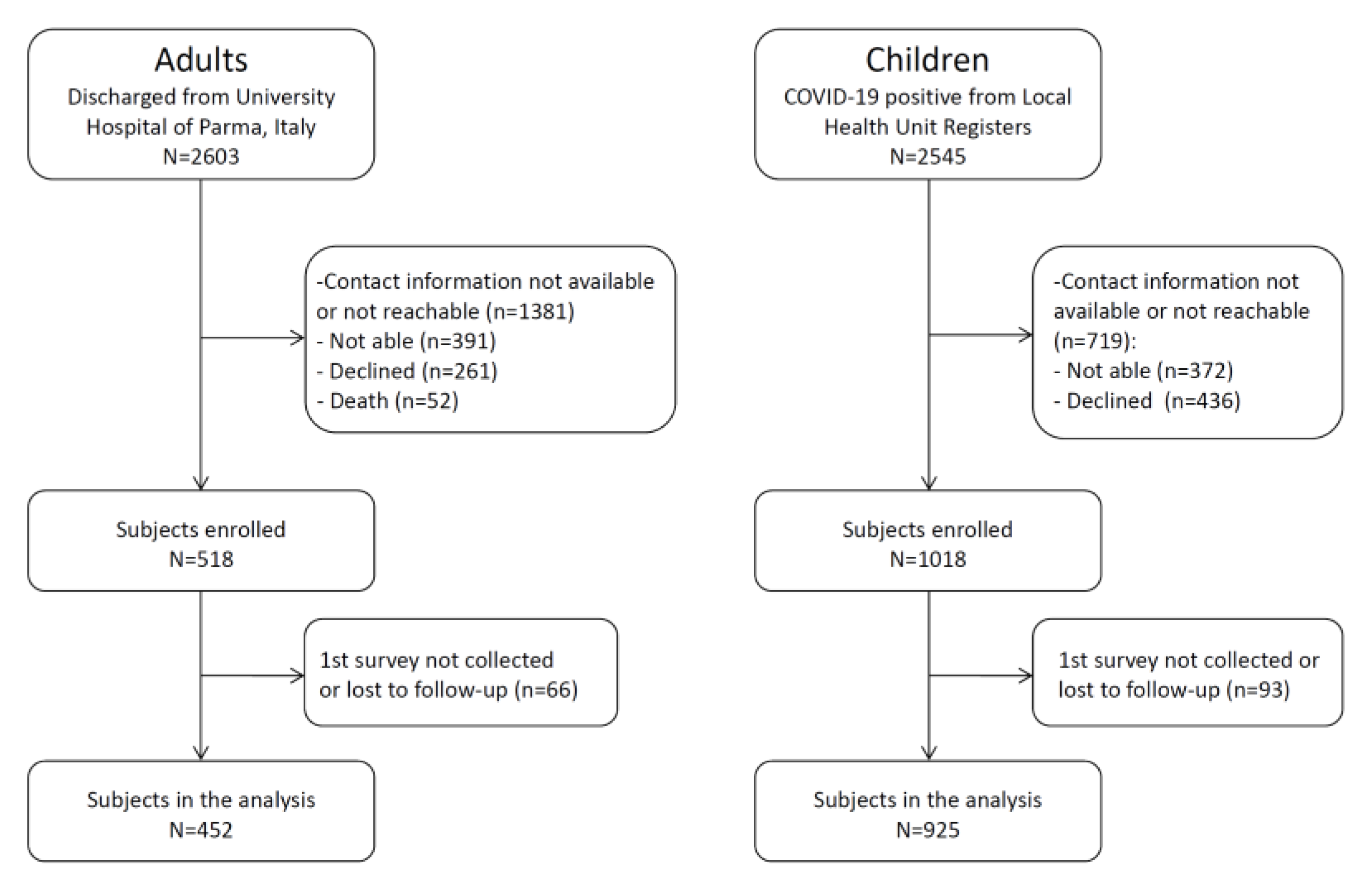
2.1. Study Design, Population and Setting
2.2. Data Collection
2.3. Outcome Measures
2.4. Statistical Analysis
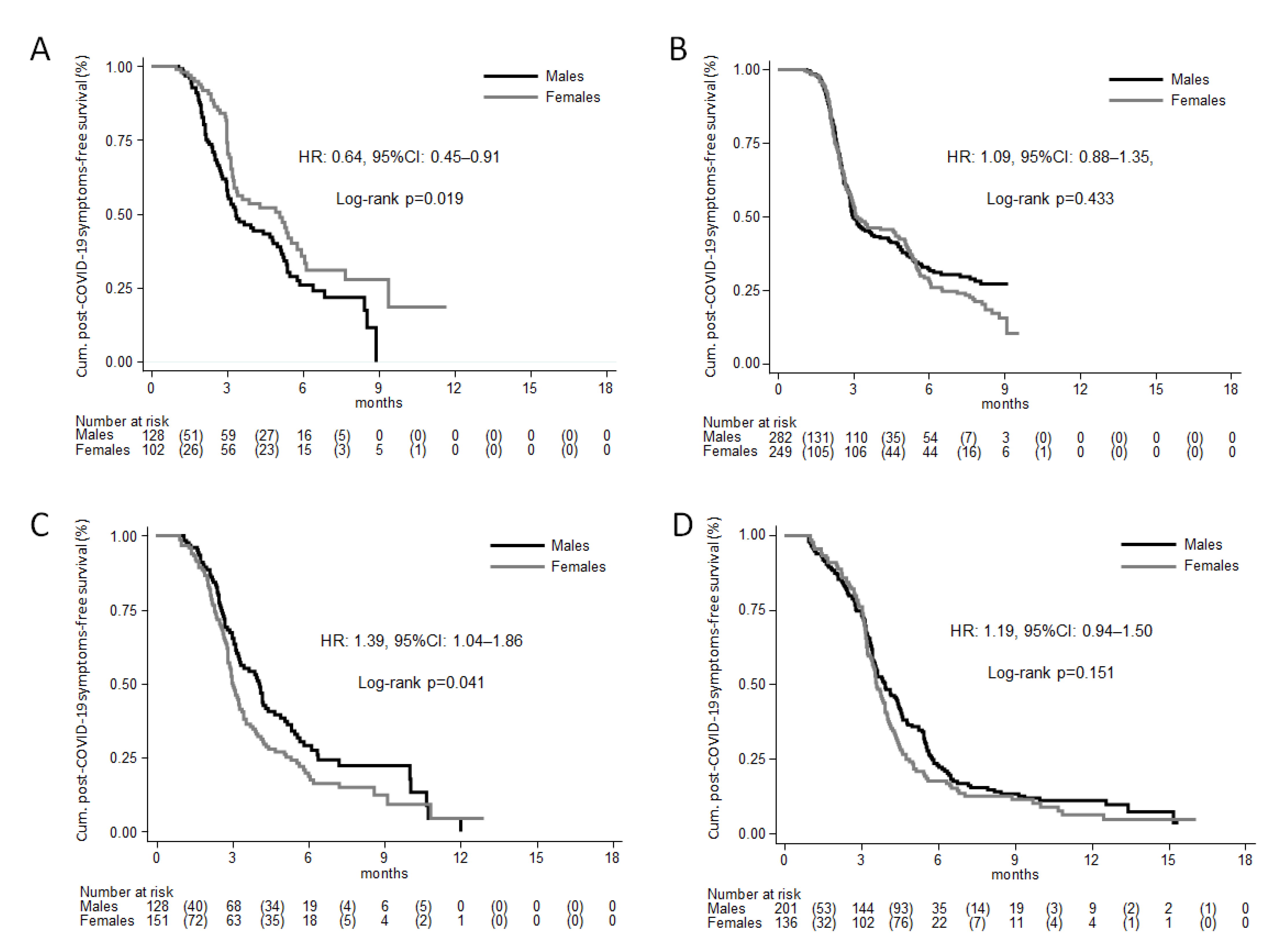
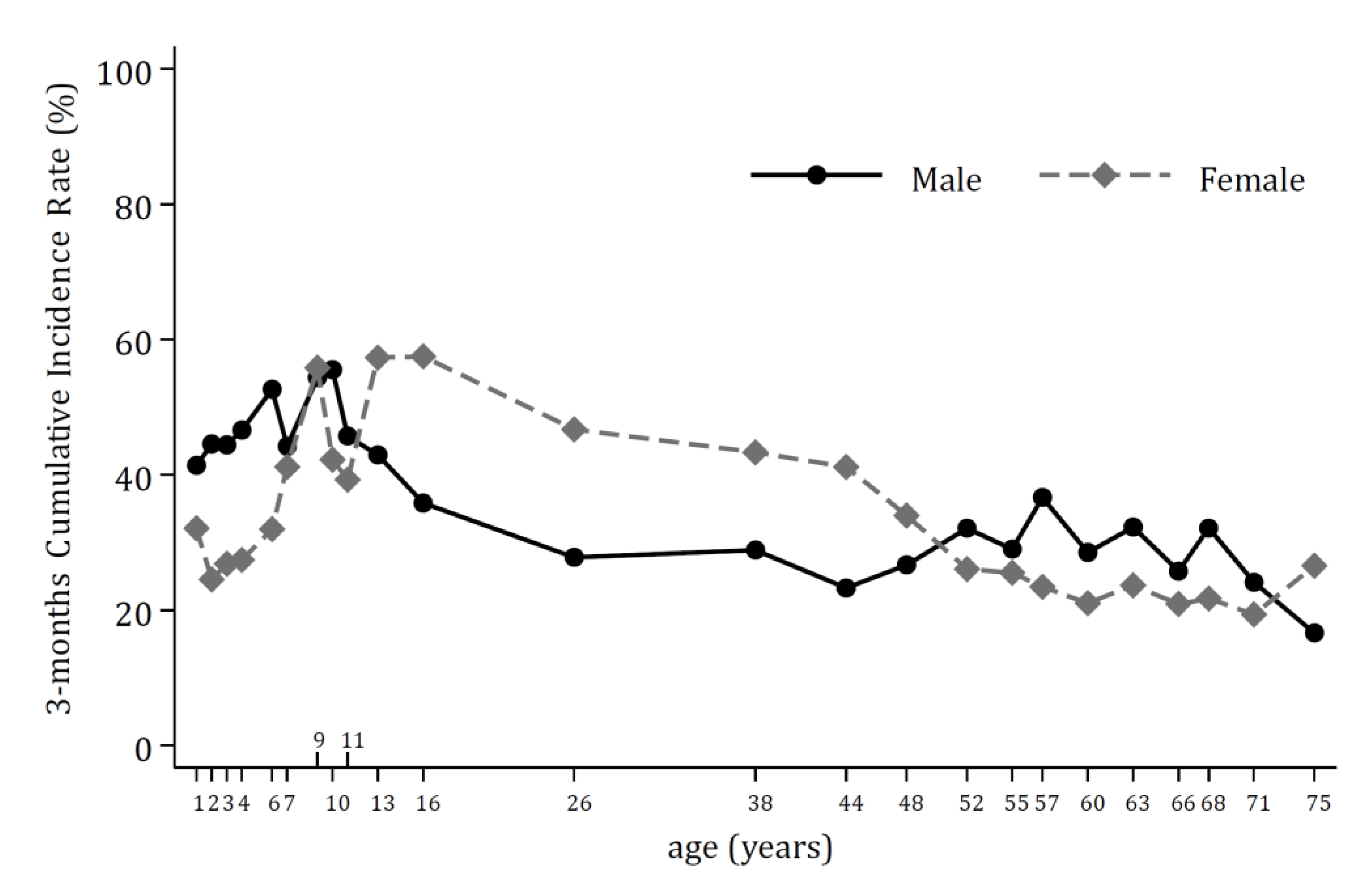
3. Results
Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buonsenso, D.; Munblit, D.; Pazukhina, E.; Ricchiuto, A.; Sinatti, D.; Zona, M.; De Matteis, A.; D’Ilario, F.; Gentili, C.; Lanni, R.; et al. FIMP-Roma. Post-COVID Condition in Adults and Children Living in the Same Household in Italy: A Prospective Cohort Study Using the ISARIC Global Follow-Up Protocol. Front. Pediatr. 2022, 10, 834875. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Bobkova, P.; Spiridonova, E.; Shikhaleva, A.; Gamirova, A.; Blyuss, O.; Nekliudov, N.; Bugaeva, P.; Andreeva, M.; DunnGalvin, A.; et al. Sechenov StopCOVID Research Team. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin. Exp. Allergy 2021, 51, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Hirt, J.; Janiaud, P.; Gloy, V.L.; Schandelmaier, S.; Pereira, T.V.; Contopoulos-Ioannidis, D.; Goodman, S.N.; Ioannidis, J.; Munkholm, K.; Hemkens, L.G. Robustness of reported postacute health outcomes in children with SARS-CoV-2 infection: A systematic review. Arch. Dis. Child. 2022, 1–8. [Google Scholar] [CrossRef]
- Pellegrino, R.; Chiappini, E.; Licari, A.; Galli, L.; Marseglia, G.L. Prevalence and clinical presentation of long COVID in children: A systematic review. Eur. J. Pediatr. 2022, 181, 3995–4009. [Google Scholar] [CrossRef]
- Behnood, S.A.; Shafran, R.; Bennett, S.; Zhang, A.; O’Mahoney, L.; Stephenson, T.; Ladhani, S.; De Stavola, B.; Viner, R.; Swann, O. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J. Infect. 2022, 84, 158–170. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long COVID outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; del Valle, N.C.A.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Brugler Yonts, A. Pediatric Long-COVID: A Review of the Definition, Epidemiology, Presentation, and Pathophysiology. Pediatr. Ann. 2022, 51, e416–e420. [Google Scholar] [CrossRef]
- Franco, J.V.A.; Garegnani, L.I.; Oltra, G.V.; Metzendorf, M.-I.; Trivisonno, L.F.; Sgarbossa, N.; Ducks, D.; Heldt, K.; Mumm, R.; Barnes, B.; et al. Short and Long-Term Wellbeing of Children following SARS-CoV-2 Infection: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14392. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Pazukhina, E.; Andreeva, M.; Spiridonova, E.; Bobkova, P.; Shikhaleva, A.; El-Taravi, Y.; Rumyantsev, M.; Gamirova, A.; Bairashevskaia, A.; Petrova, P.; et al. Sechenov StopCOVID Research Team. Prevalence and risk factors of post-COVID-19 condition in adults and children at 6 and 12 months after hospital discharge: A prospective, cohort study in Moscow (StopCOVID). BMC Med. 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Voza, A.; Riccardi, A.; Vanni, S.; De Iaco, F.; Study & Research Center of the Italian Society of Emergency Medicine (SIMEU). Unfavorable Outcome and Long-Term Sequelae in Cases with Severe COVID-19. Viruses 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O.; et al. Long COVID: Mechanisms, risk factors and recovery. Exp. Physiol. 2023, 108, 12–27. [Google Scholar] [CrossRef]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef]
- Binns, C.W.; Lee, M.K.; Doan, T.T.D.; Lee, A.; Pham, M.; Zhao, Y. COVID and Gender: A Narrative Review of the Asia-Pacific Region. Int. J. Environ. Res. Public Health 2022, 20, 245. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef]
- Maglietta, G.; Diodati, F.; Puntoni, M.; Lazzarelli, S.; Marcomini, B.; Patrizi, L.; Caminiti, C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K.; Smith, L.; Koyanagi, A.; Konrad, M.; Jacob, L. Post-COVID-19 conditions in children and adolescents diagnosed with COVID-19. Pediatr. Res. 2022, 14, 1–6. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2022, 59, 2101341. [Google Scholar] [CrossRef]
- COVID-19 Long Term Protocol—ISARIC and COVID-19 Paediatric Follow-Up. Available online: https://isaric.org/research/covid-19-clinical-research-resources/ (accessed on 20 January 2023).
- EQUATOR Network | Enhancing the QUAlity and Transparency of Health Research. Available online: https://www.equator-network.org/ (accessed on 20 January 2023).
- Sigfrid, L.; Cevik, M.; Jesudason, E.; Lim, W.S.; Rello, J.; Amuasi, J.; Bozza, F.; Palmieri, C.; Munblit, D.; Holter, J.C.; et al. What is the recovery rate and risk of long-term consequences following a diagnosis of COVID-19? A harmonised, global longitudinal observational study protocol. BMJ Open 2021, 11, e043887. [Google Scholar] [CrossRef] [PubMed]
- ISARIC Clinical Characterisation Group. The value of open-source clinical science in pandemic response: Lessons from ISARIC. Lancet Infect. Dis. 2021, 21, 1623–1624. [Google Scholar] [CrossRef]
- Caminiti, C.; Maglietta, G.; Meschi, T.; Ticinesi, A.; Silva, M.; Sverzellati, N. Effects of the COVID-19 Epidemic on Hospital Admissions for Non-Communicable Diseases in a Large Italian University-Hospital: A Descriptive Case-Series Study. J. Clin. Med. 2021, 10, 880. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Stewart, S.; Newson, L.; Briggs, T.A.; Grammatopoulos, D.; Young, L.; Gill, P. Long COVID risk—A signal to address sex hormones and women’s health. Lancet Reg. Health Eur. 2021, 11, 100242. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J. A Wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.; Gelber, R.D. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics 2004, 5, 465–481. [Google Scholar] [CrossRef]
- Sigfrid, L.; Drake, T.M.; Pauley, E.; Jesudason, E.C.; Olliaro, P.; Lim, W.S.; Gillesen, A.; Berry, C.; Lowe, D.J.; McPeake, J.; et al. ISARIC4C investigators. Long COVID in adults discharged from UK hospitals after COVID-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg. Health Eur. 2021, 8, 100186. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Malorni, W. Long COVID: To investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur. Respir. J. 2022, 59, 2102245. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Sussman, J.B.; Kent, D.M.; Hayward, R.A. Three simple rules to ensure reasonably credible subgroup analyses. BMJ 2015, 351, h5651. [Google Scholar] [CrossRef]
- Sammut, R.; Griscti, O.; Norman, I.J. Strategies to improve response rates to web surveys: A literature review. Int. J. Nurs. Stud. 2021, 123, 104058. [Google Scholar] [CrossRef]

| Adults (n = 452) | Children (n = 925) | All (n = 1377) | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 257 (57) | 482 (52) | 739 (54) |
| Female | 195 (43) | 443 (48) | 638 (46) |
| Age, years | |||
| mean (SD) | 58.4 (13.7) | 8.0 (4.2) | 24.6 (25.2) |
| median (IQR) | 59 (50–68) | 8 (6–11) | 11 (7–50) |
| min–max | 19–86 | 0–17 | 0–86 |
| Age classes, years | |||
| 0–5 | - | 230 (25) | 230 (17) |
| 6–11 | - | 531 (57) | 531 (39) |
| 12–17 | - | 164 (18) | 164 (12) |
| 18–50 | 115 (25) | - | 115 (8) |
| 51–64 | 172 (38) | - | 172 (13) |
| 65+ | 165 (37) | - | 165 (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puntoni, M.; Esposito, S.; Patrizi, L.; Palo, C.M.; Deolmi, M.; Autore, G.; Fainardi, V.; Caminiti, C.; on behalf of the University Hospital of Parma LONG-COVID Research Team. Impact of Age and Sex Interaction on Post-Acute Sequelae of COVID-19: An Italian Cohort Study on Adults and Children. J. Clin. Med. 2023, 12, 2924. https://doi.org/10.3390/jcm12082924
Puntoni M, Esposito S, Patrizi L, Palo CM, Deolmi M, Autore G, Fainardi V, Caminiti C, on behalf of the University Hospital of Parma LONG-COVID Research Team. Impact of Age and Sex Interaction on Post-Acute Sequelae of COVID-19: An Italian Cohort Study on Adults and Children. Journal of Clinical Medicine. 2023; 12(8):2924. https://doi.org/10.3390/jcm12082924
Chicago/Turabian StylePuntoni, Matteo, Susanna Esposito, Laura Patrizi, Chiara Maria Palo, Michela Deolmi, Giovanni Autore, Valentina Fainardi, Caterina Caminiti, and on behalf of the University Hospital of Parma LONG-COVID Research Team. 2023. "Impact of Age and Sex Interaction on Post-Acute Sequelae of COVID-19: An Italian Cohort Study on Adults and Children" Journal of Clinical Medicine 12, no. 8: 2924. https://doi.org/10.3390/jcm12082924
APA StylePuntoni, M., Esposito, S., Patrizi, L., Palo, C. M., Deolmi, M., Autore, G., Fainardi, V., Caminiti, C., & on behalf of the University Hospital of Parma LONG-COVID Research Team. (2023). Impact of Age and Sex Interaction on Post-Acute Sequelae of COVID-19: An Italian Cohort Study on Adults and Children. Journal of Clinical Medicine, 12(8), 2924. https://doi.org/10.3390/jcm12082924







