Abstract
In this study, we aimed to illustrate the trajectory of humoral and cellular immunity nine months after primary vaccination with the BNT162b2 mRNA vaccine among 189 healthcare workers (HCWs). Additionally, we endeavored to identify correlations between immunity parameters and a number of common variables and comorbidities. A total of 189 healthcare workers (HCWs), vaccinated against COVID-19, were finally included in the study. All of the subjects had received two doses of the BNT162b2 vaccine; had undergone antibody tests one, four and nine months post-vaccination; and had completed a medical questionnaire. Further samples taken at nine months were tested for cellular immunity. No participants had evidence of COVID-19 infection pre- or post-vaccination. An anti-S1 receptor binding domain (RBD) antibody assay was used to assess humoral response, and cellular immunity was estimated with an INF-γ release assay (IGRA). Statistical analysis was performed using STATA. We report a statistically significant antibody drop over time. Being above the age of 40 or a smoker reduces the rise of antibodies by 37% and 28%, respectively. More than half of the participants did not demonstrate T-cell activation at nine months. Female gender and antibody levels at four months predispose detection of cellular immunity at nine months post-immunization. This study furthers the qualitative, quantitative, and temporal understanding of the immune response to the BNT162b2 mRNA vaccine and the effect of correlated factors.
1. Introduction
The necessity to contain the COVID-19 pandemic impelled the emergency authorization of novel mRNA vaccines. The BNT162b2 mRNA vaccine has been administered to billions of people worldwide with a two-dose schedule proven to be 95% effective for preventing severe COVID-19 disease caused by wild-type virus and several mutations [1,2,3,4,5]. BNT162b2 has demonstrated a high efficacy rate even against variants of concern and has an acceptable safety profile [6]. Nevertheless, the decline of antibody levels post vaccination along with the increasing numbers of breakthrough infections among vaccinated individuals [7,8,9] has created uncertainty about the durability of protective immunity and has necessitated serial booster doses for the adult population.
The rise of specific antibodies against SARS-CoV-2 after natural infection or vaccination has been widely examined [10,11,12,13,14]. Evidence is scarce regarding the question as to whether these antibodies directly correlate with protection or constitute at least one of the protective immune mechanisms [15]. A large UK study (the SIREN study) has suggested that natural infection and induction of antibody response provides robust protection against asymptomatic and symptomatic reinfection [10]. Similarly, studies have demonstrated that available vaccines are able to elicit a significant humoral response in vaccinees with a peak antibody level measured one month after immunization [11,16,17,18]. Previous natural COVID-19 infection is associated with higher levels of humoral response in BNT162b2 mRNA vaccinated individuals, enabling hybrid immunity to promise long-term protection [19,20].
However, the rise of antibody titers per se is not necessarily associated with protection and the level above which we consider the antibodies to be protective is yet to be validated [21,22,23,24]. Conversely, the observation that antibody titers wane over time [21,25,26,27,28,29,30,31,32,33] has raised concerns regarding the level of residual protection and shifted the focus of scientific inquiry to other correlates of immunity to more accurately assess protection.
Vaccines are able to confer immunity by targeting not only the humoral but also the cellular branch of the immune system [34,35]. There is mounting evidence that T-cell response is elicited both in naturally infected patients and vaccinated individuals and can provide long-term protection [36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Nevertheless, the trajectory of long-term antigen-specific T-cell response following mRNA vaccination remains incompletely investigated. Cellular assays are expensive and time-consuming and require experienced lab personnel to execute. Other methods that indirectly assess cellular response, such as interferon gamma release assays (IGRA), are emerging in the literature as both sensitive and accurate in assessing T-cell antigen-specific responses in cohorts of SARS-CoV-2 convalescent and vaccinated populations [50,51,52,53,54].
The most important risk factors for serious disease from SARS-CoV-2 are old age and the presence of comorbidities [27,29,30,55,56]. Male gender, smoking, and obesity are also well-established factors for worse outcomes [57,58,59]. According to the literature, the efficacy of the BNT162b2 vaccine against SARS-CoV-2 could be correlated with the above characteristics with a difference in elicited humoral responses [60,61,62,63,64].
This study aims to elucidate aspects of the humoral and cellular response to vaccination with the BNT162b2 vaccine, and to assess the magnitude and the longitude of antibody titers measured up to nine months post-vaccination.
2. Materials and Methods
2.1. Population and Study Design
This is a single-centered, prospective, longitudinal study conducted at 251 Air Force General Hospital, in Athens. Seven hundred and twenty-nine (729) healthcare workers (HCW) were vaccinated with two doses of the BNT162b2 mRNA vaccine 21 days apart during the period of 4 January 2021–19 February 2021, when a mass vaccination campaign was initiated in-house for hospital staff. The workers all had a measurement of total antibody titers one month after receiving the second dose as per a hospital offer to check for immune response. Out of 729 individuals eligible for inclusion, 350 were selected after randomization to form our initial sample population. (Figure 1).
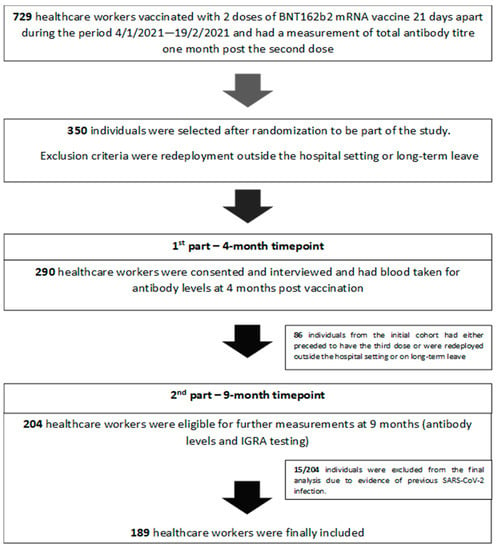
Figure 1.
Study design.
2.2. Data Extraction
The sample population was contacted in person or by telephone by study investigators and written informed consent was obtained before enrollment. Out of 350 individuals randomly selected, 290 were consented and interviewed. Data were collected using questionnaires to investigate demographic features (age and gender), anthropometric data (weight and height), smoking habits, and past medical history/regular medications. Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters (kg/m2). Morbidity recorded was classified into further subgroups including diabetes mellitus, lung disease (asthma, chronic obstructive pulmonary disease, pulmonary fibrosis), chronic renal failure, heart disease (coronary heart disease, myocardiopathy, heart failure), hypertension, dyslipidemia, immunosuppression (cancer or immunosuppressive treatment), autoimmunity.
2.3. Blood Collection Four and Nine Months Post Full Vaccination
Blood collection was scheduled four months after the second inoculation with a maximum delay of 10 days. A total of 290 blood samples were sent to the biochemistry lab for centrifuge and quantification of post-vaccination antibody levels. Samples collected were kept in the refrigerator (between +2 °C and +8 °C) and were analyzed within seven days. At the nine-month timepoint, estimation of long-term antibody levels in addition to T-cell activation profile was attempted. Of 290, 204 individuals were eligible for the second phase of the study (Figure 1), and 204 paired blood samples were analyzed. In addition to biochemistry samples sent for antibody levels, another 5 mL of whole blood from each participant (collected in five lithium heparin tubes (1 mL each)) were sent to the lab for IGRA testing. Whole blood was harvested after 16–24 h of stimulation at 37 °C and then assessed for IFN-γ.
2.4. Laboratory Methods
2.4.1. Anti-SARS-CoV-2 Antibodies
Antibody levels were measured in serum samples using the ADVIA Centaur® SARS-CoV-2 IgG (sCOVG) (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA) assay, a quantitative chemiluminescence immunoassay that uses the receptor binding domain (RBD) of the spike protein 1 as capture antigen. All samples were processed according to the manufacturer’s instructions using an automated platform (ADVIA Centaur® XP systems, Siemens (Siemens Healthineers, Erlangen, Germany)) and yielded results with 96.41% sensitivity and 99.9% specificity. A result of reactive or nonreactive was determined according to the index value established with the calibrators. A cut-off level of 1 U/mL determined a positive result.
2.4.2. IGRA
T-cell activation was evaluated using a COVI-FERON kit (SD Biosensor, Inc., Cheongju-si, Republic of Korea), an IGRA approved for use in in vitro diagnosis (IVD). The assay consists of five antigen tubes aiming to stimulate T-lymphocytes involved in cell-mediated immunity in heparinized whole blood. Nil tube estimates the background IFN-γ level of the sample. Original spike protein (OSP) antigen tube assesses the IFN-γ responses to SARS-CoV-2 spike protein (SP) antigen derived from the wild-type virus (Wuhan) and 20I/501Y.V1 (UK) variant. Variant spike protein (VSP) antigen tube assesses the IFN-γ responses to SARS-CoV-2 SP antigen derived from the 20H/501.V2 (South Africa) and 20I/501Y.V3 (Brazil) variants. A mitogen tube is used as positive control. NP Antigen tube is used to speculate IFN-γ responses to SARS-CoV-2 nucleocapsid protein (NP) antigen indicative of previous natural COVID-19 infection. Plasma from the stimulated samples was used for detection of INF-γ production using an enzyme-Linked immunosorbent assay (ELISA)-based platform. Specimens were processed as per the manufacturer’s advice. Quantitative results (INF-γ concentration in IU/mL) were recorded and further analyzed. An elevated response was defined as a value greater than at least 0.3 IU/mL, implying detectable cellular immunity with 97% sensitivity and 94.2% specificity. Finally, results were appropriately modified to represent the index and >1 was considered positive.
2.5. Ongoing Disease Surveillance
A significant benefit of all the study subjects being members of the hospital staff was that it enabled in-house surveillance for disease incidence throughout the study via several modalities: biweekly nasopharyngeal testing in high exposure placements, prompt reporting of clinical signs and symptoms and immediate testing, and close tracking and tracing of index cases and high-risk exposures. As a result, before the final analysis, 15 participants were further excluded due to evidence of COVID-19 infection (Figure 1). This was evidenced either by positive PCR or by a positive result for previous natural infection in IGRA testing (NP index). A total of 189 samples remained eligible for analysis of humoral and cellular response in COVID-19 naïve individuals. At the time of the study, the delta variant had emerged and was responsible for the main burden of infections.
2.6. Statistical Analysis
Participants were divided into two age groups (≤40 and >40 years old), and three BMI subgroups (BMI: <25 kg/m2 (Underweight/Normal), BMI: 25–29.9 kg/m2 (Overweight), BMI: ≥30 kg/m2 (Obese/Extremely Obese)). They were further classified according to their status of original SP and variant SP cellular immunity (no/yes). For descriptive analysis, continuous variables are presented as median with interquartile range (IQR) and categorical ones as absolute and relative frequencies. The antibody titers from the first, fourth, and ninth month are also presented as means with standard deviation, and the difference between the three measurements is accessed through a repeated-measures ANOVA test. Differences between the groups were compared with t-test or Mann–Whitney test for the continuous data and the chi-squared or Fisher exact test for the categorical variables, while the Shapiro–Wilk test was used to access the normality assumption of the data.
Mixed linear regression models were used to identify baseline characteristics associated with antibody titers. The natural logarithm (ln) of the antibody titers was used as the independent variable, and four multivariate models are presented for added robustness. Model 1 refers to the repeated antibody titer measurements for months 1 to 4, model 2 refers to months 4 to 9, model 3 refers to months 1 to 9, and model 4 also to months 1 to 9 including an interaction term of the variables with a significant univariate association (p-value < 0.05), that is, the age group and smoking status.
Logistic regression analysis was performed to detect factors that might be associated with original SP and variant SP cellular immunity. Antibody titers were evaluated both in their original and ln-transformed scale. Two multivariate models are presented, the first includes the antibody titers in their original scale, while the second represents their ln-transformed scale.
Statistical analysis was performed with STATA and a two-tailed p-value < 0.05 was considered significant.
3. Results
One hundred eighty-nine (189) HCWs were ultimately included in this analysis. The descriptive characteristics of the study group are summarized in Table 1. All participants were of Caucasian ethnicity, their median age was 43 years old (range: 36–50) and 48.7% (n = 92) of them were male. Additionally, 37.6% (n = 71) were younger than 40 years old, 39.2% (n = 74) and 14.8% (n = 28) were overweight and obese respectively, and 31.8% (n = 60) were current smokers. Moreover, 7.4% (n = 14) reported being hypertensive on medication, 5.8% (n = 11) reported having dyslipidemia, 9% (n = 17) endorsed autoimmunity (7 of them referred Hashimoto), and 2.1% (n = 4) claimed to have some degree of immunosuppression.

Table 1.
Baseline demographics, antibody measurements and cellular immunity.
Mean antibody levels at one, four, and nine months after the second dose of BNT162b2mRNA were 153.49 U/mL, 32.38 U/mL, and 19.65 U/mL respectively. All participants had detectable antibodies (>1 U/mL) one and four months after their second dose but 7/204 (3.4%) dropped their antibody levels to less than 1 U/mL at nine months.
A 78.9% decline in median antibody levels was calculated between the first and fourth month, and a 39.31% decline between the fourth and ninth month, revealing a continued reduction, albeit at a slower pace. ANOVA test for repeated measurements was used and corroborated a significant time effect for the mean antibody level kinetics (p < 0.001). A post hoc pairwise comparison using the Bonferroni correction revealed a statistically significant drop in antibody levels between one and four months (p < 0.001), but the drop was not statistically significant between the timepoints of four and nine months (p = 0.458).
To determine whether vaccine-induced antibody responses depended on sex, age, BMI, or specific comorbidities, we investigated the induction in antibodies one, four, and nine months after the second vaccine dose concerning these variables (Table 2). We sought to examine which characteristics might be significantly associated with antibodies at their peak detection concentration one month after the second dose. Younger participants (<40 years old) had significantly higher antibody levels at one month (mean 172 (106–210), p = 0.003), at four months (mean 32 (17–52), p < 0.001), and at nine months after a second dose (mean 5.66 (3.45–8.65), p < 0.001) than older participants. Moreover, the concentration of antibodies was increased in the non-smoker group at the first month (mean 159 (100–195), p = 0.009), at four months (mean 24 (13–49), p < 0.001), and at nine months after the second dose (mean 4.78 (2.84–8.22), p < 0.001) compared with smokers (Table 2). COVI-FERON ELISA assay demonstrated that, nine months after the second dose, the IFN-γ concentration against the original SARS-CoV-2 spike protein (OSP) was positive in 43.9% (n = 83) and the IFN-γ concentration against the variant SARS-CoV-2 spike protein (VSP) was positive in 27% (n = 51) (Table 1).

Table 2.
Antibody values at one, four, and nine months after immunization and risk factors.
3.1. Mixed Linear Regression Analysis
To investigate the possible correlation of vaccine-induced antibody responses with age, sex, BMI, smoking habits, or specific comorbidities, we conducted univariate and multivariate mixed linear model analyses. Older age (>40) and smoking habit were steadily associated with lower antibody levels in measurements at all three of the time spans (one to four months (p = 0.001, p = 0.03), four to nine months (p < 0.002, p = 0.007), and one to nine months (p = 0.002, p = 0.029)) and these correlations were statistically significant (Figure 2, Table 3, models 1–3). BMI, sex, hypertension, dyslipidemia, immunosuppression, and autoimmune disease were not significantly associated to antibody levels in any case.
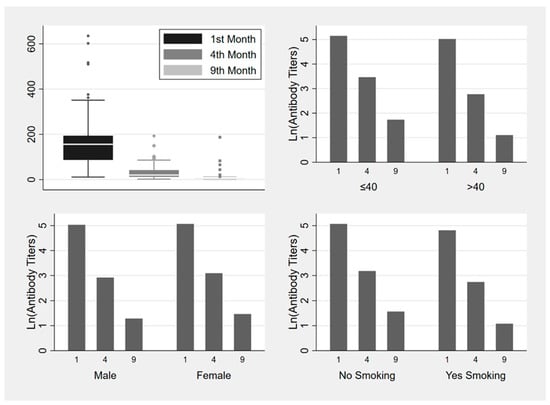
Figure 2.
Box plot and bar plots (median values) of ln (antibody titers) variance one, four and nine months after the second dose of BNT162b2 vaccine and risk factors (age, gender, smoking status).

Table 3.
Antibody kinetics dependent on time and the dependent variables (mixed linear regression).
Mixed linear model analysis showed that being above the age of 40 or being a smoker reduces the development of antibodies by 37% (β Estimate: −0.466, CI: −0.765–−0.167, p-value = 0.002) and 28% (β Estimate: −0.328, CI: −0.623–−0.033, p-value = 0.029) respectively, during the first nine months after vaccination (Table 3—model 3). The interaction effect between the two variables was also found to be significant. Specifically, it was revealed that being above the age of 40 and a smoker reduces the development of antibodies by 55% (β Estimate: −0.796, CI: −1.177–−0.416, p-value < 0.001) (Table 3—model 4).
3.2. Original SP Index
The univariate associations with the original SP index of cellular immunity were examined for all baseline demographic data, clinical characteristics, and antibody levels at one, four, and nine months on the original and ln-transformed scale. Female gender and antibody levels at one and four months on the original scale, and ln-transformed antibody levels for all months were found to have a significant univariate association as opposed to age, underlying conditions, and antibody levels at nine months (original scale) (Table 4, Part A). In the multivariable analysis, only in model 1, female gender (OR: 0.477; 95% CI: 0.238–0.956; p = 0.037) and antibody levels at four months (OR: 1.016; 95% CI: 1.002–1.031; p = 0.028) were found to be significantly associated with the presence of original SP index cellular immunity at nine months post-vaccination (Table 4, Part A). Results are similar if we account for the standardized or the normalized transformed scales of the antibody levels.

Table 4.
Predictors of positive cellular immunity (logistic regression).
3.3. Variant SP Index
Antibody levels at four months in their original scale and antibody levels at four and nine months in their ln-transformed scale were found to have a significant univariate association with the VSP index of cellular immunity (Table 4, Part B). In the multivariate analysis for the VSP index, none of the examined parameters were found to have a significant association (Table 4, Part B).
4. Discussion
In our study, anti-RBD antibody levels are observed to drop over nine months following vaccination, yet remain detectable in most cases; findings that are in agreement with other studies [31,65,66,67,68,69]. In our cohort of 189 HCWs, antibody decay was estimated to be 78.9% between one and four months after the second dose, and a further 39.3% between four and nine months (Figure 2). Similar antibody trajectories derive from other studies where the anti-S-RBD antibody fall rate six months after the initial vaccination scheme is estimated to range between 60–90% [65,69].
Several studies provide reassuring evidence that robust and long-lasting activation of spike-specific T-cells takes place and can outweigh humoral response as the main indicator of vaccine effectiveness [70,71,72]. Malipiero et al. have demonstrated that IGRA can be used as an accurate laboratory method to estimate cellular immune response to BNT162b2 mRNA vaccine in both immunocompetent and immunocompromised patients where the humoral response is undetectable [52]. Tychala et al. found a positive correlation of antibody levels with INF-γ-based cellular response five months post BNT162b2 vaccination [73].
In our study, IGRA estimation of cellular immunity nine months post vaccination shows an active cellular response to original SP in 83 participants (44%) and variant SP in 51 (26%), meaning that more than half of the participating individuals lose their highly desired cellular response by nine months (Table 1). Interestingly, we report a positive association of original SP cellular immunity with female sex and antibody levels at four months that was well-supported through multivariate regression analysis (Table 1). These findings have not been previously reported. Similar correlations are not found for cellular immunity as a response to variant SP.
A clear negative correlation is noted between increasing age and antibody titers at one, four, and nine months post vaccination, which is in line with other studies [21,31,69,74,75,76,77,78,79]. This correlation is persistent in all three measurements and is validated with logistic regression analysis and a mixed linear model. Other studies also corroborate an age-related impairment of binding and neutralizing antibodies after vaccination [80,81,82], while cellular immunity does not appear to be affected by age in our study or elsewhere [83,84,85]. We also report that being a smoker weakens antibody development by 37%, whereas being above the age of 40 and a smoker reduces the development of the antibodies by 55%. Likewise, Nomura et al. found that age and smoking habit determine antibody response three months post receipt of second vaccine [79], and the VASCO study describes a rapid decrease in antibody levels post BNT162b2 mRNA vaccine among smokers [83].
Conflicting data in the literature demonstrate either an increased capacity for females to mount humoral immune responses compared with males [62,85,86,87,88,89], or do not associate gender with antibody response [56,69,90,91,92]. In our study, we did not find an association between the female gender and antibody levels post full immunization, but we report a correlation between the female gender and the presence of activated anti-S T-cells at nine months post-immunization [31,65,93,94].
Studies have shown that mRNA COVID-19 vaccines had similar efficacy among obese and non-obese individuals [1,16,89], while others report a decreased antibody response to the first dose of BNT162b2 COVID-19 vaccine in the obesity or pre-obesity group [62,95]. In the present study, BMI is not found to significantly impair antibody or cellular response. Watanabe et al. have correlated central obesity (defined as higher waist circumference) as a factor negatively affecting the post-vaccination development of antibodies [56]. Alternatively, several studies suggest that obese individuals develop surprisingly higher neutralizing antibodies compared with their recipients with normal weight [31,96].
Regarding the effects of comorbidity, findings from several studies indicate blunted post-vaccination humoral response in people with diabetes [17,56], hypertension [56,89], dyslipidaemia [56], immunosuppression [97], autoimmune diseases and heart disease [66,93,98]. In our study, no association is found between humoral or cellular immunity and underlying medical conditions, possibly because of the small number of participants with comorbidities in this cohort. It should be acknowledged that this is a limitation of our study.
This is a single-centered study, yet the size of the sample is relatively high compared with others published so far. Another limitation of the study is that there is no baseline sample collected before vaccination and COVID-19 negative status was determined by infection surveillance. Moreover, the study population in this report is relatively young and healthy without a significant burden of comorbidities. Therefore, the generalizability of our results may not be taken for granted. Demographic and anthropometric data in this study were obtained via a standardized structured self-reporting questionnaire, introducing the possibility of recall bias. Additionally, cell-mediated immunogenicity was only measured at nine months post-vaccination, as our country’s kit for cellular immunity was not commercially available earlier. The company we sourced it from lists the date of registration in their database as 24 May 2021. In our study, vaccinees with previous COVID-19 have been excluded, thus our measurements of humoral and cellular immunity reflect the duration of vaccine-induced immunity in COVID-19 naive population. The limitations of this study prevent us from drawing definitive conclusions but do add to existing and future scientific data.
5. Conclusions
The long-term effectiveness of the primary vaccination scheme of mRNA vaccines is unknown and results from large-scale trials are warranted to indicate the optimal vaccination strategy against SARS-CoV-2 and the necessity of serial booster doses. This study provides insights into the evolution, duration, and associated factors of vaccine-induced immunity—both humoral and cellular—long after the completion of the two-dose BNT162b2 vaccine schedule.
Author Contributions
N.S. and F.S. conceived the study; F.S., N.S. and M.-C.G. designed the study; N.S., F.S., M.-C.G., E.R., Z.V., E.S., N.L., D.G. and A.Z. collected samples and questionnaires from participants; E.T., P.V., V.G., E.L. performed IgG assay; P.G. performed IGRA testing; D.K., M.-C.G. and A.P. analyzed the data. N.S., M.-C.G., D.K. drafted the manuscript; L.P. revised the manuscript; D.H. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant for scientific research from a special internal fund of the Hellenic National Defence General Staff-Medical Directorate (Funding Number: Φ.900/566356/Σ.755/05 Oκτ 21/ΓΕΕΘA/ΔΥΓ).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Scientific Board of 251 Air Force General Hospital (Approval Code: Φ.076/AΔ.4003/Σ.1385/13 Μαϊ 21/251 ΓΝA/Γραμματεία Επιστ. Συμβ. Approval Date: 13 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available upon reasonable request from the corresponding author.
Acknowledgments
We are grateful to all individuals that voluntarily participated in this study as well as for donating their time and samples. We specifically thank Joseph Abraham, a fourth-year medical student at the Albert Einstein College of Medicine, that kindly accepted to revise this manuscript for grammar and syntax.
Conflicts of Interest
The authors have no conflict of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.; Caban-Martinez, A.; Fowlkes, D.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Tang, L.; Hijano, D.R.; Gaur, A.H.; Geiger, T.L.; Neufeld, E.J.; Hoffman, J.M.; Hayden, R.T. Asymptomatic and Symptomatic SARS-CoV-2 Infections After BNT162b2 Vaccination in a Routinely Screened Workforce. JAMA 2021, 325, 2500–2502. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: Measurement, causes and impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Mizrahi, B.; Lotan, R.; Kalkstein, N.; Peretz, A.; Perez, G.; Ben-Tov, A.; Chodick, G.; Gazit, S.; Patalon, T. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat. Commun. 2021, 12, 6379. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet Lond. Engl. 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gümüş, S.; Bektöre, B.; Bozkurt, H.; Gözalan, A.; Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.27420 (accessed on 2 January 2022).
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Karachaliou, M.; Moncunill, G.; Espinosa, A.; Castaño-Vinyals, G.; Rubio, R.; Vidal, M.; Jiménez, A.; Prados, E.; Carreras, A.; Cortés, B.; et al. SARS-CoV-2 infection, vaccination, and antibody response trajectories in adults: A cohort study in Catalonia. BMC Med. 2022, 20, 347. [Google Scholar] [CrossRef]
- Yu, Y.; Esposito, D.; Kang, Z.; Lu, J.; Remaley, A.T.; De Giorgi, V.; Chen, L.; West, K.; Cao, L. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci. Rep. 2022, 12, 2628. [Google Scholar] [CrossRef]
- Koch, T.; Mellinghoff, S.C.; Shamsrizi, P.; Addo, M.M.; Dahlke, C. Correlates of Vaccine-Induced Protection against SARS-CoV-2. Vaccines 2021, 9, 238. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Karalis, V.; Ntanasis-Stathopoulos, I.; Apostolakou, F.; Gumeni, S.; Sklirou, A.D.; Skourti, S.; Gavriatopoulou, M.; Kastritis, E.; et al. Kinetics of Anti-SARS-CoV-2 Antibody Responses 3 Months Post Complete Vaccination with BNT162b2; A Prospective Study in 283 Health Workers. Cells 2021, 10, 1942. [Google Scholar] [CrossRef]
- Grupel, D.; Gazit, S.; Schreiber, L.; Nadler, V.; Wolf, T.; Lazar, R.; Lia, S.-R.; Perez, G.; Peretz, A.; Tov, A.; et al. Kinetics of SARS-CoV-2 anti-S IgG after BNT162b2 vaccination. Vaccine 2021, 39, 5337–5340. [Google Scholar] [CrossRef]
- Coppeta, L.; Ferrari, C.; Somma, G.; Mazza, A.; D’Ancona, U.; Marcuccilli, F.; Grelli, S.; Aurilio, M.T.; Pietroiusti, A.; Magrini, A.; et al. Reduced Titers of Circulating Anti-SARS-CoV-2 Antibodies and Risk of COVID-19 Infection in Healthcare Workers during the Nine Months after Immunization with the BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 141. [Google Scholar] [CrossRef]
- Stellini, R.; Gianello, R.; Gomarasca, W. Durability of anti-spike antibodies after vaccination with mRNA SARS-CoV-2 vaccine is longer in subjects with previous infection: Could the booster dose be delayed? Infection 2022, 50, 1573–1577. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.; Triccas, J.; Davenport, M. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Novello, S.; Terzolo, M.; Paola, B.; Gianetta, M.; Bianco, V.; Arizio, F.; Brero, D.; Perini, A.; Boccuzzi, A.; Caramello, V.; et al. Humoral immune response to SARS-CoV-2 in five different groups of individuals at different environmental and professional risk of infection. Sci. Rep. 2021, 11, 24503. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.N.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.W.; Le Bert, N.; Tan, C.; Tiu, C.; Zhang, J.; Tan, S.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y. The Clinical Characteristics and Risk Factors of Severe COVID-19. Gerontology 2021, 67, 255–266. [Google Scholar] [CrossRef]
- Maximiano Sousa, F.; Roelens, M.; Fricker, B.; Thiabaud, A.; Iten, A.; Cusini, A.; Flury, D.; Buettcher, M.; Zukol, F.; Balmelli, C.; et al. Risk factors for severe outcomes for COVID-19 patients hospitalised in Switzerland during the first pandemic wave, February to August 2020: Prospective observational cohort study. Swiss Med. Wkly. 2021, 151, w20547. [Google Scholar] [CrossRef]
- Gong, X.; Guo, X.; Kang, S.; Li, Y.; Gao, H.; Yuan, Y. Associated risk factors with disease severity and antiviral drug therapy in patients with COVID-19. BMC Infect. Dis. 2021, 21, 549. [Google Scholar] [CrossRef]
- Li, Y.; Ashcroft, T.; Chung, A.; Dighero, I.; Dozier, M.; Horne, M.; McSwiggan, E.; Shamsuddin, A.; Nair, H. Risk factors for poor outcomes in hospitalised COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health 2021, 11, 10001. [Google Scholar] [CrossRef]
- Halem, K.; van Bruyndonckx, R.; Hilst, J.; van der Cox, J.; Driesen, P.; Opsomer, M.; Van Steenkiste, E.; Stessel, B.; Dubois, J.; Messiaen, P. Risk factors for mortality in hospitalized patients with COVID-19 at the start of the pandemic in Belgium: A retrospective cohort study. BMC Infect. Dis. 2021, 20, 1–10. Available online: https://covid19.elsevierpure.com/en/publications/risk-factors-for-mortality-in-hospitalized-patients-with-covid-19 (accessed on 20 July 2021).
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.-L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.-M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef]
- Erice, A.; Varillas-Delgado, D.; Caballero, C. Decline of antibody titres 3 months after two doses of BNT162b2 in non-immunocompromised adults. Clin. Microbiol. Infect. 2021, 28, 139.e1–139.e4. [Google Scholar] [CrossRef]
- Tanner, R.; Villarreal-Ramos, B.; Vordermeier, H.M.; McShane, H. The Humoral Immune Response to BCG Vaccination. Front. Immunol. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Zollner, A.; Watschinger, C.; Rössler, A.; Farcet, M.R.; Penner, A.; Böhm, V.; Kiechl, S.; Stampfel, G.; Hintenberger, R.; Tilg, H.; et al. B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. eBioMedicine 2021, 70, 103539. Available online: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00332-7/fulltext (accessed on 2 January 2023). [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. Available online: https://www.science.org/doi/10.1126/science.abf4063 (accessed on 3 October 2021). [CrossRef]
- Ansari, A.; Arya, R.; Sachan, S.; Jha, S.N.; Kalia, A.; Lall, A.; Sette, A.; Grifoni, A.; Weiskopf, D.; Coshic, P.; et al. Immune Memory in Mild COVID-19 Patients and Unexposed Donors Reveals Persistent T Cell Responses After SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 749. [Google Scholar] [CrossRef]
- Bonifacius, A.; Tischer-Zimmermann, S.; Dragon, A.C.; Gussarow, D.; Vogel, A.; Krettek, U.; Gödecke, N.; Yilmaz, M.; Kraft, A.R.; Hoeper, M.M.; et al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity 2021, 54, 340–354.e6. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Karlsson, A.C.; Humbert, M.; Buggert, M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020, 5, eabe8063. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Investig. 2021, 131, 149335. [Google Scholar] [CrossRef]
- Kalimuddin, S.; Tham, C.Y.L.; Qui, M.; de Alwis, R.; Sim, J.X.Y.; Lim, J.M.E.; Tan, H.-C.; Syenina, A.; Zhang, S.L.; Le Bert, N.; et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med 2021, 2, 682–688.e4. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Methot, N.; Zhang, Y.; Dan, J.M.; Goodwin, B. Negligible impact of SARS-CoV-2 variants on CD4 + and CD8 + T cell reactivity in COVID-19 exposed donors and vaccinees. bioRxiv 2021. [Google Scholar] [CrossRef]
- Marc, G.P.; Alvarez-Paggi, D.; Polack, F.P. Mounting evidence for immunizing previously infected subjects with a single dose of SARS-CoV-2 vaccine. J. Clin. Investig. 2021, 131, e150135. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Stavridou, F.; Giannaki, M.; Paschoudi, K.; Chatzopoulou, F.; Gavriilaki, E.; Georgolopoulos, G.; Anagnostopoulos, A.; Yannaki, E. Robust SARS-CoV-2-specific T-cell immune memory persists long-term in immunocompetent individuals post BNT162b2 double shot. Heliyon 2022, 8, e09863. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.; Cutino-Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 372, 1418–1423. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Primorac, D.; Brlek, P.; Matišić, V.; Molnar, V.; Vrdoljak, K.; Zadro, R.; Parčina, M. Cellular Immunity-The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines 2022, 10, 442. [Google Scholar] [CrossRef]
- Bertoletti, A.; Le Bert, N.; Qui, M.; Tan, A.T. SARS-CoV-2-specific T cells in infection and vaccination. Cell Mol. Immunol. 2021, 18, 2307–2312. [Google Scholar] [CrossRef]
- Murugesan, K.; Jagannathan, P.; Pham, T.D.; Pandey, S.; Bonilla, H.F.; Jacobson, K.; Parsonnet, J.; Andrews, J.R.; Weiskopf, D.; Sette, A.; et al. Interferon-γ Release Assay for Accurate Detection of Severe Acute Respiratory Syndrome Coronavirus 2 T-Cell Response. Clin. Infect. Dis. 2021, 73, e3130–e3132. [Google Scholar] [CrossRef]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Najafi Fard, S.; Alonzi, T.; Castilletti, C.; Palmieri, F.; Gualano, G.; Vittozzi, P.; et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin. Microbiol. Infect. 2021, 27, e7–e286. [Google Scholar] [CrossRef]
- Malipiero, G.; Moratto, A.; Infantino, M.; D’Agaro, P.; Piscianz, E.; Manfredi, M.; Grossi, V.; Benvenuti, E.; Bulgaresi, M.; Benucci, M.; et al. Assessment of humoral and cellular immunity induced by the BNT162b2 SARS-CoV-2 vaccine in healthcare workers, elderly people, and immunosuppressed patients with autoimmune disease. Immunol. Res. 2021, 69, 576–583. [Google Scholar] [CrossRef]
- Tormo, N.; Giménez, E.; Martínez-Navarro, M.; Albert, E.; Navalpotro, D.; Torres, I.; Gimeno, C.; Navarro, D. Performance comparison of a flow cytometry immunoassay for intracellular cytokine staining and the QuantiFERON® SARS-CoV-2 test for detection and quantification of SARS-CoV-2-Spike-reactive-IFN-γ-producing T cells after COVID-19 vaccination. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 657–662. [Google Scholar] [CrossRef]
- Krüttgen, A.; Klingel, H.; Haase, G.; Haefner, H.; Imöhl, M.; Kleines, M. Evaluation of the QuantiFERON SARS-CoV-2 interferon-ɣ release assay in mRNA-1273 vaccinated health care workers. J. Virol. Methods 2021, 298, 114295. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K.; Misra, A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 505–508. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Mehraeen, E.; Karimi, A.; Barzegary, A.; Vahedi, F.; Afsahi, A.M.; Dadras, O.; Moradmand-Badie, B.; Alinaghi, S.A.S.; Jahanfar, S. Predictors of mortality in patients with COVID-19—A systematic review. Eur. J. Integr. Med. 2020, 40, 101226. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Ali, R.; Teo, H.O.; Parthasarathy, S.; Karamanis, D.; Chamorro-Pareja, N.; Kokkinidis, D.G.; Kaur, S.; Kladas, M.; Sperling, J.; et al. Obesity, Inflammation, and Mortality in COVID-19: An Observational Study from the Public Health Care System of New York City. J. Clin. Med. 2022, 11, 622. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Pfizer vaccine’s efficacy declined from 96% to 84% four months after second dose, company reports. BMJ 2021, 374, n1920. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. Available online: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(21)00208-X/abstract (accessed on 2 September 2021). [CrossRef] [PubMed]
- Sonani, B.; Aslam, F.; Goyal, A.; Patel, J.; Bansal, P. COVID-19 vaccination in immunocompromised patients. Clin. Rheumatol. 2021, 40, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.J.; Kyle, T.K.; Stanford, F.C. COVID-19 Vaccination and Obesity: Optimism and Challenges. Obesity 2021, 29, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Campo, F.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Noia, V.; Di Domenico, E.G.; et al. Antibody Persistence 6 Months Post-Vaccination with BNT162b2 among Health Care Workers. Vaccines 2021, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; Vynck, M.; De Vriese, A.S.; Reynders, M. Dynamics of the cellular and humoral immune response after BNT162b2 mRNA COVID-19 vaccination in COVID-19 naive nursing home residents. J. Infect. Dis. 2021, 13, 1690–1693. [Google Scholar]
- Bayart, J.-L.; Douxfils, J.; Gillot, C.; David, C.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Gerin, V.; et al. Waning of IgG, Total and Neutralizing Antibodies 6 Months Post-Vaccination with BNT162b2 in Healthcare Workers. Vaccines 2021, 9, 1092. [Google Scholar] [CrossRef]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef]
- Ferrari, D.; Clementi, N.; Criscuolo, E.; Ambrosi, A.; Corea, F.; Di Resta, C.; Tomaiuolo, R.; Mancini, N.; Locatelli, M.; Plebani, M.; et al. Antibody Titer Kinetics and SARS-CoV-2 Infections Six Months after Administration with the BNT162b2 Vaccine. Vaccines 2021, 9, 1357. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines 2022, 10, 64. [Google Scholar] [CrossRef]
- Painter, E.M. Demographic Characteristics of Persons Vaccinated During the First Month of the COVID-19 Vaccination Program—United States, 14 December 2020–14 January 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 174–177. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7005e1.htm (accessed on 12 March 2021). [CrossRef]
- Rossi, C.P.; Cash, E.; Aubert, C.; Coutinho, A. Role of the humoral immune response in resistance to Theiler’s virus infection. J. Virol. 1991, 65, 3895–3899. Available online: https://journals.asm.org/doi/abs/10.1128/jvi.65.7.3895-3899.1991 (accessed on 2 January 2022). [CrossRef]
- Tychala, A.; Meletis, G.; Katsimpourlia, E.; Gkeka, I.; Dimitriadou, R.; Sidiropoulou, E.; Skoura, L. Evaluation of the QuantiFERON SARS-CoV-2 assay to assess cellular immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in individuals with low and high humoral response. Hum. Vaccines Immunother. 2021, 17, 5148–5149. [Google Scholar] [CrossRef]
- Melin, J.; Svensson, M.K.; Albinsson, B.; Winqvist, O.; Pauksens, K. Humoral and cellular response to SARS-CoV-2 BNT162b2 mRNA vaccine in hemodialysis patients. BMC Immunol. 2021, 22, 70. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.-M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; The CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Coggins, S.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse effects and antibody titers in response to the bnt162b2 mrna COVID-19 vaccine in a prospective study of healthcare workers. Open Forum. Infect. Dis. 2021, 9, ofab575. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Kurihara, M.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; et al. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines 2021, 9, 1042. [Google Scholar] [CrossRef]
- Brockman, M.A.; Mwimanzi, F.; Lapointe, H.R.; Sang, Y.; Agafitei, O.; Cheung, P.K.; Ennis, S.; Ng, K.; Basra, S.; Lim, L.Y.; et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mrna vaccines among older adults. J. Infect. Dis. 2021, 225, 1129–1140. [Google Scholar] [CrossRef]
- Ikezaki, H.; Nomura, H.; Shimono, N. Dynamics of anti-Spike IgG antibody level after the second BNT162b2 COVID-19 vaccination in health care workers. J. Infect. Chemother. 2022, 28, 802–805. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2020, 106, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.; Mantovani, L.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.-D.; Liacos, C.-I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.-A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 27, e1–e1861. [Google Scholar] [CrossRef]
- Kontou, E.; Ranellou, K.; Zoulas, D.; Bletsa, A.; Rompola, E.; Piperaki, E.-T.; Athanasiou, N.; Ampelakiotou, K.; Pratikaki, M.; Stergiopoulou, C.; et al. Antibody Response Following a Two-Dose mRNA Vaccination Regimen, in Health Care Workers of a Tertiary Hospital in Athens, Greece. J. Pers. Med. 2021, 11, 576. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Giglio, R.V.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.M.; Ciaccio, A.M.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-CoV-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post—Vaccination with the second dose of BNT162b2 vaccine: A longitudinal observational cohort study in western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef]
- Padoan, A.; Dall’olmo, L.; della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef]
- Abu Jabal, K.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance 2021, 26, 2100096. [Google Scholar] [CrossRef]
- Ponticelli, D.; Antonazzo, I.C.; Caci, G.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; Ferrara, P. Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J. Travel Med. 2021, 28, taab173. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 Vaccine-Induced Immune Responses and Dynamics Vary among Age Groups, Sex and Co-Morbidities: A Longitudinal Prospective Cohort Study. SSRN Electron. J. 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3790408 (accessed on 12 February 2022).
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Early Onset of SARS-CoV-2 Antibodies after First Dose of BNT162b2: Correlation with Age, Gender and BMI. Vaccines 2021, 9, 685. [Google Scholar] [CrossRef]
- Piernas, C.; Patone, M.; Astbury, N.M.; Gao, M.; Sheikh, A.; Khunti, K.; Shankar-Hari, M.; Dixon, S.; Coupland, C.; Aveyard, P.; et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2022, 10, 571–580. [Google Scholar] [CrossRef]
- Oyaert, M.; De Scheerder, M.A.; Van Herrewege, S.; Laureys, G.; Van Assche, S.; Cambron, M.; Naesens, L.; Hoste, L.; Claes, K.; Haerynck, F.; et al. Evaluation of Humoral and Cellular Responses in SARS-CoV-2 mRNA Vaccinated Immunocompromised Patients. Front. Immunol. 2022, 13, 858399. [Google Scholar] [CrossRef]
- Schramm, R.; Costard-Jäckle, A.; Rivinius, R.; Fischer, B.; Müller, B.; Boeken, U.; Haneya, A.; Provaznik, Z.; Knabbe, C.; Gummert, J. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin. Res. Cardiol. 2021, 110, 1142–1149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).