Long-Term Mortality after New-Onset Atrial Fibrillation in COVID-19
Abstract
1. Introduction
2. Methods
2.1. Endpoints
2.2. Statistical Analysis
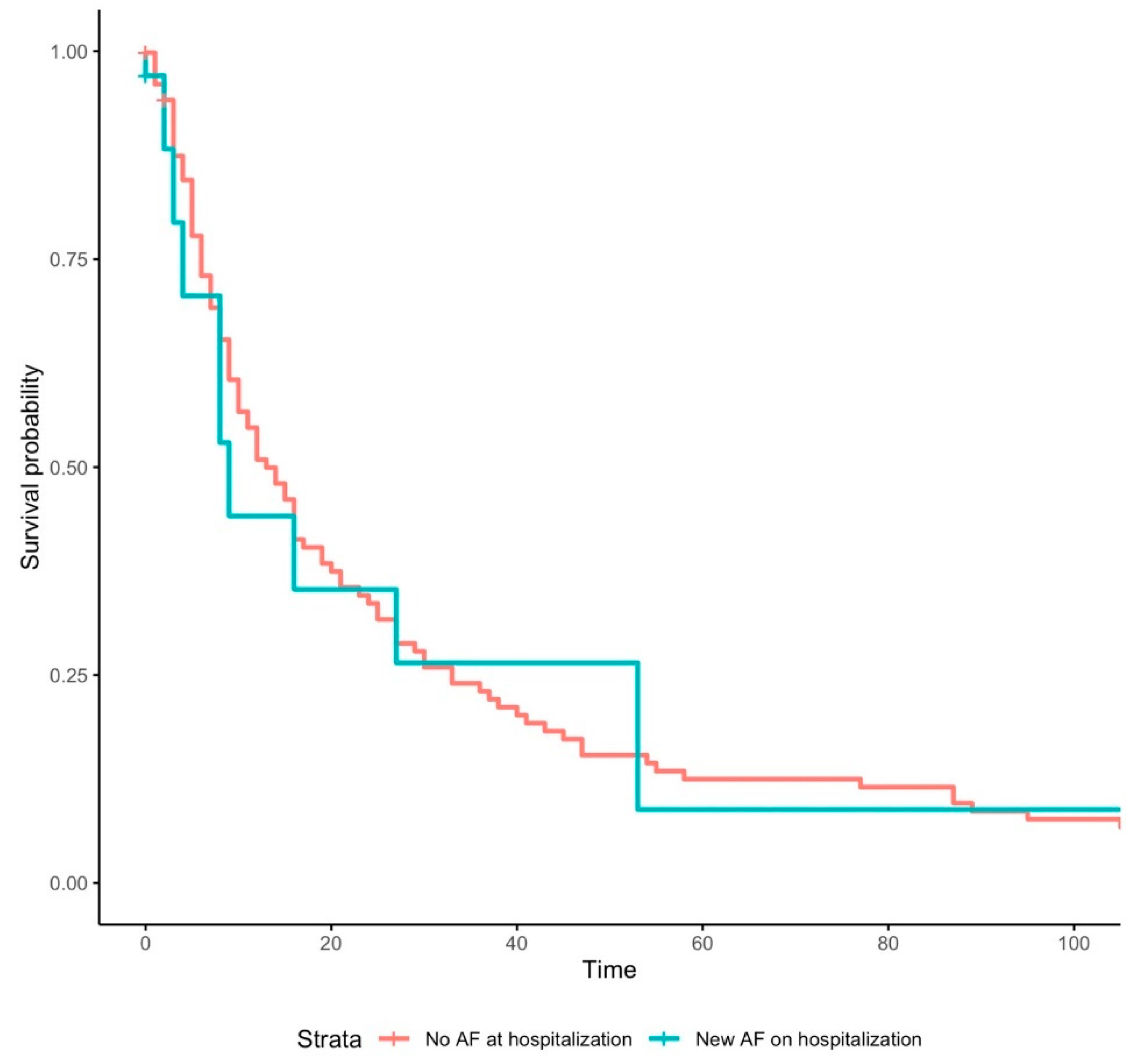
3. Results
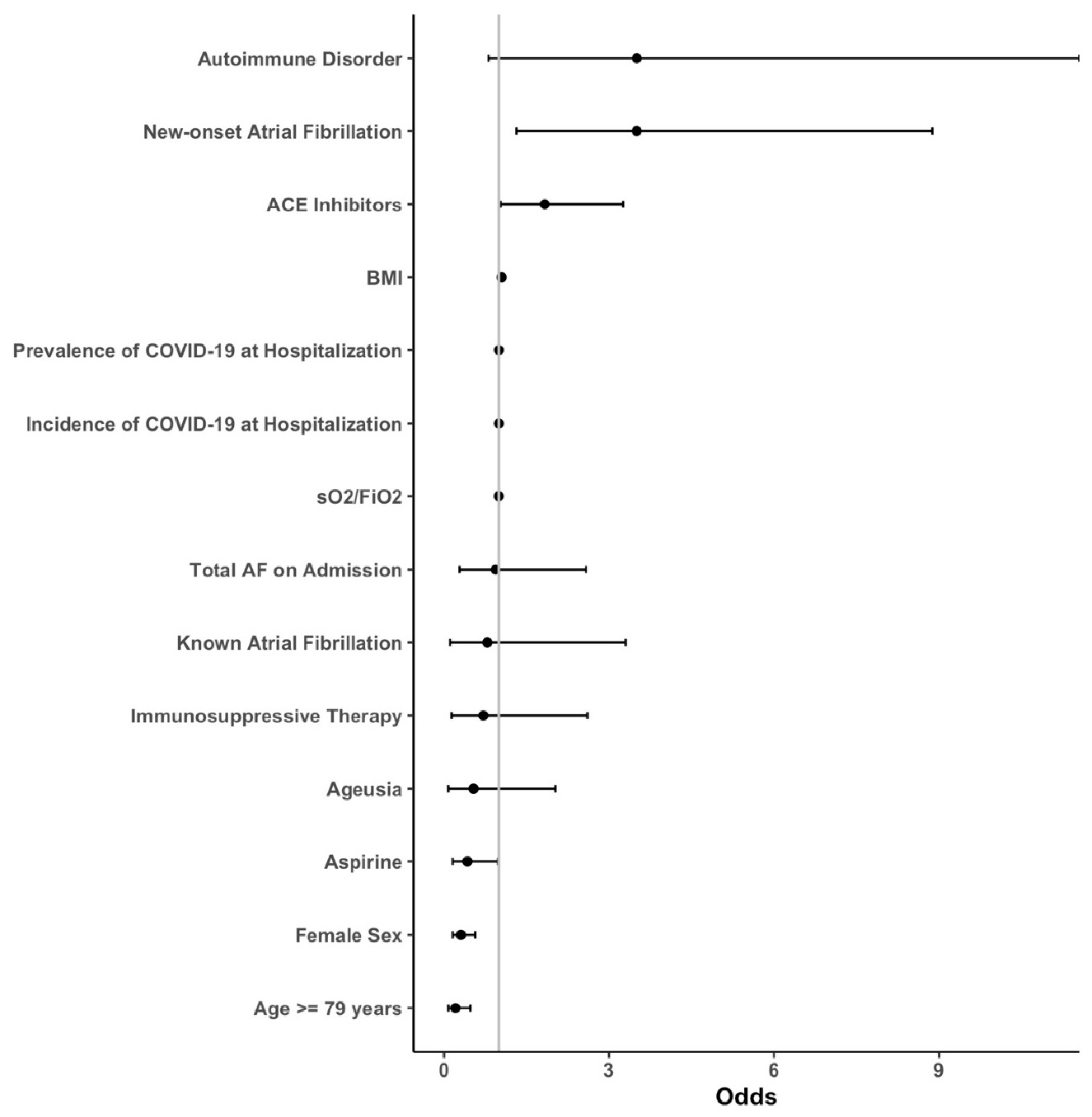
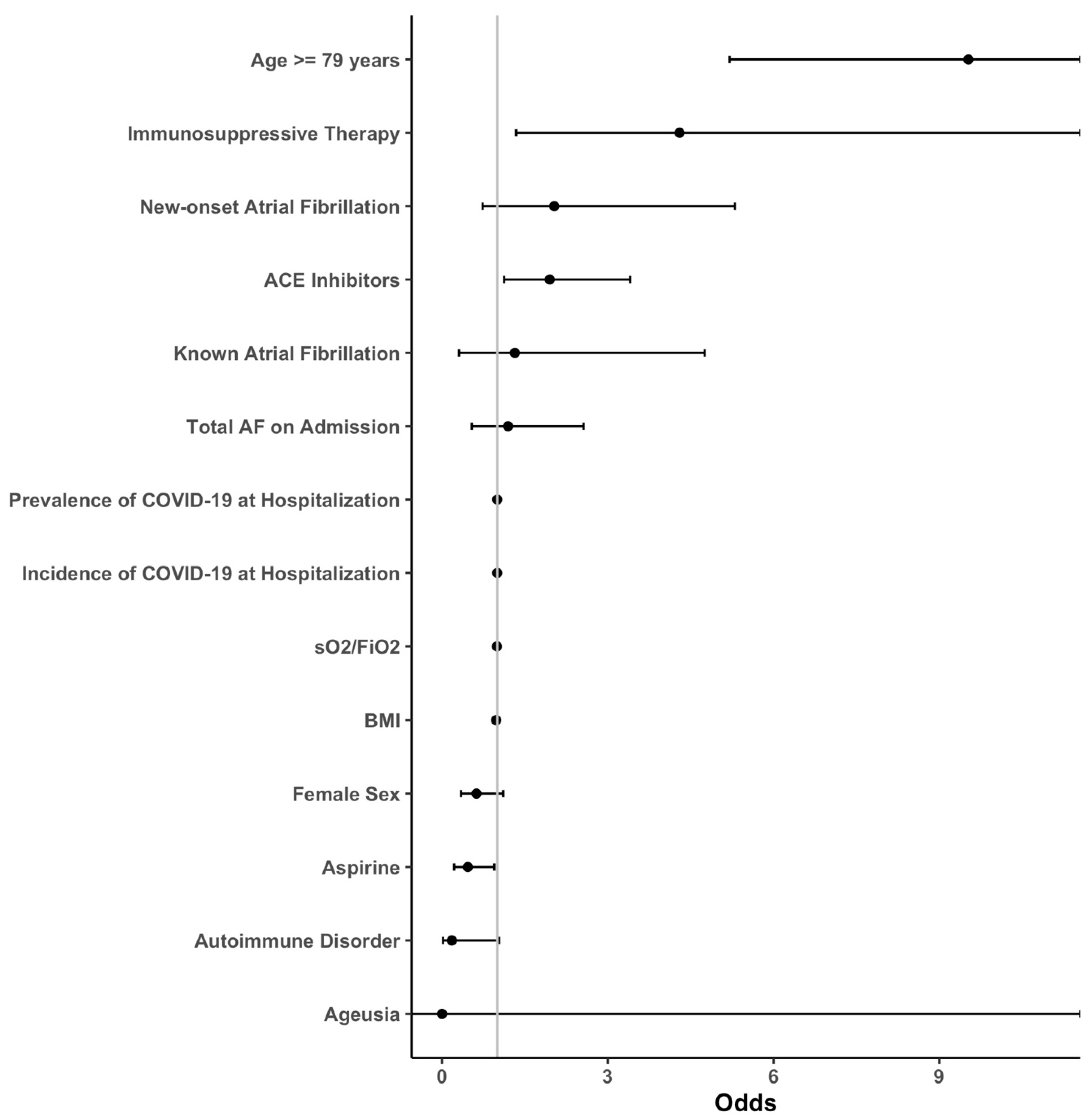
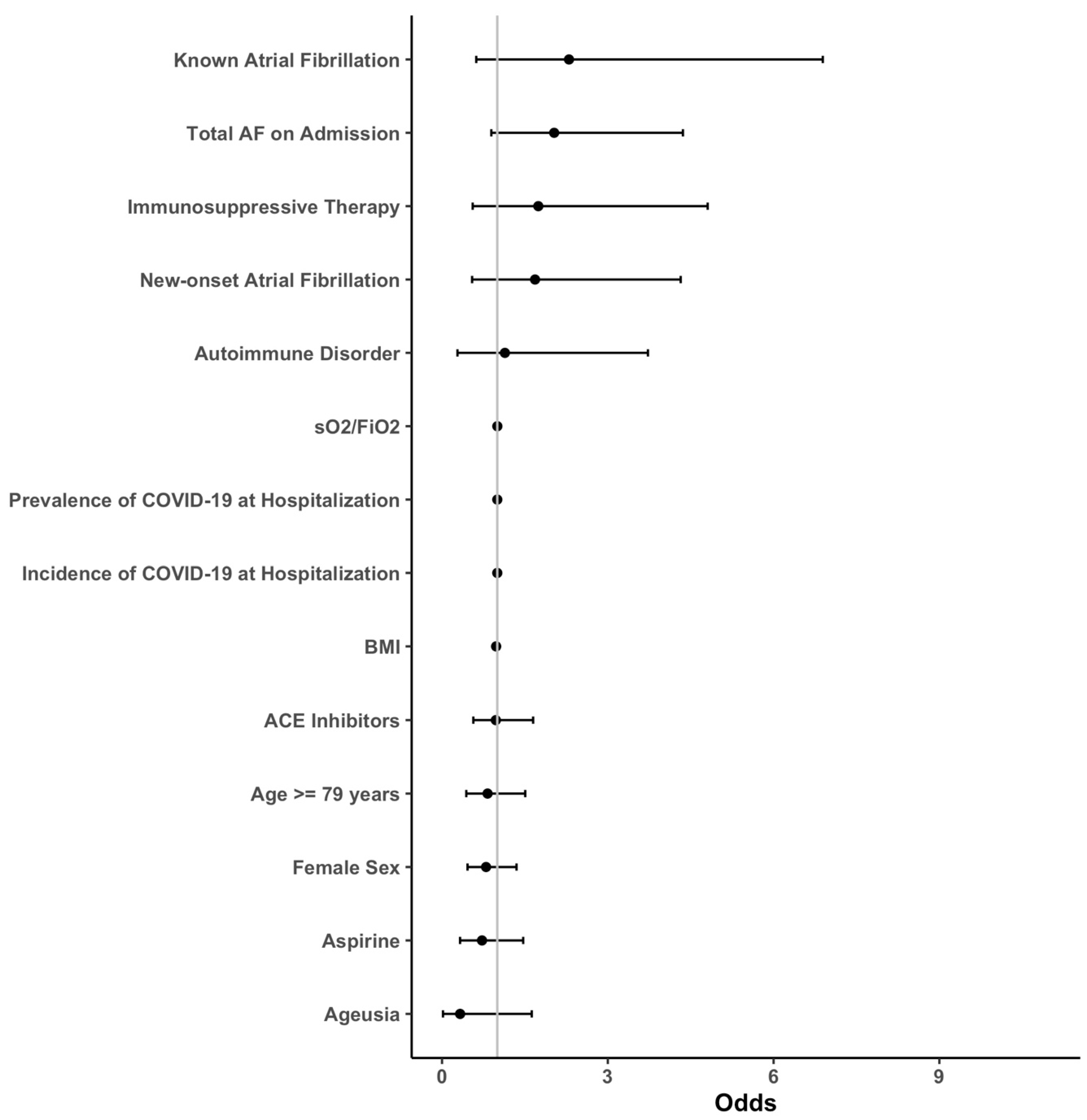
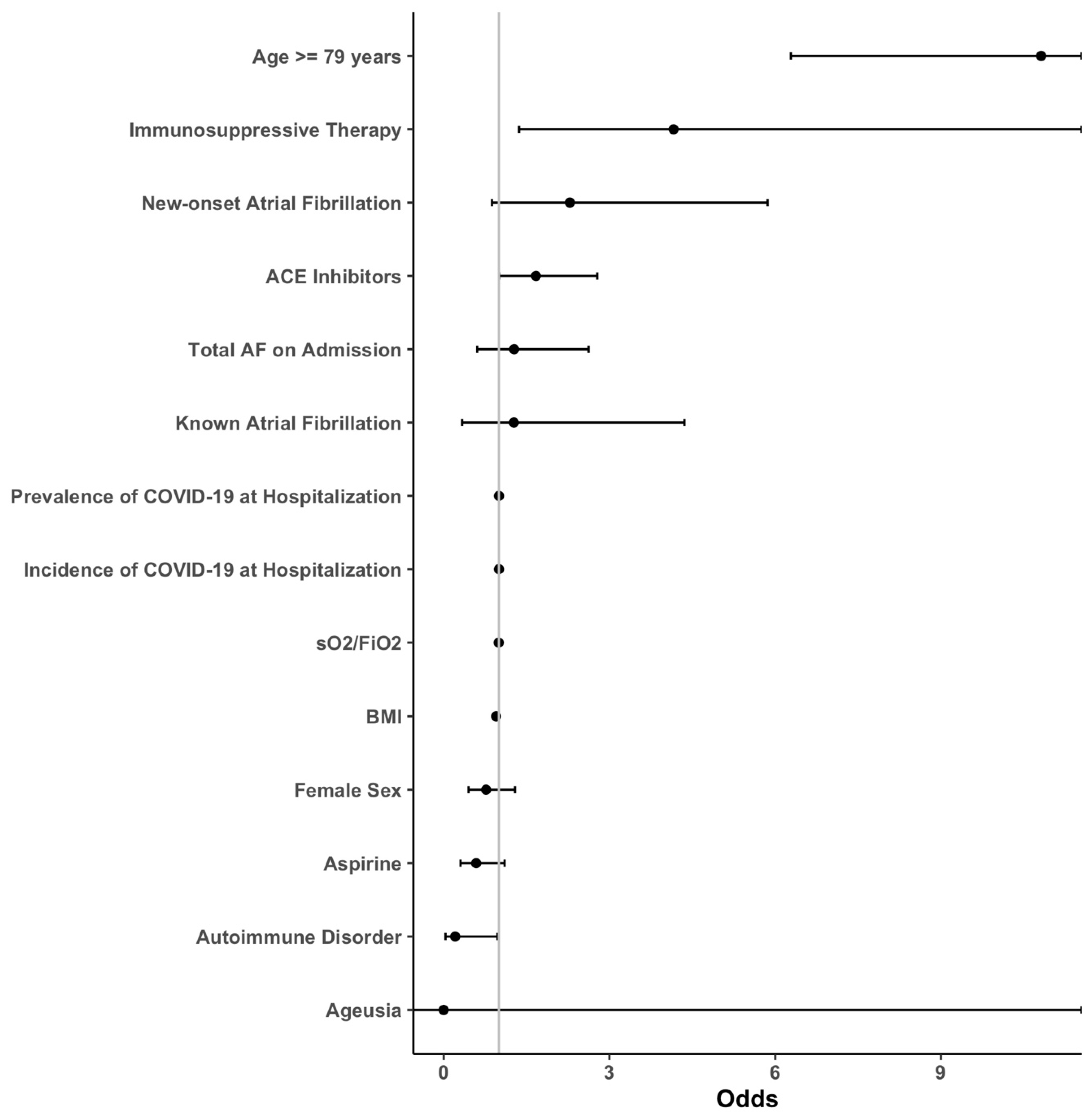
3.1. Outcomes
3.2. Subgroup Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ACE2 | Angiotensin-converting enzyme 2 |
| ACEI | Angiotensin-converting enzyme inhibitor |
| AF | Atrial fibrillation |
| ARB | Angiotensin-receptor blocker |
| ARDS | Acute respiratory distress syndrome |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CMP | Cardiomyopathy |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| CV | Cardiovascular |
| DVT | Deep vein thrombosis |
| ECG | Electrocardiogram |
| ER | Emergency room |
| ICU | Intensive care unit |
| IMC | Intermediate care unit |
| IQR | Interquartile range |
| MD | Medical doctor |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
| PCT | Procalcitonin |
| ROC | Receiver operating characteristic |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus type 2 |
| sO2 | Arterial oxygen saturation |
| TnT-hs, | Troponin T high sensitivity |
| US | United States |
References
- Poterucha, T.J.; Elias, P.; Jain, S.S.; Sayer, G.; Redfors, B.; Burkhoff, D.; Rosenblum, H.; DeFilippis, E.M.; Gupta, A.; Lawlor, M.; et al. Admission Cardiac Diagnostic Testing with Electrocardiography and Troponin Measurement Prognosticates Increased 30-Day Mortality in COVID-19. J. Am. Heart Assoc. 2021, 10, e018476. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Raharjo, S.B. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Indian Pacing Electrophysiol. J. 2020, 20, 193–198. [Google Scholar] [CrossRef]
- Russo, V.; Di Maio, M.; Mottola, F.F.; Pagnano, G.; Attena, E.; Verde, N.; Di Micco, P.; Silverio, A.; Scudiero, F.; Nunziata, L.; et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: A multicenter observational study. Eur. J. Clin. Investig. 2020, 50, e13387. [Google Scholar] [CrossRef]
- Sanz, A.P.; Tahoces, L.S.; Pérez, R.O.; Ferrer, E.G.; Recalde, Á.S.; Gómez, J.L.Z. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol. J. 2021, 28, 7. [Google Scholar]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Zanon, F.; Zuliani, G.; Roncon, L. Pre-existing atrial fibrillation is associated with increased mortality in COVID-19 Patients. J. Interv. Card. Electrophysiol. 2021, 62, 231–238. [Google Scholar] [CrossRef]
- Spinoni, E.G.; Mennuni, M.; Rognoni, A.; Grisafi, L.; Colombo, C.; Lio, V.; Renda, G.; Foglietta, M.; Petrilli, I.; D’Ardes, D.; et al. Contribution of Atrial Fibrillation to In-Hospital Mortality in Patients with COVID-19. Circ. Arrhythm. Electrophysiol. 2021, 14, e009375. [Google Scholar] [CrossRef]
- Paris, S.; Inciardi, R.M.; Lombardi, C.M.; Tomasoni, D.; Ameri, P.; Carubelli, V.; Agostoni, P.; Canale, C.; Carugo, S.; Danzi, G.; et al. Implications of atrial fibrillation on the clinical course and outcomes of hospitalized COVID-19 patients: Results of the Cardio-COVID-Italy multicentre study. EP Eur. 2021, 23, 1603–1611. [Google Scholar] [CrossRef]
- Angeli, F.; Spanevello, A.; De Ponti, R.; Visca, D.; Marazzato, J.; Palmiotto, G.; Feci, D.; Reboldi, G.; Fabbri, L.M.; Verdecchia, P. Electrocardiographic features of patients with COVID-19 pneumonia. Eur. J. Intern. Med. 2020, 78, 101–106. [Google Scholar] [CrossRef]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020, 17, 1439–1444. [Google Scholar] [CrossRef]
- Colon, C.M.; Barrios, J.G.; Chiles, J.W.; McElwee, S.K.; Russell, D.W.; Maddox, W.R.; Kay, G.N. Atrial Arrhythmias in COVID-19 Patients. JACC Clin. Electrophysiol. 2020, 6, 1189–1190. [Google Scholar] [CrossRef]
- Denegri, A.; Pezzuto, G.; D’Arienzo, M.; Morelli, M.; Savorani, F.; Cappello, C.G.; Luciani, A.; Boriani, G. Clinical and electrocardiographic characteristics at admission of COVID-19/SARS-CoV-2 pneumonia infection. Intern. Emerg. Med. 2021, 16, 1451–1456. [Google Scholar] [CrossRef]
- Kelesoglu, S.; Yilmaz, Y.; Ozkan, E.; Calapkorur, B.; Gok, M.; Dursun, Z.B.; Kilic, A.U.; Demirelli, S.; Simsek, Z.; Elcık, D. New onset atrial fibrilation and risk faktors in COVID-19. J. Electrocardiol. 2021, 65, 76–81. [Google Scholar] [CrossRef]
- Peltzer, B.; Manocha, K.K.; Ying, X.; Kirzner, J.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Safford, M.M.; et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 3077–3085. [Google Scholar] [CrossRef]
- Rav-Acha, M.; Orlev, A.; Itzhaki, I.; Zimmerman, S.F.; Fteiha, B.; Bohm, D.; Kurd, R.; Samuel, T.Y.; Asher, E.; Helviz, Y.; et al. Cardiac arrhythmias amongst hospitalised Coronavirus 2019 (COVID-19) patients: Prevalence, characterisation, and clinical algorithm to classify arrhythmic risk. Int. J. Clin. Pract. 2021, 75, e13788. [Google Scholar] [CrossRef]
- Sala, S.; Peretto, G.; De Luca, G.; Farina, N.; Campochiaro, C.; Tresoldi, M.; Dagna, L.; Zangrillo, A.; Gulletta, S.; Della Bella, P. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin. Electrophysiol. 2020, 43, 891–893. [Google Scholar] [CrossRef]
- Wetterslev, M.; Jacobsen, P.K.; Hassager, C.; Jøns, C.; Risum, N.; Pehrson, S.; Bastiansen, A.; Andreasen, A.S.; Tjelle Kristiansen, K.; Bestle, M.H.; et al. Cardiac arrhythmias in critically ill patients with coronavirus disease 2019: A retrospective population-based cohort study. Acta Anaesthesiol. Scand. 2021, 65, 770–777. [Google Scholar] [CrossRef]
- Zylla, M.M.; Merle, U.; Vey, J.A.; Korosoglou, G.; Hofmann, E.; Müller, M.; Herth, F.; Schmidt, W.; Blessing, E.; Göggelmann, C.; et al. Predictors and Prognostic Implications of Cardiac Arrhythmias in Patients Hospitalized for COVID-19. J. Clin. Med. 2021, 10, 133. [Google Scholar] [CrossRef]
- O’Shea, C.J.; Middeldorp, M.E.; Thomas, G.; Harper, C.; Elliott, A.D.; Ray, N.; Campbell, K.; Lau, D.H.; Sanders, P. Atrial fibrillation burden during the coronavirus disease 2019 pandemic. EP Eur. 2021, 23, 1493–1501. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults: National Implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Chen, Y.-J.; Lin, Y.-J.; Chen, S.-A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Romiti, G.F.; Corica, B.; Lip, G.Y.H.; Proietti, M. Prevalence and Impact of Atrial Fibrillation in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2490. [Google Scholar] [CrossRef]
- D’Andrea, A.; Russo, V.; Manzo, G.; Giordano, V.; Maio, M.D.; Crescibene, F.; D’Alto, M.; Bossone, E. Association of atrial fibrillation and left atrial volume index with mortality in patients with COVID-19 pneumonia. Eur. J. Prev. Cardiol. 2022, 29, e44–e46. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Metra, M. Atrial fibrillation in the COVID-19 era: Simple bystander or marker of increased risk? Eur. Heart J. 2020, 41, 3094. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Rosenblatt, A.G.; Ayers, C.R.; Rao, A.; Howell, S.J.; Hendren, N.S.; Zadikany, R.H.; Ebinger, J.E.; Daniels, J.D.; Link, M.S.; de Lemos, J.A.; et al. New-Onset Atrial Fibrillation in Patients Hospitalized with COVID-19: Results from the American Heart Association COVID-19 Cardiovascular Registry. Circ. Arrhythm. Electrophysiol. 2022, 15, e010666. [Google Scholar] [CrossRef]
- García-Granja, P.E.; Veras, C.; Aparisi, Á.; Amat-Santos, I.J.; Catalá, P.; Marcos, M.; Cabezón, G.; Candela, J.; Gil, J.F.; Uribarri, A.; et al. Atrial fibrillation in patients with SARS-CoV-2 infection. Med. Clín. 2021, 157, 58–63. [Google Scholar] [CrossRef]
- Yarmohammadi, H.; Morrow, J.P.; Dizon, J.; Biviano, A.; Ehlert, F.; Saluja, D.; Waase, M.; Elias, P.; Poterucha, T.J.; Berman, J.; et al. Frequency of Atrial Arrhythmia in Hospitalized Patients with COVID-19. Am. J. Cardiol. 2021, 147, 52–57. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, M.; Dong, X.; Zhang, J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Corballis, N.; Vassiliou, V.S. Renin–Angiotensin–Aldosterone Inhibitors and COVID-19 Infection. Curr. Hypertens. Rep. 2022, 24, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Baral, R.; Tsampasian, V.; Debski, M.; Moran, B.; Garg, P.; Clark, A.; Vassiliou, V.S. Association Between Renin-Angiotensin-Aldosterone System Inhibitors and Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e213594. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Lombardi, C.M.; Specchia, C.; Merlo, M.; Nuzzi, V.; Ferraro, I.; Peveri, G.; Oriecuia, C.; Pozzi, A.; Inciardi, R.M.; et al. Combined Role of Troponin and Natriuretic Peptides Measurements in Patients with COVID-19 (from the Cardio-COVID-Italy Multicenter Study). Am. J. Cardiol. 2022, 167, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shaver, C.M.; Chen, W.; Janz, D.R.; May, A.K.; Darbar, D.; Bernard, G.R.; Bastarache, J.A.; Ware, L.B. Atrial Fibrillation Is an Independent Predictor of Mortality in Critically Ill Patients. Crit. Care Med. 2015, 43, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Moss, T.J.; Calland, J.F.; Enfield, K.B.; Gomez-Manjarres, D.C.; Ruminski, C.; DiMarco, J.P.; Lake, D.E.; Moorman, J.R. New-Onset Atrial Fibrillation in the Critically Ill. Crit. Care Med. 2017, 45, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, D.B.; Benjamin, E.J.; Bajwa, E.K.; Hibbert, K.A.; Walkey, A.J. Risk factors and outcomes associated with new-onset atrial fibrillation during acute respiratory distress syndrome. J. Crit. Care 2015, 30, 994–997. [Google Scholar] [CrossRef]
- Klein Klouwenberg, P.M.C.; Frencken, J.F.; Kuipers, S.; Ong, D.S.Y.; Peelen, L.M.; van Vught, L.A.; Schultz, M.J.; van der Poll, T.; Bonten, M.J.; Cremer, O.L. Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am. J. Respir. Crit. Care Med. 2017, 195, 205–211. [Google Scholar] [CrossRef]
- Musikantow, D.R.; Turagam, M.K.; Sartori, S.; Chu, E.; Kawamura, I.; Shivamurthy, P.; Bokhari, M.; Oates, C.; Zhang, C.; Pumill, C.; et al. Atrial Fibrillation in Patients Hospitalized with COVID-19. JACC Clin. Electrophysiol. 2021, 7, 1120–1130. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chao, T.-F.; Liu, C.-J.; Chen, S.-J.; Chung, F.-P.; Liao, J.-N.; Tuan, T.-C.; Chen, T.-J.; Chen, S.-A. The association between influenza infection, vaccination, and atrial fibrillation: A nationwide case-control study. Heart Rhythm 2016, 13, 1189–1194. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Rasekhi, R.T.; Gill, D.; Babapoor, S.; Amanullah, A. Arrhythmia in COVID-19. SN Compr. Clin. Med. 2020, 2, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.; Metersky, M.; Atuegwu, N.; Anzueto, A. New Onset Atrial Fibrillation in Patients Hospitalized with Pneumonia. Eur. Respir. J. 2019, 54, OA3307. [Google Scholar]

| Total Population (n = 646) | Atrial Fibrillation (n = 116) | w/o Atrial Fibrillation (n = 530) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Female sex, no./total no. (%) | 267/646 (41.3) | 39/116 (33.6) | 228/530 (43.0) | 0.06 |
| Age, y—median (IQR) | 70 (59–80) n = 646 | 80.0 (72.0–87.0) n = 116 | 67.0 (57.0–78.0) n = 530 | <0.001 |
| BMI, kg/m2—median (IQR) | 27 (24–31) n = 646 | 26.7 (23.7–29.6) n = 116 | 27.5 (24.3–31.2) n = 530 | 0.10 |
| Symptoms, no./total no. (%) | ||||
| Fever | 317/646 (49.1) | 47/116 (40.5) | 270/530 (50.9) | 0.042 |
| Cough | 351/646 (54.3) | 53/116 (45.7) | 298/530 (56.2) | 0.039 |
| Diarrhea | 163/646 (25.2) | 27/116 (23.3) | 136/530 (25.7) | 0.59 |
| Myalgia | 101/646 (15.6) | 11/116 (9.5) | 90/530 (17.0) | 0.044 |
| Ageusia | 27/646 (4.2) | 1/116 (0.9) | 26/530 (4.9) | 0.049 |
| Vital signs, median (IQR) | ||||
| Systolic blood pressure, mmHg | 132.0 (119.0–146.0) n = 646 | 130.5 (113.5–146.0) n = 116 | 133.0 (120.0–145.3) n = 530 | 0.54 |
| Diastolic blood pressure, mmHg | 78.0 (67.5–86.0) n = 646 | 75.0 (60.0–87.0) n = 116 | 78.0 (68.0–85.3) n = 530 | 0.14 |
| Heart rate, bpm | 84.0 (74.0–95.0) n = 646 | 84.0 (72.3–93.0) n = 116 | 84.0 (74.0–96.0) n = 530 | 0.74 |
| Temperature, °C | 37.0 (36.3–37.9) n = 646 | 36.9 (36.2–37.9) n = 116 | 37 (36.3–37.9) n = 530 | 0.27 |
| Blood oxygen saturation, % | 93.7 (91.5–95.8) n = 451 | 93.8 (90.8–96.0) n = 85 | 93.7 (91.7–95.8) n = 366 | 0.85 |
| pO2, mmHg | 67.3 (60.5–76.4) n = 451 | 68.5 (60.5–81.7) n = 85 | 67.0 (60.5–75.8) n = 366 | 0.41 |
| pCO2, mmHg | 31.9 (28.5–34.9) n = 452 | 30.9 (27.5–35.6) n = 85 | 32.1 (28.8–34.8) n = 367 | 0.20 |
| P/F ratio | 383.3 (321.9–400.0) n = 508 | 375.0 (296.9–400.0) n = 89 | 383.3 (339.3–400.0) n = 419 | 0.12 |
| Laboratory findings, median (IQR) | ||||
| Troponin T hs, ng/L | 13.8 (7.9–35.5) n = 147 | 35.7 (16.4–60.4) n = 35 | 10.6 (6.9–25.2) n = 112 | <0.001 |
| Creatine kinase, U/L | 98.5 (59.3–185.5) n = 180 | 113.0 (72.0–208.0) n = 43 | 85.0 (55.0–176.0) n = 137 | 0.027 |
| NT-proBNP, ng/L | 772.5 (207.3–3623) n = 94 | 3402.0 (2000.0–6908.5) n = 26 | 342.0 (154.8–1495.5) n = 68 | <0.001 |
| CRP, mg/L | 58.1 (24.0–111.4) n = 623 | 60.9 (23.2–113.9) n = 112 | 57.3 (24.0–110.6) n = 511 | 0.49 |
| PCT, ng/mL | 0.12 (0.07–0.25) n = 526 | 0.17 (0.10–0.49) n = 96 | 0.11 (0.07–0.21) n = 430 | <0.001 |
| Creatinine, µmol/L | 84.0 (67.0–111.0) n = 621 | 102.5 (82.3–139.5) n = 112 | 82.0 (64.0–105.0) n = 509 | <0.001 |
| D-Dimer, µg FEU/L | 493.5 (297.5–1063.8) n = 448 | 460.0 (211.8–1377.3) n = 76 | 499.5 (300.8–1027.8) n = 372 | 0.43 |
| Cardiovascular risk factors, no./total no. (%) | ||||
| Hypertension | 302/644 (46.9) | 71/116 (61.2) | 231/528 (43.8) | <0.001 |
| Diabetes mellitus | 159/644 (24.6) | 35/116 (30.2) | 124/528 (23.5) | 0.13 |
| Dyslipidemia | 91/644 (14.1) | 26/116 (22.4) | 65/528 (12.3) | 0.005 |
| Cardiovascular family history | 9/644 (1.4) | 3/116 (2.6) | 6/528 (1.1) | 0.23 |
| Coexisting medical condition, no./total no. (%) | ||||
| Coronary artery disease | 79/646 (12.2) | 26/116 (22.4) | 53/530 (10.0) | <0.001 |
| Hematologic disorders | 60/645 (9.2) | 16/116 (13.8) | 44/529 (8.3) | 0.07 |
| Cancer | 70/646 (10.8) | 15/116 (12.9) | 55/530 (10.4) | 0.42 |
| Cardiomyopathy | 64/646 (9.9) | 32/116 (27.6) | 32/530 (6.0) | <0.001 |
| Chronic kidney disease | 97/645 (15.0) | 30/116 (25.9) | 67/529 (12.7) | <0.001 |
| Chronic infections | 7/646 (1.1) | 2/116 (1.7) | 5/530 (0.9) | 0.46 |
| Autoimmune disorders | 24/646 (3.7) | 3/116 (2.6) | 21/530 (4.0) | 0.48 |
| Medication on admission, no./total no. (%) | ||||
| ACE inhibitor | 245/646 (37.9) | 49/116 (42.2) | 196/530 (37.0) | 0.29 |
| Beta-blocker | 158/646 (24.5) | 57/116 (49.1) | 101/530 (19.1) | <0.001 |
| Calcium-channel antagonist | 121/646 (18.7) | 31/117 (26.7) | 90/530 (17.0) | 0.015 |
| Statin | 187/646 (28.9) | 51/116 (44.0) | 136/530 (25.7) | <0.001 |
| Aspirin | 116/646 (18.0) | 16/117 (13.8) | 100/530 (18.9) | 0.20 |
| Anticoagulation | 141/646 (21.8) | 76/116 (65.5) | 65/530 (12.3) | <0.001 |
| Immunosuppressive therapy | 32/646 (5.0) | 2/116 (1.7) | 30/530 (5.7) | 0.08 |
| Complications/management, no./total no. (%) | ||||
| Intensive care (IMC/ICU) | 177/646 (27.4) | 41/116 (35.3) | 136/530 (25.7) | 0.034 |
| Catecholamine use | 59/646 (9.1) | 12/116 (10.3) | 47/530 (8.9) | 0.62 |
| Invasive ventilation | 76/645 (11.8) | 16/116 (13.8) | 60/529 (11.3) | 0.46 |
| Noninvasive ventilation | 75/645 (11.6) | 13/116 (11.2) | 62/529 (11.7) | 0.88 |
| In-hospital mortality | 90/646 (13.9) | 33/116 (28.4) | 57/530 (10.8) | <0.001 |
| Poor clinical trajectory | 224/646 (34.7) | 58/116 (50.0) | 166/530 (31.3) | <0.001 |
| DVT | 6/646 (0.9) | 3/116 (2.6) | 3/530 (0.6) | 0.040 |
| Pulmonary embolism | 31/644 (4.8) | 1/115 (0.9) | 30/529 (5.7) | 0.029 |
| ARDS | 37/636 (5.8) | 10/114 (8.8) | 27/522 (5.2) | 0.14 |
| New-Onset AF (n = 34) | Preexisting AF (n = 82) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Female sex, no./total no. (%) | 11/34 (32.4) | 28/82 (34.1) | 0.85 |
| Age, y—median (IQR) | 75.5 (66.0–87.3) n = 34 | 81.0 (73.8–87.0) n = 82 | 0.059 |
| BMI, kg/m2—median (IQR) | 26.6 (22.8–30.3) n = 34 | 27 (23.8–29.7) n = 82 | 0.74 |
| Symptoms, no./total no. (%) | |||
| Fever | 16/34 (47.1) | 31/82 (37.8) | 0.36 |
| Cough | 17/34 (50.0) | 36/82 (43.9) | 0.55 |
| Diarrhea | 9/34 (26.5) | 18/82 (22.0) | 0.60 |
| Myalgia | 2/34 (5.9) | 9/82 (11.0) | 0.39 |
| Ageusia | 1/34 (2.9) | 0/82 (0.0) | 0.12 |
| Vital signs, median (IQR) | |||
| Systolic blood pressure, mmHg | 132.5 (120.0–154.3) n = 34 | 129.0 (112.0–146.0) n = 82 | 0.35 |
| Diastolic blood pressure, mmHg | 80.0 (59.8–89.3) n = 34 | 73.5 (59.8–86.3) n = 82 | 0.53 |
| Heart rate, bpm | 84.5 (75.3–98.5) n = 34 | 83.5 (71.8–92.3) n = 82 | 0.66 |
| Temperature, °C | 37.0 (36.0–38.0) n = 34 | 36.9 (36.4–37.9) n = 82 | 0.87 |
| Blood oxygen saturation, % | 93.8 (90.7–95.9) n = 27 | 94.1 (90.7–96.4) n = 58 | 0.96 |
| pO2, mmHg | 68.3 (60.9–79.4) n = 27 | 68.6 (60.4–82.7) n = 58 | 0.78 |
| pCO2, mmHg | 29.8 (26.7–35.3) n = 27 | 31.5 (27.7–35.8) n = 58 | 0.54 |
| P/F ratio | 333.3 (284.4–383.3) n = 27 | 387.5 (301.6–401.0) n = 62 | 0.14 |
| Laboratory findings, median (IQR) | |||
| Troponin T hs, ng/L | 25.4 (13.2–60.9) n = 13 | 37.9 (17.5–58.1) n = 22 | 0.91 |
| Creatine kinase, U/L | 121.0 (67.0–504.8) n = 18 | 108.0 (77.0–192.0) n = 25 | 0.56 |
| NT-proBNP, ng/L | 2970.0 (1170.5–10,061.5) n = 9 | 3653.0 (2250.5–6523.0) n = 17 | 0.87 |
| CRP, mg/L | 70.6 (31.7–170.0) n = 33 | 59.3 (20.2–103.8) n = 79 | 0.19 |
| PCT, ng/mL | 0.22 (0.12–0.59) n = 31 | 0.14 (0.09–0.49) n = 65 | 0.26 |
| Creatinine, µmol/L | 92.0 (71.5–117.0) n = 33 | 114.0 (89.0–144.0) n = 79 | 0.009 |
| D-dimer, µg FEU/L | 575.5 (227.8–3783.8) n = 28 | 395.5 (211.0–779.0) n = 48 | 0.12 |
| Cardiovascular risk factors, no./total no. (%) | |||
| Hypertension | 19/34 (55.9) | 52/82 (63.4) | 0.45 |
| Diabetes mellitus | 14/34 (41.2) | 21/82 (25.6) | 0.10 |
| Dyslipidemia | 8/34 (23.5) | 18/82 (22.0) | 0.85 |
| Adipositas | 6/34 (17.6) | 16/82 (19.5) | 0.82 |
| Cardiovascular family history | 1/34 (2.9) | 2/82 (2.4) | 0.88 |
| Coexisting medical condition, no./total no. (%) | |||
| Coronary artery disease | 7/34 (20.6) | 19/82 (23.2) | 0.76 |
| Hematologic disorders | 4/34 (11.8) | 12/82 (14.6) | 0.68 |
| Cancer | 2/34 (5.9) | 13/82 (15.9) | 0.15 |
| Cardiomyopathy | 5/34 (14.7) | 27/82 (32.9) | 0.046 |
| Chronic kidney disease | 2/34 (5.9) | 28/82 (34.1) | 0.002 |
| Chronic infections | 1/34 (2.9) | 1/82 (1.2) | 0.52 |
| Autoimmune disorders | 1/34 (2.9) | 2/82 (2.4) | 0.88 |
| Medication on admission, no./total no. (%) | |||
| ACE inhibitor | 14/34 (41.2) | 35/82 (42.7) | 0.88 |
| Beta blocker | 11/34 (32.4) | 46/82 (56.1) | 0.020 |
| Calcium-channel antagonist | 9/34 (26.5) | 22/82 (26.8) | 0.97 |
| Statin | 13/34 (38.2) | 38/82 (46.3) | 0.42 |
| Aspirin | 10/34 (29.4) | 6/82 (7.3) | 0.002 |
| Anticoagulation | 8/34 (23.5) | 68/82 (82.9) | <0.001 |
| Immunosuppressive therapy | 0/34 (0.0) | 2/82 (2.4) | 0.36 |
| Complications/management, no./total no. (%) | |||
| Intensive care (IMC/ICU) | 20/34 (58.8) | 21/82 (25.6) | 0.001 |
| Catecholamine use | 3/34 (8.8) | 9/82 (11.0) | 0.73 |
| Invasive ventilation | 9/34 (26.5) | 7/82 (8.5) | 0.011 |
| Noninvasive ventilation | 5/34 (14.7) | 8/82 (9.8) | 0.44 |
| In-hospital mortality | 9/34 (26.5) | 24/82 (29.3) | 0.76 |
| Poor clinical trajectory | 25/34 (73.5) | 33/82 (40.2) | 0.001 |
| DVT | 3/34 (8.8) | 0/82 (0.0) | 0.006 |
| Pulmonary embolism | 1/34 (2.9) | 0/82 (0.0) | 0.12 |
| ARDS | 8/34 (23.5) | 2/82 (2.5) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurisic, S.; Komminoth, M.; Todorov, A.; Bertschi, D.A.; Jurisic, M.; Vranjic, I.; Wiggli, B.; Schmid, H.; Gebhard, C.; Gebhard, C.E.; et al. Long-Term Mortality after New-Onset Atrial Fibrillation in COVID-19. J. Clin. Med. 2023, 12, 2925. https://doi.org/10.3390/jcm12082925
Jurisic S, Komminoth M, Todorov A, Bertschi DA, Jurisic M, Vranjic I, Wiggli B, Schmid H, Gebhard C, Gebhard CE, et al. Long-Term Mortality after New-Onset Atrial Fibrillation in COVID-19. Journal of Clinical Medicine. 2023; 12(8):2925. https://doi.org/10.3390/jcm12082925
Chicago/Turabian StyleJurisic, Stjepan, Mathis Komminoth, Atanas Todorov, Daniela A. Bertschi, Martin Jurisic, Ivica Vranjic, Benedikt Wiggli, Hansruedi Schmid, Catherine Gebhard, Caroline E. Gebhard, and et al. 2023. "Long-Term Mortality after New-Onset Atrial Fibrillation in COVID-19" Journal of Clinical Medicine 12, no. 8: 2925. https://doi.org/10.3390/jcm12082925
APA StyleJurisic, S., Komminoth, M., Todorov, A., Bertschi, D. A., Jurisic, M., Vranjic, I., Wiggli, B., Schmid, H., Gebhard, C., Gebhard, C. E., Heidecker, B., Beer, J.-H., & Patriki, D. (2023). Long-Term Mortality after New-Onset Atrial Fibrillation in COVID-19. Journal of Clinical Medicine, 12(8), 2925. https://doi.org/10.3390/jcm12082925





