Association between Systemic Factors and Vitreous Fluid Cytokines in Proliferative Diabetic Retinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Diagnostics
2.3. Systemic Factors
2.4. Vitreous Fluid Sample Collection and Cytokine Assay
2.5. Statistical Analysis
3. Results
3.1. Subjects
3.2. Vitreous Fluid Cytokine Levels
3.3. Correlation between Systemic Factors and Vitreous Fluid Cytokines
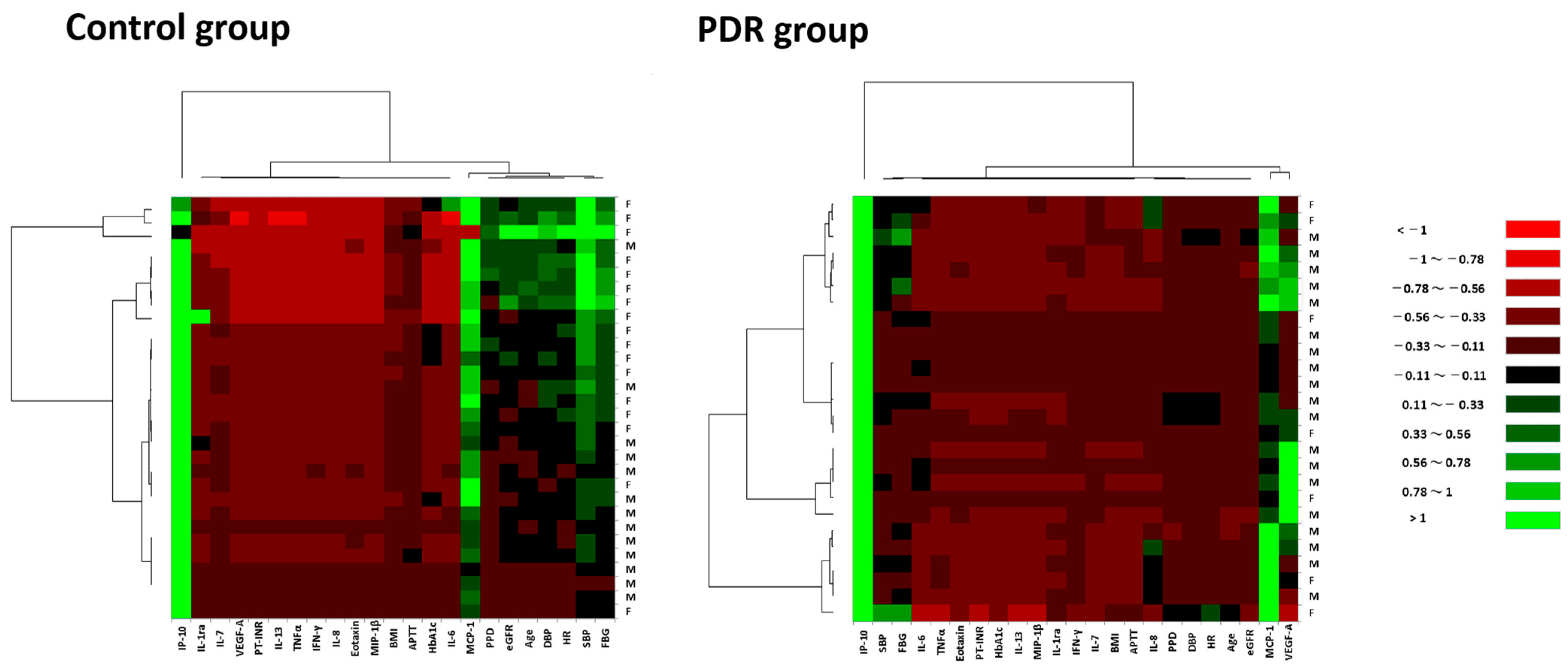
3.4. Expression Patterns of Systemic Factors and Vitreous Fluid Cytokines in Hierarchical Cluster Analysis
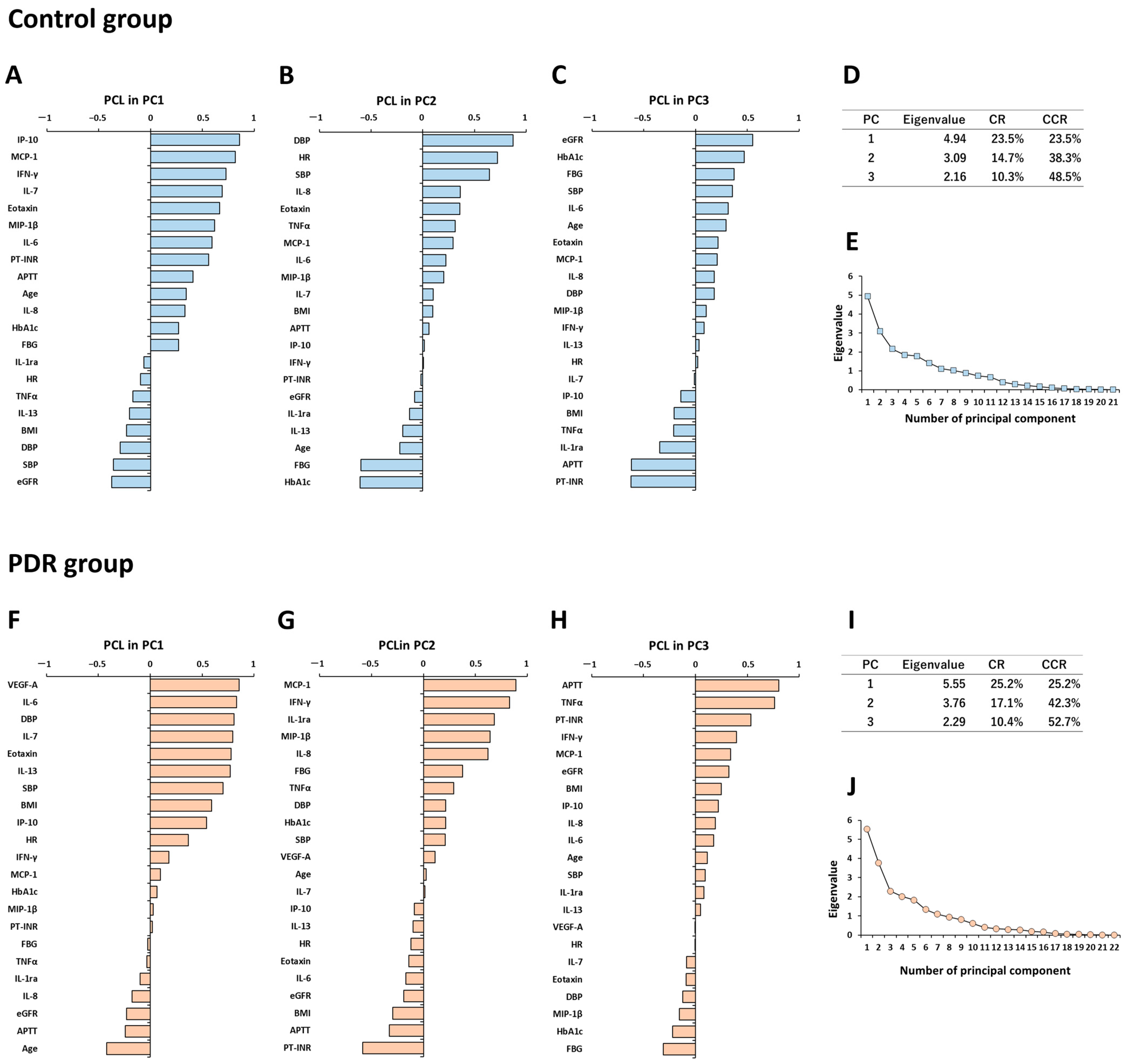
3.5. Principal Component Analysis for Expression Patterns of Systemic Factors and Vitreous Fluid Cytokines in Controls
3.6. Principal Component Analysis for Expression Patterns of Systemic Factors and Vitreous Fluid Cytokines in PDR Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dejkhamron, P.; Menon, R.K.; Sperling, M.A. Childhood diabetes mellitus: Recent advances & future prospects. Indian J. Med. Res. 2007, 125, 231–250. [Google Scholar] [PubMed]
- Kempen, J.H.; O’colmain, B.; Leske, M.; Haffner, S.; Klein, R.; Moss, S.; Taylor, R.H.; Hamman, R.F. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004, 122, 552–563. [Google Scholar] [PubMed]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef]
- Durham, J.T.; Herman, I.M. Microvascular modifications in diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Wat, N.; Wong, R.L.; Wong, I.Y. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med. J. 2016, 22, 589–599. [Google Scholar] [CrossRef]
- Gologorsky, D.; Thanos, A.; Vavvas, D. Therapeutic interventions against inflammatory and angiogenic mediators in proliferative diabetic retinopathy. Mediat. Inflamm. 2012, 2012, 629452. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris 3rd, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Sato, T.; Takeuchi, M.; Karasawa, Y.; Takayama, K.; Enoki, T. Comprehensive expression patterns of inflammatory cytokines in aqueous humor of patients with neovascular age-related macular degeneration. Sci. Rep. 2019, 9, 19447. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Enoki, T.; Karasawa, Y.; Someya, H.; Taguchi, M.; Harimoto, K.; Takayama, K.; Kanda, T.; Ito, M.; Takeuchi, M. Inflammatory Factors of Macular Atrophy in Eyes with Neovascular Age-Related Macular Degeneration Treated with Aflibercept. Front. Immunol. 2021, 2, 738521. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Taguchi, M.; Sato, T.; Karasawa, Y.; Sakurai, Y.; Harimoto, K.; Ito, M. Association of High-Mobility Group Box-1 With Th Cell-Related Cytokines in the Vitreous of Ocular Sarcoidosis Patients. Investig. Ophthalmol. Vis. Sci. 2017, 58, 528–537. [Google Scholar] [CrossRef]
- Takeuchi, M.; Karasawa, Y.; Harimoto, K.; Tanaka, A.; Shibata, M.; Sato, T.; Caspi, R.R.; Ito, M. Analysis of Th Cell-related Cytokine Production in Behcet Disease Patients with Uveitis Before and After Infliximab Treatment. Ocul. Immunol. Inflamm. 2017, 25, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Phone, A.; Lamy, R.; Ma, D.; Laotaweerungsawat, S.; Chen, Y.; Zhao, T.; Ma, W.; Zhang, F.; Psaras, C.; et al. Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakazawa, M.; Suzuki, K.; Yamazaki, H.; Miyagawa, Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn. J. Ophthalmol. 2011, 55, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sato, T.; Tanaka, A.; Muraoka, T.; Taguchi, M.; Sakurai, Y.; Karasawa, Y.; Ito, M. Elevated levels of cytokines associated with Th2 and Th17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PLoS ONE 2015, 10, e0137358. [Google Scholar] [CrossRef] [PubMed]
- Adki, K.M.; Kulkarni, Y.A. Potential Biomarkers in Diabetic Retinopathy. Curr. Diabetes Rev. 2020, 16, 971–983. [Google Scholar]
- Takeuchi, M.; Sato, T.; Sakurai, Y.; Taguchi, M.; Harimoto, K.; Karasawa, Y.; Ito, M. Association between aqueous humor and vitreous fluid levels of Th17 cell-related cytokines in patients with proliferative diabetic retinopathy. PLoS ONE 2017, 12, e0178230. [Google Scholar] [CrossRef]
- Group ETDRSR. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report no. 4. Int. Ophthalmol. Clin. 1987, 27, 265–272. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeuchi, M.; Karasawa, Y.; Ito, M. Profiles of Cytokines Secreted by ARPE-19 Cells Exposed to Light and Incubated with Anti-VEGF Antibody. Biomedicines 2021, 9, 1333. [Google Scholar] [CrossRef]
- Grover, S.; Fishman, G.A.; Anderson, R.J.; Tozatti, M.S.; Heckenlively, J.R.; Weleber, R.G.; Edwards, A.O.; Brown, J., Jr. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology 1999, 106, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1984, 102, 520–526. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Tsai, T.; Kuehn, S.; Tsiampalis, N.; Vu, M.-K.; Kakkassery, V.; Stute, G.; Dick, H.B.; Joachim, S.C. Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients. PLoS ONE 2018, 13, e0194603. [Google Scholar] [CrossRef]
- Wilks, D.S. Cluster analysis. In International Geophysics. 100; Elsevier: Amsterdam, The Netherlands, 2011; pp. 603–616. [Google Scholar]
- Zhang, X.; Saaddine, J.B.; Chou, C.-F.; Cotch, M.F.; Cheng, Y.J.; Geiss, L.S.; Gregg, E.W.; Albright, A.L.; Klein, B.E.K.; Klein, R. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010, 304, 649–656. [Google Scholar] [CrossRef]
- Stratton, I.; Kohner, E.; Aldington, S.; Turner, R.C.; Holman, R.R.; Manley, S.E.; Matthews, D.R. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [PubMed]
- Lachin, J.M.; Genuth, S.; Cleary, P.; Davis, M.D.; Nathan, D.M. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med. 2000, 342, 381–389. [Google Scholar] [PubMed]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.Y.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [PubMed]
- Man, R.E.; Sasongko, M.B.; Wang, J.J.; MacIsaac, R.; Wong, T.Y.; Sabanayagam, C.; Lamoureux, E.L. The Association of Estimated Glomerular Filtration Rate with Diabetic Retinopathy and Macular Edema. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Z.; Huang, Y.; Guo, K.; Lu, J.; Zhang, L.; Yu, H.; Bao, Y.; Jia, W. A microalbuminuria threshold to predict the risk for the development of diabetic retinopathy in type 2 diabetes mellitus patients. PLoS ONE 2012, 7, e36718. [Google Scholar] [CrossRef]
- Park, Y.H.; Shin, J.A.; Han, J.H.; Park, Y.M.; Yim, H.W. The association between chronic kidney disease and diabetic retinopathy: The Korea National Health and Nutrition Examination Survey 2008–2010. PLoS ONE 2015, 10, e0125338. [Google Scholar] [CrossRef]
- Yamamoto, M.; Fujihara, K.; Ishizawa, M.; Osawa, T.; Kaneko, M.; Ishiguro, H.; Matsubayashi, Y.; Seida, H.; Yamanaka, N.; Tanaka, S.; et al. Overt Proteinuria, Moderately Reduced eGFR and Their Combination Are Predictive of Severe Diabetic Retinopathy or Diabetic Macular Edema in Diabetes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2685–2689. [Google Scholar] [CrossRef]
- Harris, N.K.; Talwar, N.; Gardner, T.W.; Wrobel, J.S.; Herman, W.H.; Stein, J.D. Predicting development of proliferative diabetic retinopathy. Diabetes Care 2013, 36, 1562–1568. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef]
- Cohen, M.C.; Cohen, S. Cytokine function: A study in biologic diversity. Am. J. Clin. Pathol. 1996, 105, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, D.A.; Wilder, R.L.; Manolagas, S.C.; Chrousos, G.P. The pathophysiologic roles of interleukin-6 in human disease. Ann. Intern. Med. 1998, 128, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, C.; Weissinger, E.; Zuckermann, A.; Imhof, M.; Kink, F.; Schöllhammer, A.; Kopp, C.; Wolner, E. Effects of interleukin-1,-2,-4,-6, interferon-gamma and granulocyte/macrophage colony stimulating factor on human vascular endothelial cells. Immunol. Lett. 1993, 35, 109–117. [Google Scholar] [CrossRef]
- Neumann, B.; Emmanuilidis, K.; Stadler, M.; Holzmann, B. Distinct functions of interferon-gamma for chemokine expression in models of acute lung inflammation. Immunology 1998, 95, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tager, A.M.; Kradin, R.L.; LaCamera, P.; Bercury, S.D.; Campanella, G.S.; Leary, C.P.; Polosukhin, V.; Zhao, L.-H.; Sakamoto, H.; Blackwell, T.S.; et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am. J. Respir. Cell Mol. Biol. 2004, 31, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 2018, 33, 141–156. [Google Scholar] [CrossRef]
- Wong, M.Y.Z.; Man, R.E.K.; Fenwick, E.K.; Gupta, P.; Li, L.J.; van Dam, R.M.; Chong, M.F.; Lamoureux, E.L. Dietary intake and diabetic retinopathy: A systematic review. PLoS ONE 2018, 13, e0186582. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoshimura, Y.; Kawasaki, R.; Kamada, C.; Tanaka, S.; Horikawa, C.; Ohashi, Y.; Araki, A.; Ito, H.; Akanuma, Y.; et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology 2013, 24, 204–211. [Google Scholar] [CrossRef]
- Osaadon, P.; Fagan, X.; Lifshitz, T.; Levy, J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 2014, 28, 510–520. [Google Scholar] [CrossRef]
- Bhisitkul, R.B. Vascular endothelial growth factor biology: Clinical implications for ocular treatments. Br. J. Ophthalmol. 2006, 90, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Canataroglu, H.; Varinli, I.; Ozcan, A.A.; Canataroglu, A.; Doran, F.; Varinli, S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul. Immunol. Inflamm. 2005, 13, 375–381. [Google Scholar] [CrossRef]
- Kakehashi, A.; Inoda, S.; Mameuda, C.; Kuroki, M.; Jono, T.; Nagai, R.; Horiuchi, S.; Kawakami, M.; Kanazawa, Y. Relationship among VEGF, VEGF receptor, AGEs, and macrophages in proliferative diabetic retinopathy. Diabetes Res. Clin. Pract. 2008, 79, 438–445. [Google Scholar] [CrossRef]
- Hong, K.H.; Ryu, J.; Han, K.H. Monocyte chemoattractant protein-1–induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005, 105, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Martidis, A.; Duker, J.S.; Greenberg, P.B.; Rogers, A.H.; Puliafito, C.A.; Reichel, E.; Baumal, C. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002, 109, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Kaiser, P.K. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am. J. Ophthalmol. 2005, 139, 290–294. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef]
- Jolliffe, I. Principal component analysis. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1094–1096. [Google Scholar]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Klein, B.E. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII: The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008, 115, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.B.; Brownlee, M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA 2010, 303, 2291–2292. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Lai, T.Y.; Koizumi, H.; Farah, M.E.; Ferrara, D.; Pelayes, D.; Sato, T.; Meyer, C.H.; Murray, T. Anterior chamber paracentesis during intravitreal injections in observational trials: Effectiveness and safety and effects. Int. J. Retina Vitr. 2019, 5, 8. [Google Scholar] [CrossRef] [PubMed]
| Category | PDR | Control | p Value | Unit | Reference Range | ||
|---|---|---|---|---|---|---|---|
| n | 26 | 30 | |||||
| Detectable | Mean ± SD | Detectable | Mean ± SD | ||||
| Rate (%) | rate (%) | ||||||
| Age | 26 (100) | 58.6 ± 13.5 | 30 (100) | 70.7 ± 7.94 | 0.001 | year | |
| Gender (M/F) | 18 (69.2)/7 | 13 (43.3)/17 | 0.063 | ||||
| Laterality | |||||||
| (B/R/L) | 1/14/10 | 0/14/16 | 0.378 | ||||
| LogMAR VA | 26 (100) | 1.62 ± 0.72 | 30 (100) | 0.45 ± 0.40 | 2.05 × 10−6 | ||
| IOP | 26 (100) | 14.5 ± 3.56 | 30 (100) | 15.0 ± 2.30 | 0.464 | mmHg | 10 to 21 |
| CRT | 5 (19.2) | 307.0 ± 67.4 | 30 (100) | 429.2 ± 88.4 | 0.015 | μm | |
| Subgroup | |||||||
| PRP (+/−) | 16 (61.5)/10 | − | |||||
| Focal PC (+/−) | 0 (0)/26 | − | |||||
| DME (+/−) | 21 (80.8)/5 | − | |||||
| TRD (+/−) | 16 (61.5)/10 | − | |||||
| VH (+/−) | 23 (88.5)/3 | − | |||||
| IVB (+/−) | 0 (0)/26 | − | |||||
| NVG (+/−) | 0 (0)/26 | − | |||||
| MH/ERM | − | 14/16 | |||||
| BMI | 26 (100) | 24.3 ± 4.76 | 30 (100) | 23.4 ± 4.24 | 0.455 | 18.5 to 25.0 | |
| SBP | 26 (100) | 134.5 ± 18.9 | 30 (100) | 139.7 ± 19.7 | 0.270 | mmHg | 140 > |
| DBP | 26 (100) | 78.6 ± 14.6 | 30 (100) | 79.2 ± 10.2 | 0.511 | mmHg | 90 > |
| PPD | 26 (100) | 55.8 ± 15.4 | 30 (100) | 60.5 ± 14.3 | 0.133 | mmHg | |
| HR | 26 (100) | 78.2 ± 10.5 | 30 (100) | 76.3 ± 14.2 | 0.376 | beats/min | 45 to 85 |
| PT-INR | 26 (100) | 0.97 ± 0.04 | 30 (100) | 1.04 ± 0.30 | 0.098 | 0.85 to 1.15 | |
| APTT | 26 (100) | 27.6 ± 2.27 | 30 (100) | 28.7 ± 3.78 | 0.364 | sec | 24 to 34 |
| FBS | 26 (100) | 159.4 ± 59.4 | 30 (100) | 102.8 ± 11.6 | 8.21 × 10−6 | mg/dL | 70 to 109 |
| HbA1c | 26 (100) | 7.40 ± 1.47 | 30 (100) | 5.98 ± 0.61 | 5.39 × 10−5 | % | 4.6 to 6.2 |
| BUN | 26 (100) | 22.2 ± 10.6 | 30 (100) | 15.1 ± 3.32 | 0.002 | mg/dL | 8 to 20 |
| Cre | 26 (100) | 1.28 ± 0.79 | 30 (100) | 0.75 ± 0.21 | 0.002 | mg/dL | 0.65 to 1.07 |
| eGFR | 26 (100) | 58.4 ± 28.9 | 30 (100) | 69.5 ± 13.1 | 0.037 | mL/min/1.73 m2 | ≥60 |
| CRP | 26 (100) | 0.13 ± 0.31 | 30 (100) | 0.08 ± 0.30 | 0.450 | mg/dL | 0.3 > |
| U-glucose | 26 (100) | 1.38 ± 1.65 | 30 (100) | 0 | 0.001 | mg/dL | 2 to 20 |
| U-protein | 26 (100) | 1.35 ± 1.16 | 30 (100) | 0.11 ± 0.28 | 6.57 × 10−5 | mg/dL | 0 to 10 |
| Category | PDR | Control | p Value | Detection Range | ||||
|---|---|---|---|---|---|---|---|---|
| n | 26 | 30 | ||||||
| Detectable | Mean ± SD | Detectable | Mean ± SD | |||||
| Rate (%) | Rate (%) | |||||||
| PDGF-BB | 8 (30.8) | 15.4 ± 42.2 | 0 | 0 | 0.054 | 1.00 | to | 52,401 |
| IL-1β | 5 (19.2) | 0.09 ± 0.21 | 0 | 0 | 0.226 | 0.06 | to | 4598 |
| IL-1ra | 17 (65.4) | 23.9 ± 32.8 | 13 (43.3) | 16.3 ± 47.1 | 0.034 | 2.52 | to | 247,147 |
| IL-2 | 1 (3.85) | 0.07 ± 0.37 | 0 | 0 | 0.531 | 0.54 | to | 26,397 |
| IL-4 | 9 (34.6) | 0.18 ± 0.32 | 16 (53.3) | 0.10 ± 0.12 | 0.518 | 0.08 | to | 4035 |
| IL-5 | 2 (7.69) | 1.24 ± 4.46 | 0 | 0 | 0.513 | 3.69 | to | 81,206 |
| IL-6 | 23 (88.5) | 133.2 ± 159.1 | 28 (93.3) | 8.53 ± 19.4 | 0.001 | 0.37 | to | 21,699 |
| IL-7 | 26 (100) | 35.9 ± 30.7 | 25 (83.3) | 17.8 ± 12.2 | 0.008 | 0.49 | to | 41,077 |
| IL-8 | 26 (100) | 95.7 ± 102.5 | 22 (73.3) | 2.57 ± 3.23 | 3.97 × 10−8 | 0.75 | to | 27,477 |
| IL-9 | 6 (23.1) | 2.01 ± 4.30 | 0 | 0 | 0.152 | 0.92 | to | 45,633 |
| IL-10 | 6 (23.1) | 2.58 ± 6.00 | 0 | 0 | 0.152 | 0.74 | to | 23,402 |
| IL-12 | 7 (26.9) | 13.2 ± 27.2 | 0 | 0 | 0.090 | 1.23 | to | 21,022 |
| IL-13 | 16 (61.5) | 11.1 ± 16.8 | 3 (10.0) | 0.11 ± 0.38 | 0.001 | 0.32 | to | 9203 |
| IL-15 | 9 (34.6) | 2.77 ± 4.09 | 0 | 0 | 0.031 | 1.62 | to | 251,038 |
| IL-17A | 4 (15.4) | 1.05 ± 2.82 | 0 | 0 | 0.330 | 1.71 | to | 41,194 |
| Eotaxin | 23 (88.5) | 17.6 ± 13.2 | 29 (96.7) | 5.85 ± 3.34 | 0.001 | 0.07 | to | 7488 |
| bFGF | 8 (30.8) | 9.86 ± 21.4 | 0 | 0 | 0.054 | 3.02 | to | 3939 |
| G-CSF | 12 (46.2) | 24.8 ± 59.2 | 3 (10.0) | 1.08 ± 3.32 | 0.019 | 1.67 | to | 138,193 |
| GM-CSF | 1 (3.85) | 0.05 ± 0.26 | 0 | 0 | 0.531 | 0.38 | to | 10,559 |
| IFN-γ | 25 (96.2) | 37.1 ± 27.7 | 27 (90.0) | 5.32 ± 4.52 | 4.80 × 10−7 | 0.74 | to | 20,941 |
| IP-10 | 26 (100) | 4568.3 ± 4746.5 | 30 (100) | 979.0 ± 810.6 | 8.70 × 10−6 | 2.75 | to | 48,834 |
| MCP-1 | 26 (100) | 717.4 ± 460.1 | 30 (100) | 209.0 ± 86.1 | 7.10 × 10−7 | 0.44 | to | 11,213 |
| MIP-1α | 26 (100) | 2.09 ± 1.65 | 21 (70.0) | 0.16 ± 0.13 | 7.27 × 10−8 | 0.05 | to | 1045 |
| MIP-1β | 26 (100) | 11.5 ± 9.16 | 27 (90.0) | 3.34 ± 2.98 | 3.99 × 10−5 | 0.29 | to | 6180 |
| RANTES | 9 (34.6) | 8.18 ± 18.0 | 1 (3.33) | 0.09 ± 0.50 | 0.042 | 1.41 | to | 7569 |
| TNFα | 21 (80.8) | 15.9 ± 21.3 | 1 (3.33) | 0.15 ± 0.80 | 3.83 × 10−6 | 2.73 | to | 63,996 |
| VEGF-A | 22 (84.6) | 870.0 ± 1680.8 | 1 (3.33) | 0.16 ± 0.89 | 1.55 × 10−6 | 2.42 | to | 178,228 |
| Control Group | |||||||||||||||||||||||
| Age | BMI | SBP | DBP | PPD | HR | PT-INR | APTT | RBG | HbA1c | eGFR | IL-1ra | IL-6 | IL-7 | IL-8 | IL-13 | Eotaxin | IFN-γ | IP-10 | MCP-1 | MIP-1β | TNFα | VEGF-A | |
| Age | − | 0.236 | 0.982 | 0.591 | 0.674 | 0.506 | 0.114 | 0.053 | 0.180 | 0.462 | 0.240 | 0.213 | 0.546 | 0.735 | 0.975 | 0.094 | 0.157 | 0.615 | 0.060 | 0.528 | 0.943 | 0.093 | 0.955 |
| BMI | − | 0.504 | 0.499 | 0.513 | 0.868 | 0.489 | 0.047 | 0.988 | 0.821 | 0.080 | 0.989 | 0.775 | 0.907 | 0.755 | 0.210 | 0.823 | 0.618 | 0.727 | 0.325 | 0.756 | 0.778 | 0.612 | |
| SBP | − | 1.14 × 10−5 | 4.65 × 10−9 | 0.001 | 0.557 | 0.367 | 0.293 | 0.129 | 0.471 | 0.565 | 0.323 | 0.734 | 0.645 | 0.980 | 0.839 | 0.796 | 0.422 | 0.855 | 0.467 | 0.866 | 0.778 | ||
| DBP | ** | − | 0.085 | 3.18 × 10−4 | 0.894 | 0.373 | 0.008 | 0.022 | 0.407 | 0.393 | 0.952 | 0.831 | 0.317 | 0.781 | 0.223 | 0.874 | 0.925 | 0.927 | 0.933 | 0.395 | 0.279 | ||
| PPD | ** | − | 0.190 | 0.842 | 0.737 | 0.941 | 0.686 | 0.281 | 0.944 | 0.196 | 0.774 | 0.923 | 0.650 | 0.722 | 0.857 | 0.292 | 0.703 | 0.338 | 0.280 | 0.735 | |||
| HR | ** | ** | − | 0.278 | 0.231 | 0.195 | 0.111 | 0.589 | 0.110 | 0.824 | 0.896 | 0.665 | 0.229 | 0.880 | 0.945 | 0.993 | 0.735 | 0.982 | 0.462 | 0.334 | |||
| PT-INR | − | 0.007 | 0.379 | 0.831 | 0.532 | 0.170 | 0.317 | 0.420 | 0.097 | 0.354 | 0.999 | 0.806 | 0.735 | 0.959 | 0.626 | 0.278 | 0.278 | ||||||
| APTT | * | ** | − | 0.800 | 0.085 | 0.887 | 0.240 | 0.144 | 0.526 | 0.862 | 0.711 | 0.404 | 0.342 | 0.316 | 0.372 | 0.509 | 0.907 | 0.348 | |||||
| RBG | ** | − | 0.109 | 0.221 | 0.763 | 0.512 | 0.853 | 0.708 | 0.888 | 0.900 | 0.127 | 0.368 | 0.305 | 0.918 | 0.151 | 0.334 | |||||||
| HbA1c | * | − | 0.902 | 0.915 | 0.722 | 0.501 | 0.429 | 0.767 | 0.678 | 0.586 | 0.313 | 0.462 | 0.085 | 0.168 | N/A | ||||||||
| eGFR | − | 0.241 | 0.845 | 0.952 | 0.427 | 0.742 | 0.597 | 0.293 | 0.899 | 0.265 | 0.218 | 0.955 | 0.280 | ||||||||||
| IL-1ra | − | 0.342 | 0.492 | 0.985 | 0.994 | 0.002 | 0.502 | 0.017 | 0.704 | 0.504 | 0.416 | 0.145 | |||||||||||
| IL-6 | − | 0.174 | 0.074 | 0.426 | 0.158 | 0.010 | 0.058 | 0.002 | 0.030 | 0.534 | 0.094 | ||||||||||||
| IL-7 | − | 0.158 | 0.091 | 0.006 | 0.011 | 5.56 × 10−4 | 0.049 | 0.250 | 0.611 | 0.305 | |||||||||||||
| IL-8 | − | 0.944 | 0.202 | 0.034 | 0.230 | 0.062 | 0.616 | 0.649 | 0.148 | ||||||||||||||
| IL-13 | − | 0.803 | 0.884 | 0.264 | 0.202 | 0.639 | 0.746 | 0.746 | |||||||||||||||
| Eotaxin | ** | ** | − | 0.133 | 7.60 × 10−7 | 0.142 | 0.035 | 0.462 | 0.121 | ||||||||||||||
| IFN-γ | ** | * | * | − | 0.040 | 2.43 × 10−11 | 0.017 | 0.534 | 0.611 | ||||||||||||||
| IP-10 | * | ** | ** | * | − | 0.020 | 0.033 | 0.612 | 0.121 | ||||||||||||||
| MCP-1 | ** | * | ** | * | − | 0.004 | 0.462 | 0.189 | |||||||||||||||
| MIP-1β | * | * | * | * | ** | − | 0.572 | 0.866 | |||||||||||||||
| TNFα | − | 0.856 | |||||||||||||||||||||
| VEGF-A | − | ||||||||||||||||||||||
| PDR group | |||||||||||||||||||||||
| Age | BMI | SBP | DBP | PPD | HR | PT-INR | APTT | RBG | HbA1c | eGFR | IL-1ra | IL-6 | IL-7 | IL-8 | IL-13 | Eotaxin | IFN-γ | IP-10 | MCP-1 | MIP-1β | TNFα | VEGF-A | |
| Age | − | 0.060 | 0.763 | 0.002 | 0.029 | 0.587 | 0.534 | 0.428 | 0.431 | 0.192 | 0.861 | 0.978 | 0.261 | 0.764 | 0.056 | 0.462 | 0.055 | 0.208 | 0.295 | 0.578 | 0.442 | 0.231 | 0.809 |
| BMI | − | 0.032 | 0.241 | 0.272 | 0.564 | 0.408 | 0.764 | 0.775 | 0.121 | 0.775 | 0.048 | 0.043 | 0.069 | 0.925 | 0.436 | 0.428 | 0.735 | 0.025 | 0.812 | 0.714 | 0.826 | 0.330 | |
| SBP | * | − | 0.006 | 0.002 | 0.149 | 0.612 | 0.404 | 0.029 | 0.561 | 0.587 | 0.959 | 0.021 | 0.022 | 0.254 | 0.031 | 0.039 | 0.170 | 0.002 | 0.187 | 0.190 | 0.250 | 0.213 | |
| DBP | ** | ** | − | 0.219 | 0.079 | 0.293 | 0.074 | 0.405 | 0.454 | 0.330 | 0.918 | 0.024 | 0.056 | 0.568 | 0.297 | 0.011 | 0.129 | 0.035 | 0.162 | 0.438 | 0.173 | 0.381 | |
| PPD | * | ** | − | 0.966 | 0.890 | 0.339 | 0.256 | 0.428 | N/A | 0.824 | 0.589 | 0.360 | 0.180 | 0.162 | 0.966 | 0.873 | 0.204 | 0.751 | 0.541 | 0.907 | 0.586 | ||
| HR | − | 0.740 | 0.789 | 0.930 | 0.160 | 0.838 | 0.876 | 0.071 | 0.640 | 0.973 | 0.822 | 0.054 | 0.675 | 0.060 | 0.883 | 0.846 | 0.188 | 0.274 | |||||
| PT-INR | − | 0.004 | 0.018 | 0.469 | 0.097 | 0.155 | 0.572 | 0.589 | 0.674 | 0.140 | 0.883 | 0.389 | 0.694 | 0.341 | 0.394 | 0.396 | 0.699 | ||||||
| APTT | ** | − | 0.232 | 0.130 | 0.125 | 0.717 | 0.999 | 0.392 | 0.739 | 0.786 | 0.477 | 0.651 | 0.365 | 0.581 | 0.507 | 0.410 | 0.478 | ||||||
| RBG | * | * | − | 0.138 | 0.746 | 0.519 | 0.849 | 0.849 | 0.527 | 0.475 | 0.690 | 0.689 | 0.660 | 0.398 | 0.525 | 0.932 | 0.474 | ||||||
| HbA1c | − | 0.532 | 0.364 | 0.636 | 0.697 | 0.369 | 0.291 | 0.614 | 0.976 | 0.694 | 0.904 | 0.987 | 0.527 | 0.158 | |||||||||
| eGFR | − | 0.301 | 0.669 | 0.648 | 0.360 | 0.217 | 0.619 | 0.613 | 0.761 | 0.966 | 0.650 | 0.900 | 0.635 | ||||||||||
| IL-1ra | * | − | 0.082 | 0.031 | 0.136 | 0.200 | 0.596 | 1.45 × 10−4 | 0.529 | 8.95 × 10−4 | 0.693 | 7.73 × 10−4 | 0.341 | ||||||||||
| IL-6 | * | * | * | − | 6.69 × 10−5 | 0.559 | 0.075 | 0.018 | 0.893 | 2.95 × 10−6 | 0.904 | 0.126 | 0.673 | 0.156 | |||||||||
| IL-7 | * | * | ** | − | 0.516 | 8.87 × 10−6 | 0.262 | 0.485 | 0.014 | 0.943 | 0.046 | 0.371 | 0.024 | ||||||||||
| IL-8 | − | 0.108 | 0.561 | 0.022 | 0.204 | 0.002 | 0.001 | 0.040 | 0.313 | ||||||||||||||
| IL-13 | * | ** | − | 0.264 | 0.921 | 0.157 | 0.577 | 0.080 | 0.805 | 0.002 | |||||||||||||
| Eotaxin | * | * | * | − | 0.225 | 0.006 | 0.846 | 0.946 | 0.045 | 0.002 | |||||||||||||
| IFN-γ | ** | * | − | 0.039 | 5.40 × 10−9 | 0.124 | 2.50 × 10−7 | 0.268 | |||||||||||||||
| IP-10 | * | ** | * | ** | * | ** | * | − | 0.021 | 0.017 | 0.159 | 0.246 | |||||||||||
| MCP-1 | ** | ** | ** | * | − | 0.003 | 7.70 × 10−5 | 0.343 | |||||||||||||||
| MIP-1β | * | ** | * | ** | − | 0.702 | 0.131 | ||||||||||||||||
| TNFα | ** | * | * | ** | ** | − | 0.049 | ||||||||||||||||
| VEGF-A | * | ** | ** | * | − | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, T.; Okazawa, R.; Nagura, K.; Someya, H.; Nishio, Y.; Enoki, T.; Ito, M.; Takeuchi, M. Association between Systemic Factors and Vitreous Fluid Cytokines in Proliferative Diabetic Retinopathy. J. Clin. Med. 2023, 12, 2354. https://doi.org/10.3390/jcm12062354
Sato T, Okazawa R, Nagura K, Someya H, Nishio Y, Enoki T, Ito M, Takeuchi M. Association between Systemic Factors and Vitreous Fluid Cytokines in Proliferative Diabetic Retinopathy. Journal of Clinical Medicine. 2023; 12(6):2354. https://doi.org/10.3390/jcm12062354
Chicago/Turabian StyleSato, Tomohito, Rina Okazawa, Koichi Nagura, Hideaki Someya, Yoshiaki Nishio, Toshio Enoki, Masataka Ito, and Masaru Takeuchi. 2023. "Association between Systemic Factors and Vitreous Fluid Cytokines in Proliferative Diabetic Retinopathy" Journal of Clinical Medicine 12, no. 6: 2354. https://doi.org/10.3390/jcm12062354
APA StyleSato, T., Okazawa, R., Nagura, K., Someya, H., Nishio, Y., Enoki, T., Ito, M., & Takeuchi, M. (2023). Association between Systemic Factors and Vitreous Fluid Cytokines in Proliferative Diabetic Retinopathy. Journal of Clinical Medicine, 12(6), 2354. https://doi.org/10.3390/jcm12062354






