Qualification of Hemophilia Treatment Centers to Enable Multi-Center Studies of Gene Expression Signatures in Blood Cells from Pediatric Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Workflow for Qualification of Local Operators in HTC Associated Qualifying Laboratories
2.2. Healthy Blood Donors
2.3. Preparation of PBMC from Peripheral Blood and In Vitro Re-Stimulation
2.4. RNA Preparation and RNA Quality Control at the Central Laboratory
2.5. cDNA Synthesis and Real-Time qPCR
2.6. Assessment of Results and Follow-On Activities
- (A)
- PBMC viability: we used a cutoff of ≥89% viable PBMC as previously suggested by Smith et al. [17] for effective separation of optimal PBMC samples from those that could not respond effectively to antigen.
- (B)
- PBMC yield: we requested a yield of ≥1 × 106/mL PBMC as this was the minimum yield required for the subsequent gene expression studies
- (C)
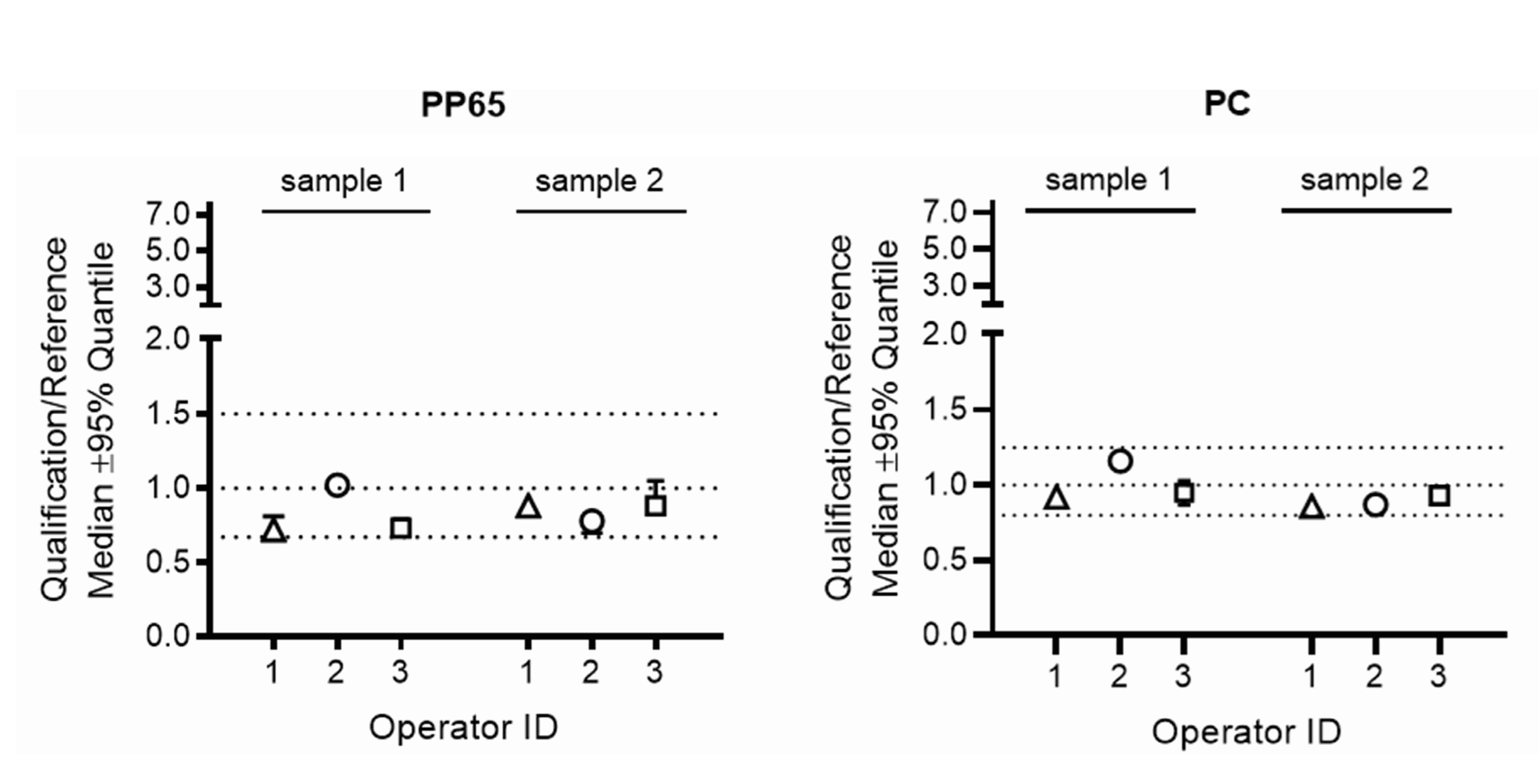
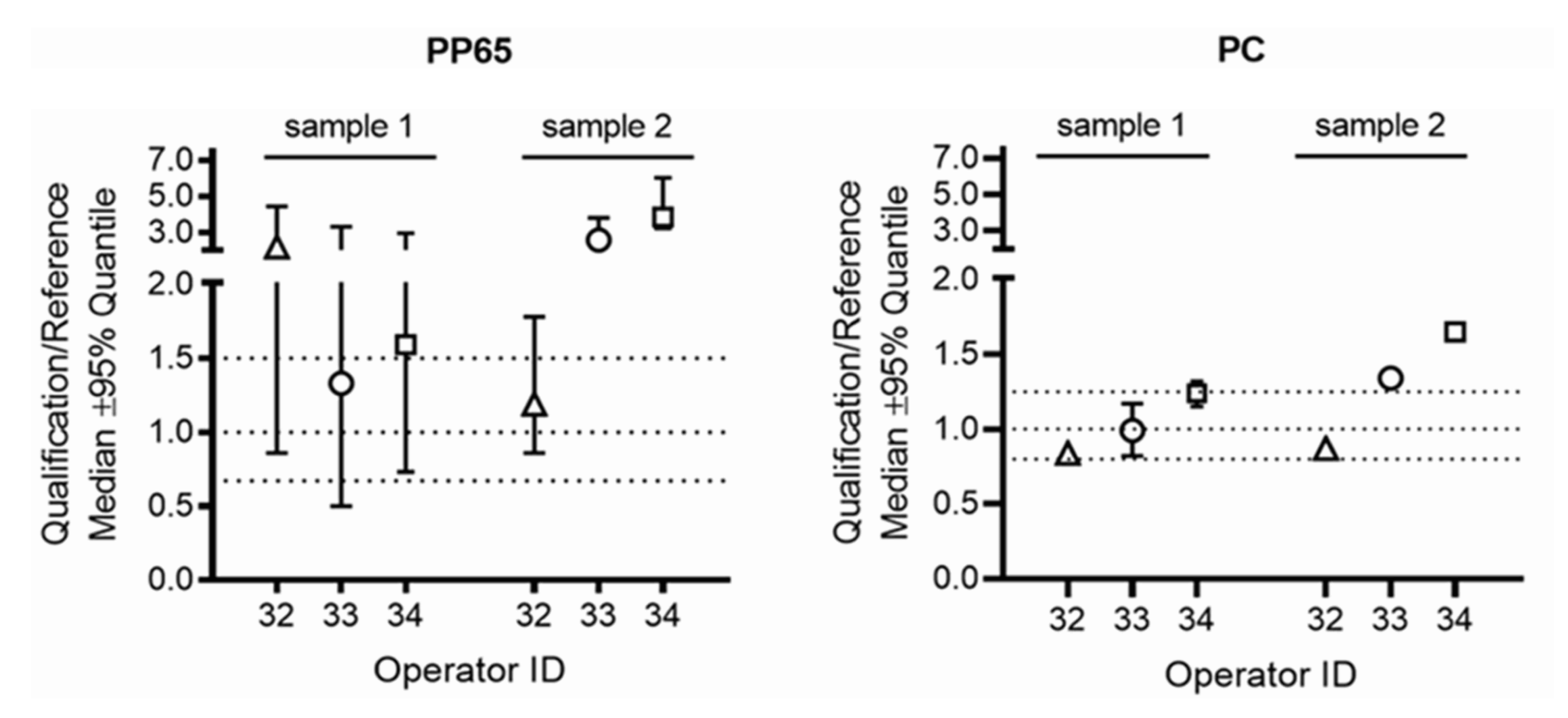
- Relative IFNG gene expression: the ratio for the relative IFNG gene expression in samples processed by the operator in the qualifying HTC laboratory over the gene expression in the corresponding samples processed by the operator in the respective reference laboratory had to be within pre-defined ranges (0.67–1.5 for PBMC stimulated with CMV-pp65 and 0.8–1.25 for PBMC stimulated with Dynabeads Human T-Activator CD3/CD28). These ranges were calculated by assessing the variability for relative gene expression when three different operators in the reference laboratories processed blood of six different healthy blood donors using the workflow implemented for the qualification of local operators in HTCs, as described above.
3. Results
3.1. Qualification of Local Operators in HTC Associated Qualifying Laboratories
3.2. PBMC Viability and Yield
3.3. Gene Expression Data
3.4. RNA Yield and RNA Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusher, J.M.; Arkin, S.; Abildgaard, C.F.; Schwartz, R.S.; Kogenate Previously Untreated Patient Study Group. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A—Safety, efficacy, and development of inhibitors. N. Engl. J. Med. 1993, 328, 453–459. [Google Scholar] [CrossRef]
- Wight, J.; Paisley, S. The epidemiology of inhibitors in haemophilia A: A systematic review. Haemophilia 2003, 9, 418–435. [Google Scholar] [CrossRef]
- Auerswald, G.; Thompson, A.A.; Recht, M.; Brown, D.; Liesner, R.; Guzman-Becerra, N.; Dyck-Jones, J.; Ewenstein, B.; Abbuehl, B. Experience of Advate rAHF-PFM in previously untreated patients and minimally treated patients with haemophilia A. Thromb. Haemost. 2012, 107, 1072–1182. [Google Scholar] [CrossRef]
- Morfini, M.; Haya, S.; Tagariello, G.; Pollmann, H.; Quintana, M.; Siegmund, B.; Stieltjes, N.; Dolan, G.; Tusell, J. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia 2007, 13, 606–612. [Google Scholar] [CrossRef]
- Mahlangu, J.; Oldenburg, J.; Callaghan, M.U.; Shima, M.; Santagostino, E.; Moore, M.; Recht, M.; Garcia, C.; Yang, R.; Lehle, M.; et al. Bleeding events and safety outcomes in persons with haemophilia A with inhibitors: A prospective, multi-centre, non-interventional study. Haemophilia 2018, 24, 921–929. [Google Scholar] [CrossRef]
- Nerich, V.; Tissot, E.; Faradji, A.; Demesmay, K.; Bertrand, M.A.; Lorenzini, J.L.; Briquel, M.E.; Pouzol, P.; Woronoff-Lemsi, M.C. Cost-of-illness study of severe haemophilia A and B in five French haemophilia treatment centres. Pharm. World Sci. 2008, 30, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Mannucci, P.M.; Garagiola, I.; El-Beshlawy, A.; Elalfy, M.; Ramanan, V.; Eshghi, P.; Hanagavadi, S.; Varadarajan, R.; Karimi, M.; et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. N. Engl. J. Med. 2016, 374, 2054–2064. [Google Scholar] [CrossRef]
- Whelan, S.F.J.; Hofbauer, C.J.; Horling, F.M.; Allacher, P.; Wolfsegger, M.J.; Oldenburg, J.; Male, C.; Windyga, J.; Tiede, A.; Schwarz, H.P.; et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood 2013, 121, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, C.J.; Whelan, S.F.J.; Hirschler, M.; Allacher, P.; Horling, F.M.; Lawo, J.P.; Oldenburg, J.; Tiede, A.; Male, C.; Windyga, J.; et al. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood 2015, 125, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, A.; Valsecchi, C.; Garagiola, I.; Palla, R.; Mannucci, P.M.; Rosendaal, F.R.; Peyvandi, F.; SIPPET Study Group. Nonneutralizing antibodies against factor VIII and risk of inhibitor development in severe hemophilia A. Blood 2017, 129, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Reipert, B.M.; Gangadharan, B.; Hofbauer, C.J.; Berg, V.; Schweiger, H.; Bowen, J.; Blatny, J.; Fijnvandraat, K.; Mullins, E.S.; Klintman, J.; et al. The prospective Hemophilia Inhibitor PUP Study reveals distinct antibody signatures prior to FVIII inhibitor development. Blood Adv. 2020, 4, 5785–5796. [Google Scholar] [CrossRef]
- Hofbauer, C.J.; Male, C.; Brown, D.; Santagostino, E.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. Exploration of biomarkers for early recognition of FVIII inhibitor development in previously untreated severe hemophilia A patients: Hemophilia Inhibitor PUP Study and beyond. J. Thromb. Haemost. 2013, 11 (Suppl. S2), 133. [Google Scholar]
- Hofbauer, C.J.; Hirschler, M.; Male, C.; Thom, K.; Scheiflinger, F.; Reipert, B.M. Down-regulation of Th17-like pro-inflammatory cytokine patterns is associated with the eradication of neutralizing antibodies against factor VIII in a patient with severe hemophilia A. In Proceedings of the VIIth World Immune Regulation Meeting (WIRM), Davos, Switzerland, 13–16 March 2013. [Google Scholar]
- Hofbauer, C.J.; Male, C.; Brown, D.; Santagostino, E.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. Immune monitoring of FVIII inhibitor development in the Hemophilia Inhibitor PUP Study (HIPS). Hemophilia 2014, 20 (Suppl. S3), 45. [Google Scholar]
- Karim, A.F.; Soltis, A.R.; Sukumar, G.; Königs, C.; Ewing, N.P.; Dalgard, C.L.; Wilkerson, M.D.; Pratt, K.P. Hemophilia a Inhibitor Subjects Show Unique PBMC Gene Expression Profiles That Include Up-Regulated Innate Immune Modulators. Front. Immunol. 2020, 11, 1219. [Google Scholar] [CrossRef]
- Kern, F.; Bunde, T.; Faulhaber, N.; Kiecker, F.; Khatamzas, E.; Rudawski, I.M.; Pruss, A.; Gratama, J.W.; Volkmer-Engert, R.; Ewert, R.; et al. Cytomegalovirus (CMV) Phosphoprotein 65 Makes a Large Contribution to Shaping the T Cell Repertoire in CMV-Exposed Individuals. J. Infect. Dis. 2002, 185, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Joseph, H.R.; Green, T.; Field, J.A.; Wooters, M.; Kaufhold, R.M.; Antonello, J.; Caulfield, M.J. Establishing acceptance criteria for cell-mediated-immunity assays using frozen Peripheral Blood Mononuclear Cells stored under optimal and suboptimal conditions. Clin. Vaccine Immunol. 2007, 14, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Song, L.Y.; Wilkening, C.; Sevin, A.; Blais, B.; Louzao, R.; Stein, D.; Defechereux, P.; Durand, D.; Riedel, E.; et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin. Vaccine Immunol. 2009, 16, 1176–1186. [Google Scholar] [CrossRef]
- Ducar, C.; Smith, D.; Pinzona, C.; Stirewalt, M.; Cooper, C.; McElratha, M.J.; Hural, J.; NIAID HIV Vaccine Trials Network. Benefits of a comprehensive quality program for cryopreserved PBMC covering 28 clinical trials sites utilizing an integrated, analytical web-based portal. J. Immunol. Methods 2014, 409, 9–20. [Google Scholar] [CrossRef]
- Dyer, W.B.; Pett, S.L.; Sullivan, J.S.; Emery, S.; Cooper, D.A.; Kelleher, A.D.; Lloyd, A.; Lewin, S.R. Substantial improvements in performance indicators achieved in a peripheral blood mononuclear cell cryopreservation quality assurance program using single donor samples. Clin. Vaccine Immunol. 2007, 14, 52–59. [Google Scholar] [CrossRef]
- Todd, C.A.; Sanchez, A.M.; Garcia, A.; Denny, T.N.; Sarzotti-Kelsoe, M. Implementation of Good Clinical Laboratory Practice (GCLP) guidelines within the External Quality Assurance Program Oversight Laboratory (EQAPOL). J. Immunol. Methods 2014, 409, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Corkum, C.P.; Ings, D.P.; Burgess, C.; Karwowska, S.; Kroll, W.; Michalak, T.I. Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube (CPT™) and standard density gradient. BMC Immunol. 2015, 16, 48. [Google Scholar] [CrossRef]
- Hønge, B.L.; Petersen, M.S.; Olesen, R.; Møller, B.K.; Erikstrup, C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLoS ONE 2017, 12, e0187440. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Dela Rosa, C.; Goodell, V.; Kuan, L.Y.Y.; Chang, J.C.C.; Kuus-Reichel, K.; Clay, T.M.; Lyerly, K.; Bhatia, S.; Ghanekar, S.A.; et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J. Immunol. Methods 2006, 308, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Schürch, C.M.; Noble, K.; Kim, K.; Krutzik, P.O.; O’Donnell, E.; Tuig, J.V.; Nolan, G.P.; McIlwain, D.R. Functional comparison of PBMCs isolated by Cell Preparation Tubes (CPT) vs. Lymphoprep Tubes. BMC Immunol. 2020, 21, 15. [Google Scholar] [CrossRef]
- Wills, M.R.; Carmichael, A.J.; Mynard, K.; Jin, X.; Weekes, M.P.; Plachter, B.; Sissons, J.G. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: Frequency, specificity, and T cell receptor usage of pp65-specific CTL. J. Virol. 1996, 70, 7569–7579. [Google Scholar] [CrossRef] [PubMed]
- Beninga, J.; Kropff, B.; Mach, M. Comparative analysis of fourteen individual human cytomegalovirus proteins for helper T cell response. J. Gen. Virol. 1995, 76, 153–160. [Google Scholar] [CrossRef]

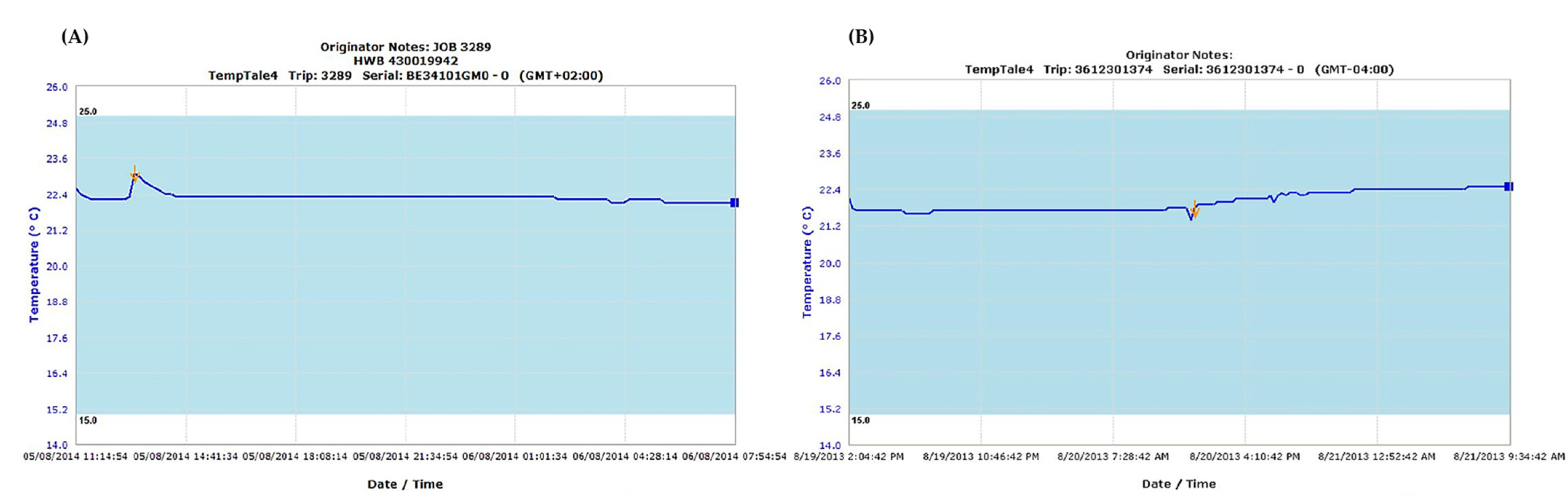
| Hemophilia Treatment Center | Associated Qualifying Laboratory | Reference Laboratory |
|---|---|---|
| University Texas, Houston, TX, USA | University Texas, Houston, TX, USA | University Texas, Houston, TX, USA |
| Baylor College of Medicine, Houston, TX, USA | University Texas, Houston, TX, USA | University Texas, Houston, TX, USA |
| Cincinnati Children’s Hospital, Cincinnati, OH, USA | Cincinnati Children’s Hospital, Cincinnati, OH, USA | University Texas, Houston, TX, USA |
| Emory University, Atlanta, GA, USA | Emory University, Atlanta, GA, USA | University Texas, Houston, TX, USA |
| Indiana Hemophilia and Thrombosis Center, Indianapolis, IN, USA | Cincinnati Children’s Hospital, Cincinnati, OH, USA | University Texas, Houston, TX, USA |
| Miami University, Miami, FL, USA | Miami University, Miami, FL, USA | University Texas, Houston, TX, USA |
| Oregon Health & Science University, Portland, OR, USA | Oregon Health & Science University, Portland, OR, USA | University Texas, Houston, TX, USA |
| Tulane University, New Orleans, LA, USA | Tulane University, New Orleans, LA, USA | University Texas, Houston, TX, USA |
| University of Iowa, Iowa City, IA, USA | University of Iowa, Iowa City, IA, USA | University Texas, Houston, TX, USA |
| University of Kentucky, Lexington, KY | Cincinnati Children’s Hospital, Cincinnati, OH, USA | University Texas, Houston, TX, USA |
| University North Carolina, Chapel Hill, NC, USA | University North Carolina, Chapel Hill, NC, USA | University Texas, Houston, TX, USA, USA |
| University of Utah, Salt Lake City, UT, USA | University of Utah, Salt Lake City, UT, USA | University Texas, Houston, TX, USA |
| Weill Cornell Medicine, New York City, NY, USA | Weill Cornell Medicine, New York City, NY, USA | University Texas, Houston, TX, USA |
| University Pittsburgh Medical Center, Pittsburgh, PA, USA | University Pittsburgh Medical Center, Pittsburgh, PA, USA | University Texas, Houston, TX, USA |
| North Texas Comprehensive Hemophilia Center, Children’s Medical Center, Dallas, TX, USA | North Texas Comprehensive Hemophilia Center, Children’s Medical Center, Dallas, TX, USA | University Texas, Houston, TX, USA |
| University of Amsterdam, Amsterdam, The Netherlands | Sanquin Research, Amsterdam, The Netherlands | Baxalta Innovations GmbH, Vienna, Austria |
| Lund University, Malmö, Sweden | Lund University, Malmö, Sweden | Baxalta Innovations GmbH, Vienna, Austria |
| University of Milan, Milan, Italy | University of Milan, Milan, Italy | Baxalta Innovations GmbH, Vienna, Austria |
| Masaryk University, Brno, Czech Republic | Baxalta Innovations GmbH, Vienna, Austria | Baxalta Innovations GmbH, Vienna, Austria |
| Medical University Vienna, Vienna, Austria | Baxalta Innovations GmbH, Vienna, Austria | Baxalta Innovations GmbH, Vienna, Austria |
| Operator ID | Retraining/Failed Qualification | Reason for Failure | Resolution |
|---|---|---|---|
| 8, 9, 24, 25, 32, 33, 34 | PBMC yield | Low PBMC yields caused by suboptimal recovery of PBMC from CPT tubes, wrong dilution of cells prior to cell counting or failure in calculating cell concentrations based on the counting result | Retraining on SOPs, all operators passed on second attempt |
| 14, 32 | PBMC cell viability | Faulty viability assessment | Retraining on SOPs, all operators passed on second attempt |
| 32, 33, 34 | IFNG gene expression | IFNG gene expression ratios compared to reference lab were not acceptable | Retraining on SOPs, all operators passed on second attempt |
| PASS | FAIL | |||
|---|---|---|---|---|
| HTC Operators | Reference Lab | HTC Operators | Reference Lab | |
| PBMC viability [%] | 97.7 | 98.0 | 60.0 | 97.0 |
| Median [min, max] | [89.0, 100.0] | [92.0, 100.0] | [52.0, 66.0] | [97.0, 98.0] |
| PBMC yield [×106/mL] | 4.8 | 5.0 | 0.2 | 3.6 |
| Median [min, max] | [2.0, 10.0] | [1.3, 12.5] | [0.1, 0.8] | [2.2, 7.0] |
| PBMC Stimulus | RNA Conc [ng/mL] | RIN | ||
|---|---|---|---|---|
| HTC Operators | Reference Lab | HTC Operators | Reference Lab | |
| CMV pp65 | 13.0 [1.1, 37] | 12.0 [6.0, 32.0] | 9.4 [8.1, 10.0] | 9.3 [8.4, 10.0] |
| n | 39 | 39 | 35 | 36 |
| Negative control | 15.0 [1.0, 63.0] | 12.0 [0.2, 40.0] | 9.4 [8.0, 10.0] | 9.5 [7.6, 10.0] |
| n | 39 | 39 | 35 | 36 |
| Positive control | 14.0 [1.0, 52.0] | 11.0 [1.1, 41.0] | 9.5 [7.7, 10.0] | 9.4 [8.0, 10.0] |
| n | 39 | 39 | 33 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reipert, B.M.; Hofbauer, C.J.; Gangadharan, B.; Berg, V.; Donnachie, E.; Meeks, S.; Mancuso, M.E.; Bowen, J.; Brown, D.L. Qualification of Hemophilia Treatment Centers to Enable Multi-Center Studies of Gene Expression Signatures in Blood Cells from Pediatric Patients. J. Clin. Med. 2023, 12, 2080. https://doi.org/10.3390/jcm12052080
Reipert BM, Hofbauer CJ, Gangadharan B, Berg V, Donnachie E, Meeks S, Mancuso ME, Bowen J, Brown DL. Qualification of Hemophilia Treatment Centers to Enable Multi-Center Studies of Gene Expression Signatures in Blood Cells from Pediatric Patients. Journal of Clinical Medicine. 2023; 12(5):2080. https://doi.org/10.3390/jcm12052080
Chicago/Turabian StyleReipert, Birgit M., Christoph J. Hofbauer, Bagirath Gangadharan, Verena Berg, Elizabeth Donnachie, Shannon Meeks, Maria Elisa Mancuso, Joel Bowen, and Deborah L. Brown. 2023. "Qualification of Hemophilia Treatment Centers to Enable Multi-Center Studies of Gene Expression Signatures in Blood Cells from Pediatric Patients" Journal of Clinical Medicine 12, no. 5: 2080. https://doi.org/10.3390/jcm12052080
APA StyleReipert, B. M., Hofbauer, C. J., Gangadharan, B., Berg, V., Donnachie, E., Meeks, S., Mancuso, M. E., Bowen, J., & Brown, D. L. (2023). Qualification of Hemophilia Treatment Centers to Enable Multi-Center Studies of Gene Expression Signatures in Blood Cells from Pediatric Patients. Journal of Clinical Medicine, 12(5), 2080. https://doi.org/10.3390/jcm12052080






