Management of Stable Vitiligo—A Review of the Surgical Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results
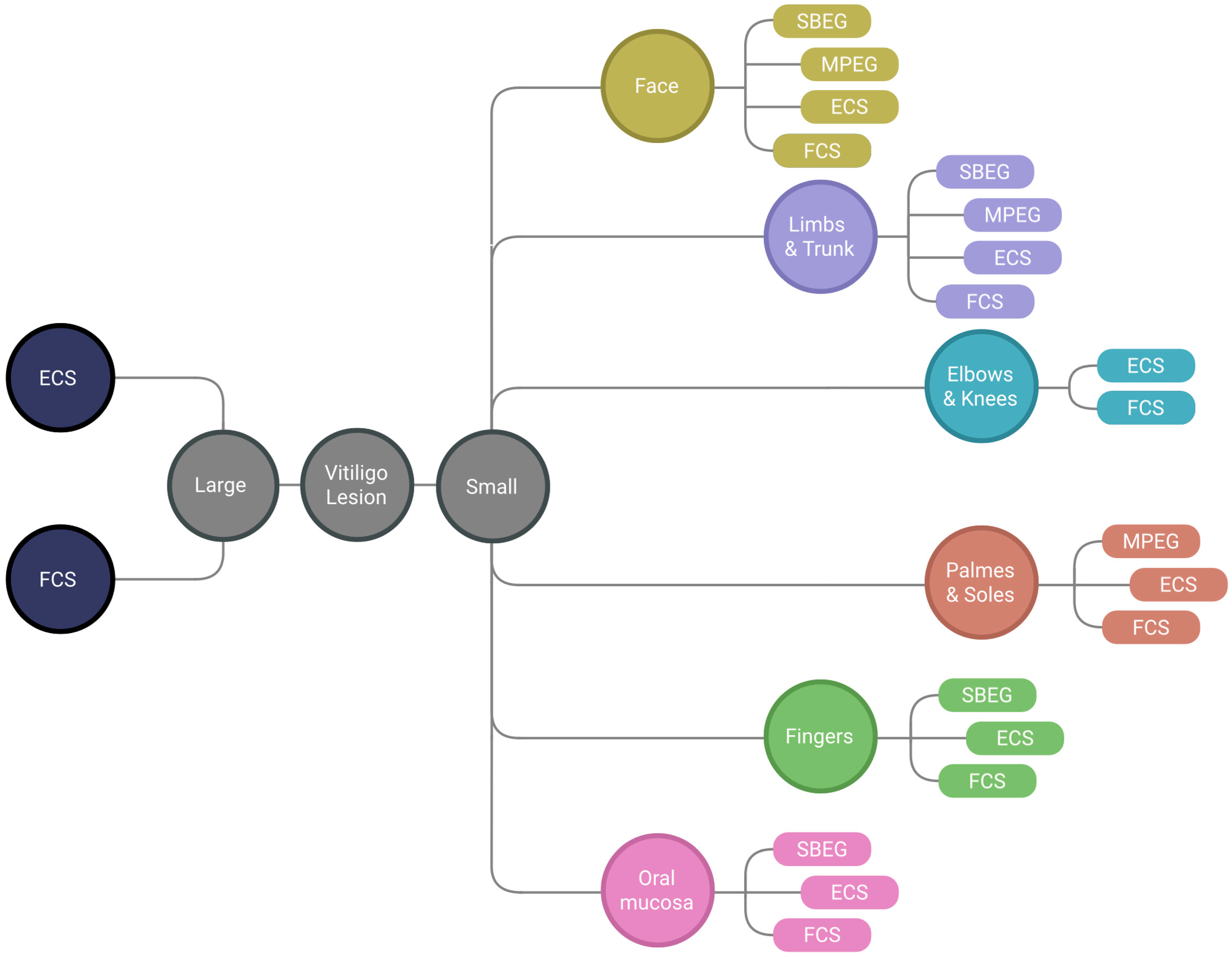
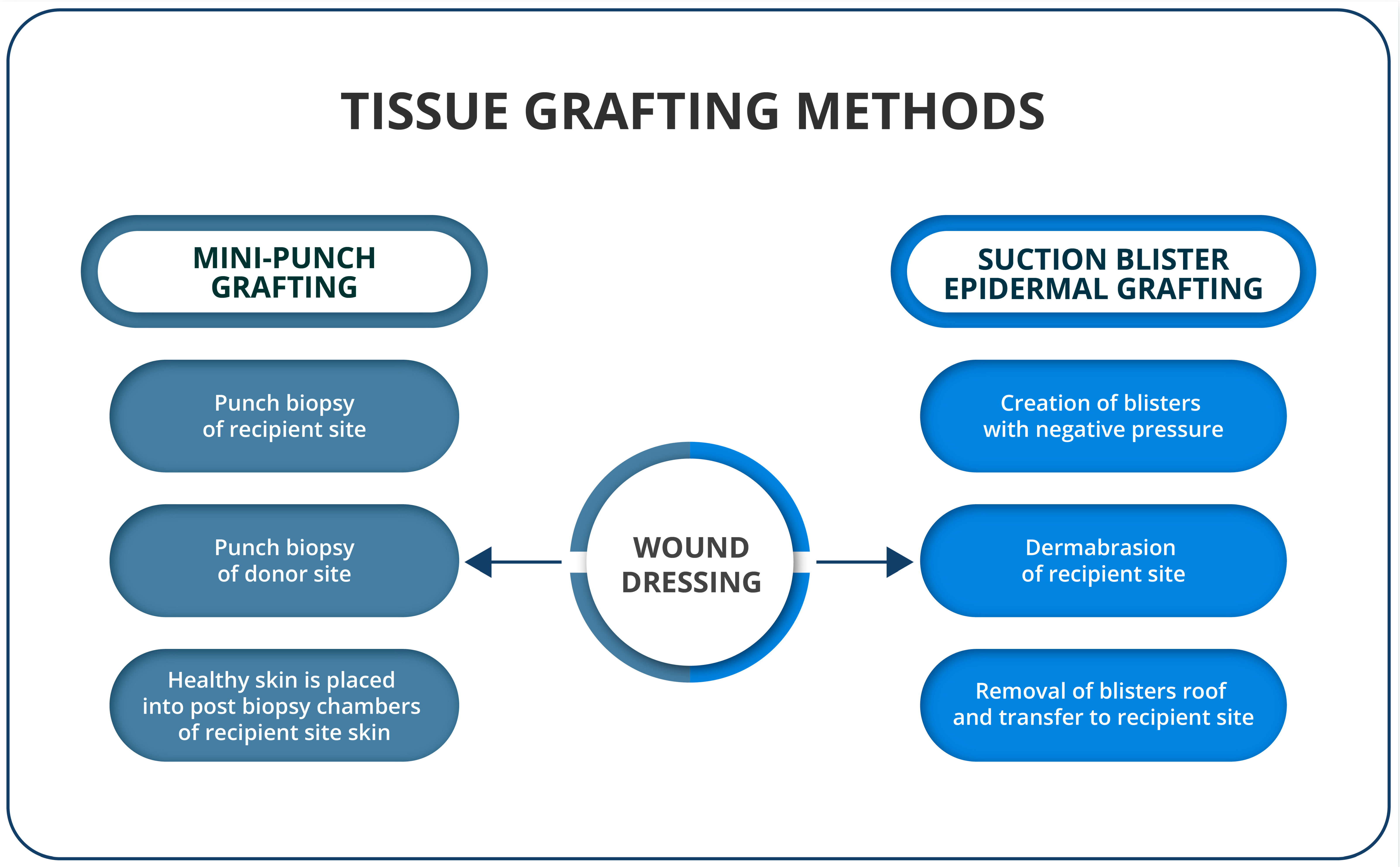
3.1. Tissue Grafting Methods
3.1.1. Suction Blister Epidermal Grafting (SBEG)
3.1.2. Mini-Punch Grafting (MPG)
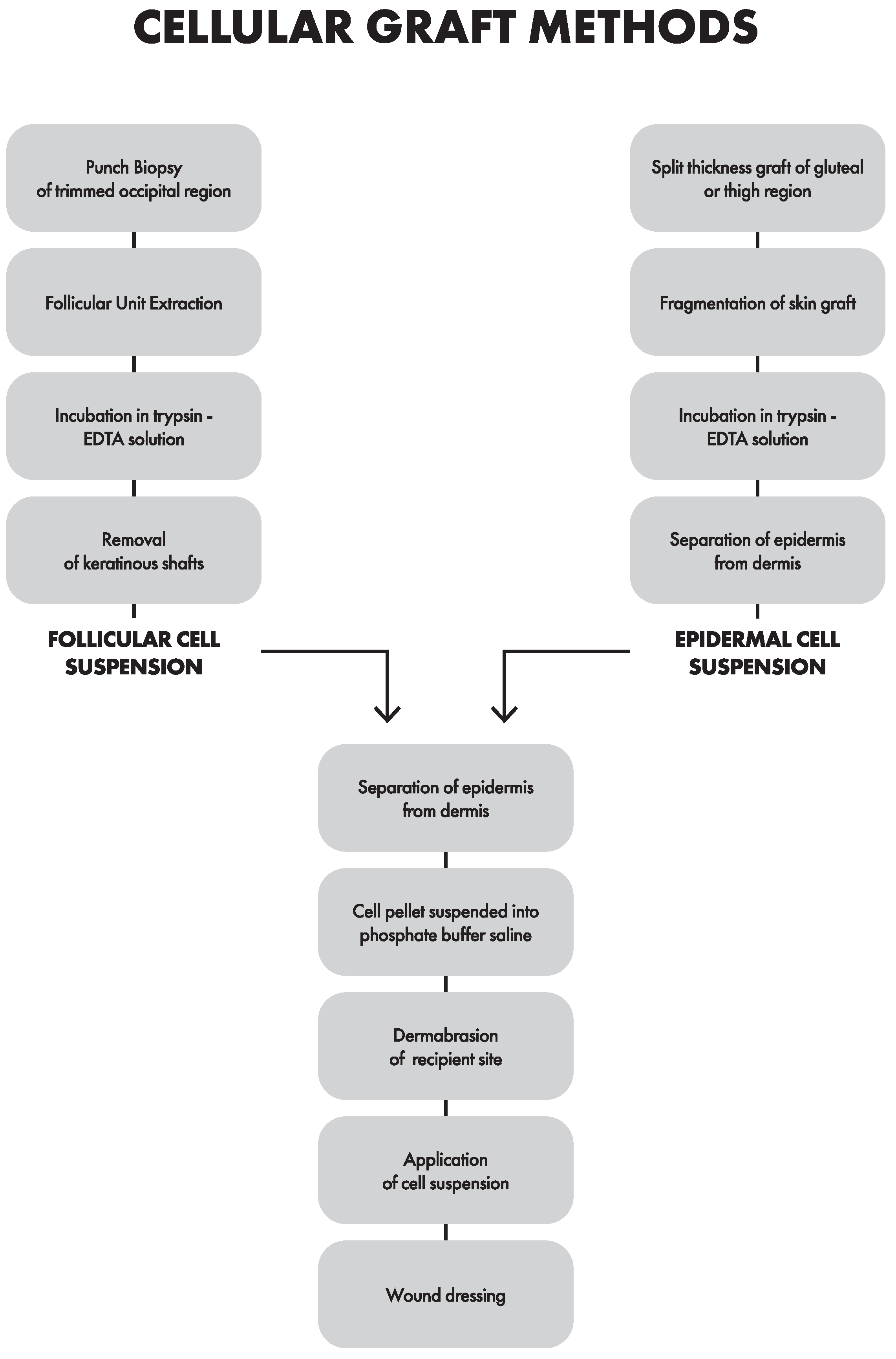
3.2. Cellular Grafting Methods
3.2.1. Epidermal Cell Suspension (ECS)
3.2.2. Follicular Cell Suspension (FCS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joge, R.R.; Kathane, P.U.; Joshi, S.H. Vitiligo: A Narrative Review Article. Cureus 2022, 14, e29307. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet 2015, 386, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Lim, H.W.; Suzuki, T.; Katayama, I.; Hamzavi, I.; Lan, C.C.E.; Goh, B.K.; Anbar, T.; Silva de Castro, C.; Lee, A.Y.; et al. Revised Classification/Nomenclature of Vitiligo and Related Issues: The Vitiligo Global Issues Consensus Conference: Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012, 25, E1–E13. [Google Scholar] [CrossRef]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Prim. 2015, 1, 15011. [Google Scholar] [CrossRef]
- Marchioro, H.Z.; Silva de Castro, C.C.; Fava, V.M.; Sakiyama, P.H.; Dellatorre, G.; Miot, H.A. Update on the Pathogenesis of Vitiligo. An. Bras. Dermatol. 2022, 97, 478–490. [Google Scholar] [CrossRef]
- Lyu, C.; Sun, Y. Immunometabolism in the Pathogenesis of Vitiligo. Front. Immunol. 2022, 13, 1055958. [Google Scholar] [CrossRef]
- Czajkowski, R.; Męcińska-Jundziłł, K. Current Aspects of Vitiligo Genetics. Pdia 2014, 4, 247–255. [Google Scholar] [CrossRef]
- Nath, S.K.; Majumder, P.P.; Nordlund, J.J. Genetic Epidemiology of Vitiligo: Multilocus Recessivity Cross-Validated. Am. J. Hum. Genet. 1994, 55, 981–990. [Google Scholar]
- Hirschhorn, J.N.; Daly, M.J. Genome-Wide Association Studies for Common Diseases and Complex Traits. Nat. Rev. Genet. 2005, 6, 95–108. [Google Scholar] [CrossRef]
- Spritz, R.A.; Andersen, G.H.L. Genetics of Vitiligo. Dermatol. Clin. 2017, 35, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, J.F.; Veillette, A. Association of Inhibitory Tyrosine Protein Kinase P50csk with Protein Tyrosine Phosphatase PEP in T Cells and Other Hemopoietic Cells. EMBO J. 1996, 15, 4909–4918. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.A.; Jordan, C.; Ho, T.; Upadhyay, P.R.; Fleischer, A.; Hamzavi, I. The Enigma and Challenges of Vitiligo Pathophysiology and Treatment. Pigment Cell Melanoma Res. 2020, 33, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Li, C. Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. 2019, 25, 1017–1023. [Google Scholar] [CrossRef]
- Khaitan, B.K.; Sindhuja, T. Autoimmunity in Vitiligo: Therapeutic Implications and Opportunities. Autoimmun. Rev. 2022, 21, 102932. [Google Scholar] [CrossRef]
- Benzekri, L.; Gauthier, Y. Clinical Markers of Vitiligo Activity. J. Am. Acad. Dermatol. 2017, 76, 856–862. [Google Scholar] [CrossRef]
- Ramos, M.G.; Ramos, D.G.; Ramos, C.G. Evaluation of Treatment Response to Autologous Transplantation of Noncultured Melanocyte/Keratinocyte Cell Suspension in Patients with Stable Vitiligo. An. Bras. Dermatol. 2017, 92, 312–318. [Google Scholar] [CrossRef]
- Taieb, A.; Alomar, A.; Böhm, M.; Dell’Anna, M.L.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.J.; Jouary, T.; et al. Guidelines for the Management of Vitiligo: The European Dermatology Forum Consensus: EDF Vitiligo Guidelines. Br. J. Dermatol. 2013, 168, 5–19. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, Y. Advances in Vitiligo: Update on Therapeutic Targets. Front. Immunol. 2022, 13, 986918. [Google Scholar] [CrossRef]
- Daniel, B.S.; Wittal, R. Vitiligo Treatment Update: Therapies for Vitiligo. Australas. J. Dermatol. 2015, 56, 85–92. [Google Scholar] [CrossRef]
- Speeckaert, R.; van Geel, N. Vitiligo: An Update on Pathophysiology and Treatment Options. Am. J. Clin. Dermatol. 2017, 18, 733–744. [Google Scholar] [CrossRef]
- Frączek, A.; Kasprowicz-Furmańczyk, M.; Placek, W.; Owczarczyk-Saczonek, A. Surgical Treatment of Vitiligo. Int. J. Environ. Res. Public Health 2022, 19, 4812. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, A.; Felsten, L.M.; Daly, M.; Petronic-Rosic, V. Vitiligo: A Comprehensive Overview. J. Am. Acad. Dermatol. 2011, 65, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, V.; Atkar, R.; Batchelor, J.; McDonald, B.; Novakovic, L.; Patel, J.V.; Ravenscroft, J.; Rush, E.; Shah, D.; Shah, R.; et al. British Association of Dermatologists Guidelines for the Management of People with Vitiligo 2021. Br. J. Dermatol. 2022, 186, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Placek, W.; Flisiak, I.; Krasowska, D.; Maj, J.; Marchlewicz, M.; Reich, A.; Wolska, H.; Rudnicka, L. Vitiligo. Diagnostic and Therapeutic Recommendations of the Polish Dermatological Society. Dermatol. Rev./Prz. Dermatol. 2019, 106, 1–15. [Google Scholar] [CrossRef]
- Craiglow, B.G.; King, B.A. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015, 151, 1110. [Google Scholar] [CrossRef]
- Abduelmula, A.; Mufti, A.; Chong, D.H.; Sood, S.; Sachdeva, M.; Yeung, J. Management of Vitiligo with Topical Janus Tyrosine Kinase Inhibitor Therapy: An Evidence-Based Review. JAAD Int. 2022, 9, 156–158. [Google Scholar] [CrossRef]
- White, C.; Miller, R. A Literature Review Investigating the Use of Topical Janus Kinase Inhibitors for the Treatment of Vitiligo. J. Clin. Aesthet. Dermatol. 2022, 15, 20–25. [Google Scholar]
- Harris, J.E.; Rashighi, M.; Nguyen, N.; Jabbari, A.; Ulerio, G.; Clynes, R.; Christiano, A.M.; Mackay-Wiggan, J. Rapid Skin Repigmentation on Oral Ruxolitinib in a Patient with Coexistent Vitiligo and Alopecia Areata (AA). J. Am. Acad. Dermatol. 2016, 74, 370–371. [Google Scholar] [CrossRef]
- FDA Approves Topical Treatment Addressing Repigmentation in Vitiligo in Patients Aged 12 and Older. FDA 2022.
- Kadekaro, A.L.; Leachman, S.; Kavanagh, R.J.; Swope, V.; Cassidy, P.; Supp, D.; Sartor, M.; Schwemberger, S.; Babcock, G.; Wakamatsu, K.; et al. Melanocortin 1 Receptor Genotype: An Important Determinant of the Damage Response of Melanocytes to Ultraviolet Radiation. FASEB J. 2010, 24, 3850–3860. [Google Scholar] [CrossRef] [PubMed]
- Bertolani, M.; Rodighiero, E.; de Felici del Giudice, M.B.; Lotti, T.; Feliciani, C.; Satolli, F. Vitiligo: What’s Old, What’s New. Dermatol. Rep. 2021, 13, 9142. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Papaccio, F.; Picardo, M. Regenerative Medicine-Based Treatment for Vitiligo: An Overview. Biomedicines 2022, 10, 2744. [Google Scholar] [CrossRef]
- Taneja, N.; Sreenivas, V.; Sahni, K.; Gupta, V.; Ramam, M. Disease Stability in Segmental and Non-Segmental Vitiligo. Indian Dermatol. Online J. 2022, 13, 60. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, P.; Khunger, N. Clinicopathologic Analysis of Stable and Unstable Vitiligo: A Study of 66 Cases. Am. J. Dermatopathol. 2016, 38, 608–613. [Google Scholar] [CrossRef]
- Thakur, V.; Bishnoi, A.; Vinay, K.; Kumaran, S.M.; Parsad, D. Vitiligo: Translational Research and Effective Therapeutic Strategies. Pigment Cell Melanoma Res. 2021, 34, 814–826. [Google Scholar] [CrossRef]
- Abdallah, M.; Lotfi, R.; Othman, W.; Galal, R. Assessment of Tissue FoxP3+, CD4+ and CD8+ T-Cells in Active and Stable Nonsegmental Vitiligo. Int. J. Dermatol. 2014, 53, 940–946. [Google Scholar] [CrossRef]
- Geel, N.; Passeron, T.; Wolkerstorfer, A.; Speeckaert, R.; Ezzedine, K. Reliability and Validity of the Vitiligo Signs of Activity Score (VSAS). Br. J. Dermatol. 2020, 183, 883–890. [Google Scholar] [CrossRef]
- Lin, F.; Hu, W.; Xu, W.; Zhou, M.; Xu, A. CXCL9 as a Key Biomarker of Vitiligo Activity and Prediction of the Success of Cultured Melanocyte Transplantation. Sci. Rep. 2021, 11, 18298. [Google Scholar] [CrossRef]
- Shabaka, F.H.; Rashed, L.A.; Said, M.; Ibrahim, L. Sensitivity of Serum S100B Protein as a Disease Activity Marker in Egyptian Patients with Vitiligo (Case-Control Study). Arch. Physiol. Biochem. 2022, 128, 930–937. [Google Scholar] [CrossRef]
- Speeckaert, R.; Speeckaert, M.; De Schepper, S.; van Geel, N. Biomarkers of Disease Activity in Vitiligo: A Systematic Review. Autoimmun. Rev. 2017, 16, 937–945. [Google Scholar] [CrossRef] [PubMed]
- McCrary, M.R.; Gibbs, D.C.; Alharthi, M.; Krueger, L.D. Utilization of Our Toolkit: A Systematic Review and Meta-Analysis of Surgical Therapies in Vitiligo Treatment. Dermatol. Surg. 2022, 48, 815–821. [Google Scholar] [CrossRef]
- Anbar, T.S.; El- Ammawi, T.S.; Mohammed, S.S.; Abdel-Rahman, A.T. Noncultured Epidermal Suspensions Obtained from Partial-Thickness Epidermal Cuts and Suction Blister Roofs for Vitiligo Treatment: A Prospective Comparative Study. J. Cosmet. Dermatol. 2020, 19, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Thakur, V.; Vinay, K. Transplantation of Non-cultured Epidermal Cell Suspension in a Case of Segmental Vitiligo with Post-treatment Scarring. J. Cosmet. Dermatol. 2022, 21, 2680–2681. [Google Scholar] [CrossRef] [PubMed]
- Kar, B.R.; Raj, C. Suction Blister Epidermal Grafting for Vitiligo Involving Angles of Lip: Experience of 112 Patients. J. Cutan. Aesthet. Surg. 2018, 11, 13. [Google Scholar] [CrossRef]
- Lahiri, K. Evolution and Evaluation of Autologous Mini Punch Grafting in Vitiligo. Indian J. Dermatol. 2009, 54, 159. [Google Scholar] [CrossRef]
- Bhingradia, Y.M.; Patel, N.K. Pinhole Technique for Cobblestoning in Patients Post Mini-Punch Grafting for Stable Vitiligo. IJDVL 2021, 87, 861–863. [Google Scholar] [CrossRef]
- Singh, B.S.T.P.; Agrawal, I.; Kar, B.R. Use of Disposable Syringe for Transfer of Graft in Suction Blister Epidermal Grafting. J. Am. Acad. Dermatol. 2022, 86, e43–e44. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Mohammad, T.F.; Hamzavi, I.H. Vitiligo Surgery: Shuffling Melanocytes. J. Investig. Dermatol. Symp. Proc. 2017, 18, S34–S37. [Google Scholar] [CrossRef]
- Ghasemi, M.; Bajouri, A.; Shafiiyan, S.; Aghdami, N. Hair Follicle as a Source of Pigment-Producing Cells for Treatment of Vitiligo: An Alternative to Epidermis? Tissue Eng. Regen. Med. 2020, 17, 815–827. [Google Scholar] [CrossRef]
- Falabella, R. Epidermal Grafting. An Original Technique and Its Application in Achromic and Granulating Areas. Arch. Dermatol. 1971, 104, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, M.; Li, M.; Du, J. A Self-Controlled Comparative Study of Mini Punch Graft versus Suction Blister Epidermal Graft in the Treatment of Stable Vitiligo. J. Dermatol. Treat. 2021, 32, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T. Surgical Procedures and Innovative Approaches for Vitiligo Regenerative Treatment and Melanocytorrhagy. J. Dermatol. 2022, 49, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.-R.; Wang, C.-H.; Lin, Y.-J.; Huang, Y.-H.; Chang, Y.-C.; Chung, W.-H.; Ng, C.Y. A Comparative Study of Suction Blister Epidermal Grafting and Automated Blister Epidermal Micrograft in Stable Vitiligo. Sci. Rep. 2022, 12, 393. [Google Scholar] [CrossRef]
- Dalla, A.; Parsad, D.; Vinay, K.; Thakur, V.; Sendhil Kumaran, M. A Prospective Study to Assess the Efficacy of Various Surgical Modalities in Treatment of Stable Vitiligo Patches over Resistant Sites. Int. J. Dermatol. 2020, 59, 837–842. [Google Scholar] [CrossRef]
- Patel, N.S.; Paghdal, K.V.; Cohen, G.F. Advanced Treatment Modalities for Vitiligo. Dermatol. Surg. 2012, 38, 381–391. [Google Scholar] [CrossRef]
- Khalili, M.; Amiri, R.; Mohammadi, S.; Iranmanesh, B.; Aflatoonian, M. Efficacy and Safety of Traditional and Surgical Treatment Modalities in Segmental Vitiligo: A Review Article. J. Cosmet. Dermatol. 2022, 21, 2360–2373. [Google Scholar] [CrossRef]
- Anbar, T.S.; El-Fakahany, H.M.; El-khayyat, M.A.; Abdel-Rahman, A.T.; Saad, E.K. Factors Affecting the Outcome of the Suction Blisters Using Two Different Harvesting Techniques in Vitiligo Patients. J. Cosmet. Dermatol. 2020, 19, 1723–1729. [Google Scholar] [CrossRef]
- Griffith, J.L.; Al-Jamal, M.; Hamzavi, I.H. Classification of Surgical Therapies in Vitiligo. In Vitiligo; Gupta, S., Olsson, M.J., Parsad, D., Lim, H.W., van Geel, N., Pandya, A.G., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2018; pp. 193–207. ISBN 978-1-118-93730-3. [Google Scholar]
- Iwanowski, T.; Szlązak, P.; Rustowska, A.; Sokołowska-Wojdyło, M. Efficacy of Suction Blister Epidermal Grafting with Concomitant Phototherapy in Vitiligo Treatment. Pdia 2018, 35, 592–598. [Google Scholar] [CrossRef]
- Angeletti, F.; Kaufmann, R. Suction Blister Epidermal Graft (SBEG)—An Easy Way to Apply This Method. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 468–471. [Google Scholar] [CrossRef]
- Dellatorre, G.; Bertolini, W.; Castro, C.C.S. de Optimizing Suction Blister Epidermal Graft Technique in the Surgical Treatment of Vitiligo. An. Bras. Dermatol. 2017, 92, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.J.; Bae, J.M.; Lee, R.W.; Kim, S.H.; Parsad, D.; Pourang, A.; Hamzavi, I.; Shourick, J.; Ezzedine, K. Surgical Interventions for Patients With Vitiligo: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2021, 157, 307. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.F.; Hamzavi, I.H. Surgical Therapies for Vitiligo. Dermatol. Clin. 2017, 35, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.A.M.; Fezeaa, T.A.; El Khazragy, N.; Soltan, M.Y. Effect of Platelet-rich Plasma on the Outcome of Mini-punch Grafting Procedure in Localized Stable Vitiligo: Clinical Evaluation and Relation to Lesional Basic Fibroblast Growth Factor. Dermatol. Ther. 2021, 34, e14738. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Abdel-Raouf, H.; Al-Khayyat, M.; Abdel-Azeem, E.; Hanna, M.R.; Cota, C.; Picardo, M.; Anbar, T.S. Vitiligo: Characterization of Melanocytes in Repigmented Skin after Punch Grafting. J. Eur. Acad. Dermatol. Venereol 2015, 29, 581–590. [Google Scholar] [CrossRef]
- Lahiri, K.; Malakar, S.; Sarma, N.; Banerjee, U. Repigmentation of Vitiligo with Punch Grafting and Narrow-Band UV-B (311 Nm)—A Prospective Study. Int. J. Dermatol. 2006, 45, 649–655. [Google Scholar] [CrossRef]
- Hirobe, T.; Enami, H. Excellent Color-Matched Repigmentation of Human Vitiligo Can Be Obtained by Mini-Punch Grafting Using a Machine in Combination with Ultraviolet Therapy. Dermatol. Sin. 2018, 36, 203–206. [Google Scholar] [CrossRef]
- Chavez-Alvarez, S.; Herz-Ruelas, M.; Ocampo-Candiani, J.; Villarreal-Martinez, A.; Vazquez-Martinez, O. Stable Segmental Vitiligo Treated with Punch Mini-grafts and Narrow Band UVB Phototherapy. Australas. J. Dermatol. 2020, 61, 83–85. [Google Scholar] [CrossRef]
- Martin, K.R. Silicon: The Health Benefits of a Metalloid. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; Volume 13, pp. 451–473. ISBN 978-94-007-7499-5. [Google Scholar]
- Anbar, T.; Abd El Raheem, T.; Bassiouny, D.A.; Fawzy, M.M.; El Maadawi, Z.; Farouk, N.; Hassan, M. Value of Silicone Gel in Prevention of Cobblestoning Following Punch Minigrafting in Vitiligo. J. Dermatol. Treat. 2022, 33, 306–313. [Google Scholar] [CrossRef]
- Ragab, M.; El Zagh, O.; Farid, C. Transverse Needling After Autologous Mini-Punch Grafts Improves Repigmentation in Stable Non-Segmental Vitiligo. CCID 2021, 14, 827–835. [Google Scholar] [CrossRef]
- Gauthier, Y.; Surleve-Bazeille, J.-E. Autologous Grafting with Noncultured Melanocytes: A Simplified Method for Treatment of Depigmented Lesions. J. Am. Acad. Dermatol. 1992, 26, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Vachiramon, V.; Triyangkulsri, K.; Saengwimol, D.; Chanprapaph, K. Outcome of Repeated Use of Donor Site for Noncultured Epidermal Cellular Grafting in Stable Vitiligo: A Retrospective Study. BioMed Res. Int. 2019, 2019, 7623607. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Hussein, T.; Shakshouk, H.R. Noncultured Extracted Hair Follicle Outer Root Sheath Cell Suspension versus Noncultured Epidermal Cell Suspension in the Treatment of Stable Vitiligo. J. Cutan. Aesthet. Surg. 2019, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, Y.M.; Abd Elazim, N.E.; Abdel-Motaleb, A.A.; Mohammed, R.A.A.; Tohamy, A.M.A. The Effect of NB-UVB on Noncultured Melanocyte and Keratinocyte Transplantation in Treatment of Generalized Vitiligo Using Two Different Donor-to-Recipient Ratios. J. Cosmet. Dermatol. 2019, 18, 638–646. [Google Scholar] [CrossRef]
- Altalhab, S.; AlJasser, M.I.; Mulekar, S.V.; Al Issa, A.; Mulekar, S.; Diaz, J.; Diallo, A.; Ezzedine, K. Six-Year Follow-up of Vitiligo Patients Successfully Treated with Autologous Non-Cultured Melanocyte-Keratinocyte Transplantation. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 1172–1176. [Google Scholar] [CrossRef]
- Razmi, T.M.; Kumar, R.; Rani, S.; Kumaran, S.M.; Tanwar, S.; Parsad, D. Combination of Follicular and Epidermal Cell Suspension as a Novel Surgical Approach in Difficult-to-Treat Vitiligo: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 301. [Google Scholar] [CrossRef]
- Narayan, V.S.; Bol, L.L.C.; Geel, N.; Bekkenk, M.W.; Luiten, R.M.; Wolkerstorfer, A. Donor to Recipient Ratios in the Surgical Treatment of Vitiligo and Piebaldism: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1077–1086. [Google Scholar] [CrossRef]
- Lamoria, A.; Agrawal, A.; Rao, P.; Kachhawa, D. A Comparative Study between Follicular Unit Transplantation and Autologous Non-Cultured Non-Trypsinized Epidermal Cells Grafting (Jodhpur Technique) in Stable Vitiligo. J. Cutan Aesthet. Surg. 2020, 13, 204. [Google Scholar] [CrossRef]
- Tyagi, S.; Malhotra, S.K.; Kaur, T. Comparative Evaluation of Efficacy of Non-Cultured Epidermal Cell Suspension and Epidermal Curettage in Stable Vitiligo. J. Cutan. Aesthet. Surg. 2021, 14, 32–40. [Google Scholar] [CrossRef]
- Garg, S.; Dosapaty, N.; Arora, A.K. Laser Ablation of the Recipient Area With Platelet-Rich Plasma–Enriched Epidermal Suspension Transplant in Vitiligo Surgery: A Pilot Study. Dermatol. Surg. 2019, 45, 83–89. [Google Scholar] [CrossRef]
- Rasheed, H.M.; Esmat, S.M.; Hegazy, R.A.; Gawdat, H.I.; Bassiouny, D.M.; Doss, S.S.; Parsad, D.; Elkhouly, N.S. Effect of Different Methods of Trypsinization on Cell Viability and Clinical Outcome in Vitiligo Patients Undergoing Noncultured Epidermal Cellular Suspension. Dermatol. Surg. 2020, 46, 1307–1314. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Griffith, J.L.; Huggins, R.H.; Henderson, M.D.; Kerr, H.A.; Jacobsen, G.; Mulekar, S.V.; Lim, H.W.; Hamzavi, I.H. Long-Term Follow-up of Patients Undergoing Autologous Noncultured Melanocyte-Keratinocyte Transplantation for Vitiligo and Other Leukodermas. J. Am. Acad. Dermatol. 2017, 77, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, X.; Hong, W.; Fu, L.; Qian, G.; Xu, A. A Retrospective Study of Long Term Follow-up of 2283 Vitiligo Patients Treated by Autologous, Non-Cultured Melanocyte–Keratinocyte Transplantation. Aging 2021, 13, 5415–5425. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, S.D.; Rasal, Y.D. Innovative Technique for Securing Autologous Melanocyte-keratinocyte Cell Transplant (MKCT) Suspension in Stable Vitiligo. J. Cutan. Aesthet. Surg. 2021, 14, 132. [Google Scholar] [CrossRef]
- Esmat, S.; Bassiouny, D.; Saleh, M.A.; AbdelHalim, D.; Hegazy, R.; ElHawary, M.; Gawdat, H.; Gouda, H.; Khorshied, M.; Samir, N. Studying the Effect of Adding Growth Factors to the Autologous Melanocyte Keratinocyte Suspension in Segmental Vitiligo. Dermatol. Ther. 2020, 33, e13368. [Google Scholar] [CrossRef]
- Vanscheidt, W.; Hunziker, T. Repigmentation by Outer-Root-Sheath-Derived Melanocytes: Proof of Concept in Vitiligo and Leucoderma. Dermatology 2009, 218, 342–343. [Google Scholar] [CrossRef]
- Vashisht, K.R.; Arava, S.K.; Tembhre, M.K.; Parihar, A.S.; Sharma, V.K.; Das, B.K.; Sreenivas, V.; Sethuraman, G.; Gupta, S. A Randomized Pilot Study to Compare Hair Follicle Cell Suspensions Prepared Using Trypsin Alone versus Trypsin in Combination with Collagenase Type I for Transplantation in Vitiligo. Clin. Exp. Dermatol. 2020, 45, 172–179. [Google Scholar] [CrossRef]
- Gunaabalaji, D.R.; Pangti, R.; Challa, A.; Chauhan, S.; Sahni, K.; Arava, S.K.; Sethuraman, G.; Vishnubhatla, S.; Sharma, V.K.; Gupta, S. Comparison of Efficacy of Noncultured Hair Follicle Cell Suspension and Noncultured Epidermal Cell Suspension in Repigmentation of Leukotrichia and Skin Patch in Vitiligo: A Randomized Trial. Int. J. Dermatol. 2020, 59, 1393–1400. [Google Scholar] [CrossRef]
- Kumar, P.; Bhari, N.; Tembhre, M.K.; Mohanty, S.; Arava, S.; Sharma, V.K.; Gupta, S. Study of Efficacy and Safety of Noncultured, Extracted Follicular Outer Root Sheath Cell Suspension Transplantation in the Management of Stable Vitiligo. Int. J. Dermatol. 2018, 57, 245–249. [Google Scholar] [CrossRef]
- Kerure, A.S.; Deshmukh, N.; Agrawal, S.; Patwardhan, N.G. Follicular Unit Extraction [FUE]—One Procedure, Many Uses. Indian Dermatol. Online J. 2021, 12, 381–388. [Google Scholar] [CrossRef]
- Mokhtar, M.; El-Ashmawy, A.A.; Mostafa, W.A.; Gamei, M.M. Clinical and Dermoscopic Evaluation of Follicular Unit Transplantation vs. Mini-Punch Grafting in the Repigmentation of Resistant and Stable Vitiligo: A Comparative Study. J. Cosmet. Dermatol. 2022, 21, 5837–5851. [Google Scholar] [CrossRef]
- Nirmal, B.; Antonisamy, B.; Peter, C.V.D.; George, L.; George, A.A.; Dinesh, G.M. Cross-Sectional Study of Dermatoscopic Findings in Relation to Activity in Vitiligo: BPLeFoSK Criteria for Stability. J. Cutan. Aesthet. Surg. 2019, 12, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Vinay, K.; Bishnoi, A.; Kumaran, M.S.; Dogra, S.; Parsad, D. Dermatoscopic Assessment of Treatment Response in Patients Undergoing Autologous Non-Cultured Epidermal Cell Suspension for the Treatment of Stable Vitiligo: A Prospective Study. Dermatol. Ther. 2021, 34, e15099. [Google Scholar] [CrossRef] [PubMed]
- Kumar Jha, A.; Sonthalia, S.; Lallas, A.; Chaudhary, R.K.P. Dermoscopy in Vitiligo: Diagnosis and Beyond. Int. J. Dermatol. 2018, 57, 50–54. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Chang, C.-C.; Cheng, K.-L. Wood’s Lamp for Vitiligo Disease Stability and Early Recognition of Initiative Pigmentation after Epidermal Grafting: Determine Vitiligo Disease Stability before Epidermal Grafting with a Wood’s Lamp. Int. Wound J. 2017, 14, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Baweja, S.; Chand, S. A Prospective Observational Comparative Study of Novel Autologous Negative Pressure Epidermal Harvesting System (ANPEHS or EHS) and Suction Blister Grafting (SBG) in Treatment of Stable Vitiligo. J. Cutan. Aesthet. Surg. 2020, 13, 283. [Google Scholar] [CrossRef]
- Ezz-Eldawla, R.; Abu El-Hamd, M.; Saied, S.M.; Hassanien, S.H. A Comparative Study between Suction Blistering Graft, Mini Punch Graft, and Hair Follicle Transplant in Treatment of Patients with Stable Vitiligo. J. Dermatol. Treat. 2019, 30, 492–497. [Google Scholar] [CrossRef]
- Li, J.; Fu, W.-W.; Zheng, Z.-Z.; Zhang, Q.-Q.; Xu, Y.; Fang, L. Suction Blister Epidermal Grafting Using a Modified Suction Method in the Treatment of Stable Vitiligo: A Retrospective Study. Dermatol. Surg. 2011, 37, 999–1006. [Google Scholar] [CrossRef]
| Higher Rate of Relapse | Lower Rate of Relapse |
|---|---|
| Male gender | Affected body surface area of less than 1% |
| Age of surgery older than 23 years | Mechanical dermabrasion |
| Non-segmental vitiligo | Segmental- or focal-type vitiligo |
| Age of onset older than 13 years Fingertip involvement | Fingertip involvement Higher rate of repigmentation |
| New lesion onset | |
| Laser dermabrasion | |
| Family history of vitiligo |
| Method | Strengths | Limitations |
|---|---|---|
| Suction Blister Epidermal Grafting |
|
|
| Mini-Punch Grafting |
|
|
| Epidermal Cell Suspension |
|
|
| Follicular Cell Suspension |
|
|
| Negative Prognostic Factors in Dermoscopy | Positive Prognostic Factors in Dermoscopy | |
|---|---|---|
|
| |
|
| |
| Prior to treatment |
|
|
| ||
|
| |
| Following treatment |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochocka, M.; Wełniak, A.; Białczyk, A.; Marek-Jozefowicz, L.; Tadrowski, T.; Czajkowski, R. Management of Stable Vitiligo—A Review of the Surgical Approach. J. Clin. Med. 2023, 12, 1984. https://doi.org/10.3390/jcm12051984
Grochocka M, Wełniak A, Białczyk A, Marek-Jozefowicz L, Tadrowski T, Czajkowski R. Management of Stable Vitiligo—A Review of the Surgical Approach. Journal of Clinical Medicine. 2023; 12(5):1984. https://doi.org/10.3390/jcm12051984
Chicago/Turabian StyleGrochocka, Małgorzata, Adam Wełniak, Aleksandra Białczyk, Luiza Marek-Jozefowicz, Tadeusz Tadrowski, and Rafał Czajkowski. 2023. "Management of Stable Vitiligo—A Review of the Surgical Approach" Journal of Clinical Medicine 12, no. 5: 1984. https://doi.org/10.3390/jcm12051984
APA StyleGrochocka, M., Wełniak, A., Białczyk, A., Marek-Jozefowicz, L., Tadrowski, T., & Czajkowski, R. (2023). Management of Stable Vitiligo—A Review of the Surgical Approach. Journal of Clinical Medicine, 12(5), 1984. https://doi.org/10.3390/jcm12051984





