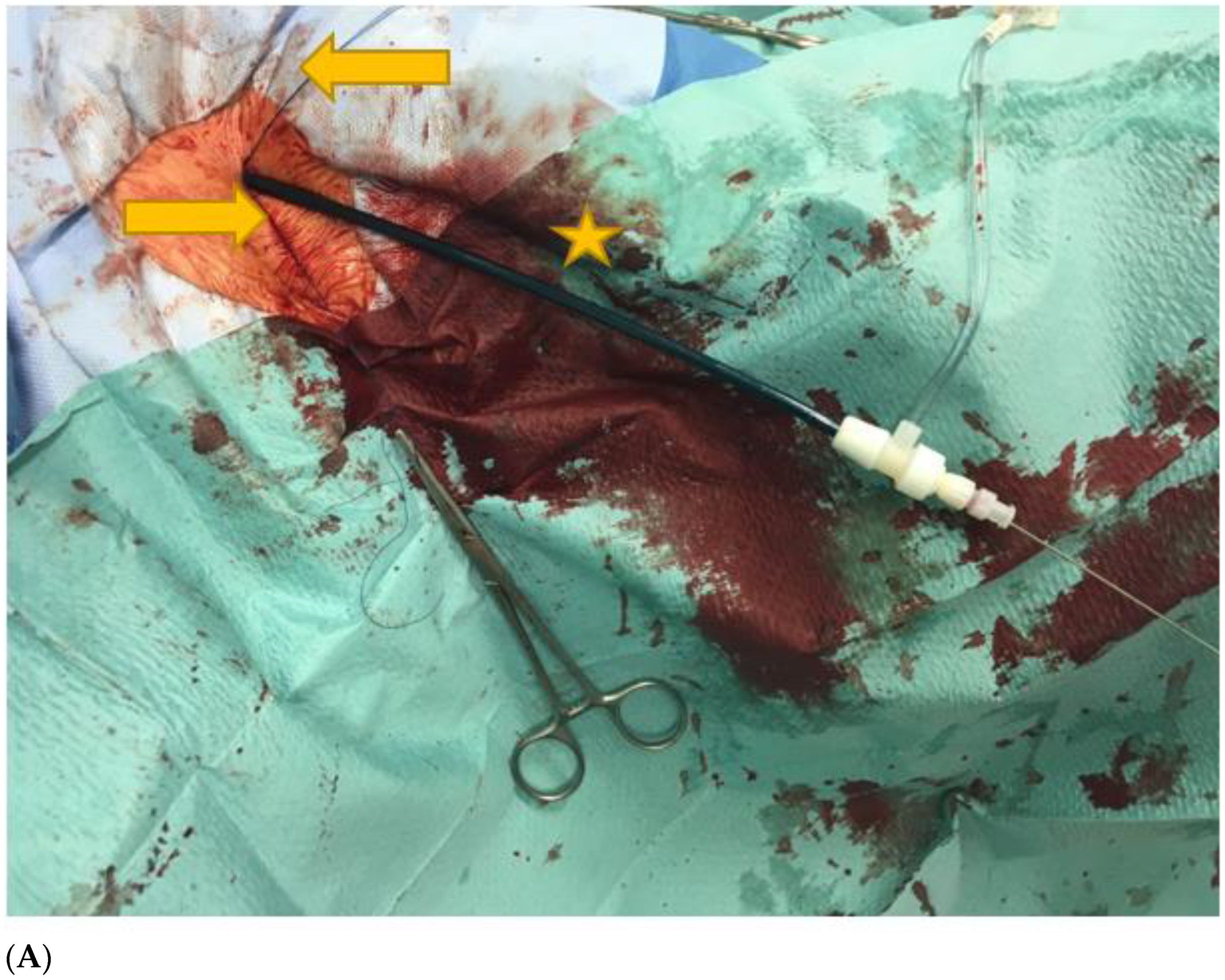
Feasibility and Safety of Percutaneous Axillary Artery Access in a Prospective Series of 100 Complex Aortic and Aortoiliac Interventions
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Patient Selection
2.2. Interventional Details and Postoperative Management
2.3. Outcome Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient and Procedural Characteristics
3.2. Acute Procedural Outcomes
3.3. All-Cause Death through 30 Days
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AxA | axillary artery |
| ER | endovascular repair |
| PVCD | percutaneous vascular closure devices |
| TAAA | thoraco-abdominal aortic aneurysms |
| UEA | upper extremity access |
| IBD | iliac branch devices |
| CTA | computer tomography angiography |
| BEVAR | branched endovascular aortic repair |
| FEVAR | fenestrated endovascular aortic repair |
| ChEVAR | chimney endovascular aortic repair |
| FBEVAR | fenestrated-branched endovascular aortic repair |
| EVAR | endovascular aortic repair |
| ICU | intensive care unit |
References
- Tenorio, E.R.; Dias-Neto, M.F.; Lima, G.B.B.; Estrera, A.L.; Oderich, G.S. Endovascular repair for thoracoabdominal aortic aneurysms: Current status and future challenges. Ann. Cardiothorac. Surg. 2021, 10, 744–767. [Google Scholar] [CrossRef] [PubMed]
- Meertens, M.M.; van Herwaarden, J.A.; de Vries, J.P.P.; Verhagen, H.J.; van der Laan, M.J.; Reijnen, M.M.; Schurink, G.W.; Mees, B.M. Multicenter experience of upper extremity access in complex endovascular aortic aneurysm repair. J. Vasc. Surg. 2022, 76, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, U.; Deuschl, F.; Schofer, N.; Frerker, C.; Schmidt, T.; Kuck, K.; Kreidel, F.; Schirmer, J.; Mizote, I.; Reichenspurner, H.; et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int. J. Cardiol. 2017, 232, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Puippe, G.D.; Kobe, A.; Rancic, Z.; Pfiffner, R.; Lachat, M.; Pfammatter, T. Safety of percutaneous axillary artery access with a suture-mediated closing device for parallel endograft aortic procedures—A retrospective pilot study. Vasa 2018, 47, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Wooster, M.; Powell, A.; Back, M.; Illig, K.; Shames, M. Axillary Artery Access as an Adjunct for Complex Endovascular Aortic Repair. Ann. Vasc. Surg. 2015, 29, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.; Nation, D.A.; Timaran, D.E.; Gomez, L.F.; Baig, M.S.; Valentine, R.J.; Timaran, C.H. Upper extremity access for fenestrated endovascular aortic aneurysm repair is not associated with increased morbidity. J. Vasc. Surg. 2015, 61, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Branzan, D.; Steiner, S.; Haensig, M.; Scheinert, D.; Schmidt, A. Percutaneous Axillary Artery Access for Endovascular Treatment of Complex Thoraco-abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Agrusa, C.J.; Connolly, P.H.; Ellozy, S.H.; Schneider, D.B. Safety and Effectiveness of Percutaneous Axillary Artery Access for Complex Aortic Interventions. Ann. Vasc. Surg. 2019, 61, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Grandi, A.; Melloni, A.; Kahlberg, A.; Melissano, G.; Chiesa, R. Percutaneous AXillary Artery (PAXA) Access at the First Segment During Fenestrated and Branched Endovascular Aortic Procedures. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Southmayd, G.; Hoque, A.; Kaki, A.; Tayal, R.; Rab, S.T. Percutaneous large-bore axillary access is a safe alternative to surgical approach: A systematic review. Catheter. Cardiovasc. Interv. 2020, 96, 1481–1488. [Google Scholar] [CrossRef] [PubMed]

| Variables | No. | % |
|---|---|---|
| Sex | ||
| Male | 75 | 75.0 |
| Female | 25 | 25.0 |
| Age, years | ||
| Mean ± SD | 73.8 ± 8.2 | |
| Median (range) | 76.0 (54–87) | |
| History of hypertension | 98 | 98.0 |
| COPD | 24 | 24.0 |
| Active smoking | 51 | 51.0 |
| CHD | 30 | 30.0 |
| Diabetes mellitus | 28 | 28.0 |
| Chronic renal insufficiency * | 68 | 68.0 |
| Hyperlipidaemia | 88 | 88.0 |
| BMI (kg/m²) | ||
| Mean ± SD | 26.6 ± 4.6 | |
| Median (range) | 25.8 (20.0–42.4) | |
| PCI-pre-OP | 10 | 10.0 |
| CABG pre-OP | 5 | 5.0 |
| Creatinine pre-OP (µmol/L) | ||
| Mean ± SD | 125.3 ± 113.2 | |
| Median (range) | 95.5 (42.0–763.0) | |
| ASA Score | ||
| ASA II | 22 | 22.0 |
| ASA III | 77 | 77.0 |
| ASA IV | 1 | 1.0 |
| Antiplatelets | 81 | 81.0 |
| Anticoagulant | 21 | 21.0 |
| AxA Description Pre-OP | ||
|---|---|---|
| Diameter AxA (mm) | ||
| Mean ± SD | 7.26 ± 1.29 | |
| Median (range) | 7.27 (4.50–10.80) | |
| Calcification > 50% circumference | 0 | 0 |
| Stenosis > 50% | 0 | 0 |
| Previous percutaneous access | 0 | 0 |
| Pacemaker on the punctured side | 4 | 4.0 |
| Dialysis AVF on the punctured side | 5 | 5.0 |
| Side of puncture AxA | ||
| Left | 93 | 93.0 |
| Right | 7 | 7.0 |
| Aneurysms Characteristics | No. | % |
|---|---|---|
| Acute | 18 | 18.0 |
| Rupture | 9 | 9.0 |
| Penetrating aortic ulcer | 3 | 3.0 |
| Pain | 6 | 6.0 |
| Chronic | 72 | 72.0 |
| Crawford Classification | ||
| Type II | 32 | 32.0 |
| Type III | 33 | 33.0 |
| Type IV | 25 | 25.0 |
| Maximum aortic diameter, mm | ||
| Mean ± SD | 65.7 ± 14.4 | |
| Median (range) | 66.0 (25–102) | |
| Previous repair of the aorta | 42 | 42.0 |
| TEVAR | 20 | 20.0 |
| EVAR | 22 | 22.0 |
| TAAA | ||
| Atherosclerotic | 82 | 82.0 |
| Dissection | 8 | 8.0 |
| Previous coil of segmental arteries | 55 | 55.0 |
| Treatment Characteristics | No. | % |
|---|---|---|
| FEVAR (with no. of fenestrations) | 30 | 30.0 |
| 2 | 5 | 5.0 |
| 4 | 25 | 25.0 |
| BEVAR (with no. of branches) | 45 | 45.0 |
| 2 | 3 | 3.0 |
| 3 | 7 | 7.0 |
| 4 | 34 | 34.0 |
| 5 | 1 | 1.0 |
| FBEVAR (with no. of fenestrations) | 6 | 6.0 |
| 4 | 5 | 5.0 |
| 5 | 1 | 1.0 |
| ChEVAR (with no. of fenestrations) | 9 | 9.0 |
| 3 | 3 | 3.0 |
| 4 | 5 | 5.0 |
| 5 | 1 | 1.0 |
| Other | 10 | 10.0 |
| General anesthesia | 99 | 99.0 |
| Operative time (minutes) | ||
| Mean ± SD | 191.0 ± 69.0 | |
| Median (range) | 193 (53–480) | |
| Fluoroscopy time (minutes) | ||
| Mean ± SD | 50.3 ± 22.7 | |
| Median (range) | 49 (16–142) | |
| Radiation dose (Gycm²) | ||
| Mean ± SD | 1567.6 ± 2156.2 | |
| Median (range) | 429.7 (52.0–8635.6) | |
| ID of introducer (French) | ||
| 6F | 1 | 1.0 |
| 7F | 18 | 18.0 |
| 8F | 15 | 15.0 |
| 9F | 5 | 5.0 |
| 12F | 60 | 60.0 |
| 14F | 1 | 1.0 |
| Median (range) | 12 (6–14) |
| Variables | No. | % |
|---|---|---|
| Primary hemostasis AxA | 92 | 92.0 |
| Stenosis/occlusion AxA | 6 | 6.0 |
| Bleeding | 2 | 2.0 |
| Uncovered stent | 5 | 5.0 |
| Covered stent | 3 | 3.0 |
| Surgical repair | 0 | 0 |
| Hematoma | 6 | 6.0 |
| PSA | 3 | 3.0 |
| Arm ischemia | 0 | 0 |
| Peripheral nerve injury | 0 | 0 |
| DVT | 0 | 0 |
| Stroke | 7 | 7.0 |
| SCI | 11 | 11.0 |
| Death within 30 days | 8 | 8.0 |
| ICU stay (days) | ||
| Median (range) | 2 (0–34) | |
| Hospital stay (days) | ||
| Median (range) | 12 (1–95) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittig, T.; Sabanov, A.; Schmidt, A.; Scheinert, D.; Steiner, S.; Branzan, D. Feasibility and Safety of Percutaneous Axillary Artery Access in a Prospective Series of 100 Complex Aortic and Aortoiliac Interventions. J. Clin. Med. 2023, 12, 1959. https://doi.org/10.3390/jcm12051959
Wittig T, Sabanov A, Schmidt A, Scheinert D, Steiner S, Branzan D. Feasibility and Safety of Percutaneous Axillary Artery Access in a Prospective Series of 100 Complex Aortic and Aortoiliac Interventions. Journal of Clinical Medicine. 2023; 12(5):1959. https://doi.org/10.3390/jcm12051959
Chicago/Turabian StyleWittig, Tim, Arsen Sabanov, Andrej Schmidt, Dierk Scheinert, Sabine Steiner, and Daniela Branzan. 2023. "Feasibility and Safety of Percutaneous Axillary Artery Access in a Prospective Series of 100 Complex Aortic and Aortoiliac Interventions" Journal of Clinical Medicine 12, no. 5: 1959. https://doi.org/10.3390/jcm12051959
APA StyleWittig, T., Sabanov, A., Schmidt, A., Scheinert, D., Steiner, S., & Branzan, D. (2023). Feasibility and Safety of Percutaneous Axillary Artery Access in a Prospective Series of 100 Complex Aortic and Aortoiliac Interventions. Journal of Clinical Medicine, 12(5), 1959. https://doi.org/10.3390/jcm12051959