1. Introduction
Thoracic endovascular aortic repair (TEVAR) with landing in the ascending aorta (AA) is a treatment option for a variety of proximal aortic pathologies in selected patients for whom open surgery carries high risk [
1,
2,
3,
4,
5]. The outcome of any TEVAR procedure critically depends on the morphology of the proximal landing zone (PLZ), with non-optimal aortic sizing and endograft sizing reportedly associated with increasing rates of endoleaks (ELs), endograft migration, and reintervention [
6,
7,
8,
9,
10]. The pulsatile morphology of the AA, and its variable segmental geometry during the cardiac cycle, may be disadvantageous for proximal endograft alignment and may thereby lead to poor TEVAR outcomes [
11,
12,
13]. Few studies have reported the dynamic slice anatomy and motility of selected parts of the AA and aortic arch, and the segmental anatomy of the entire AA remains under-reported [
14,
15,
16,
17]. TEVAR in the AA shows promising outcomes but is associated with high rates of ELs and substantial rates of retrograde aortic dissection (RAD) and conversion [
2,
4,
18,
19]. Thus, the dynamic segmental anatomy of the AA must be further investigated to advance TEVAR in the AA and to improve the current clinical and technical outcomes of this procedure.
The objective of the present study was to assess the dynamic segmental anatomy of the entire AA, enabling the determination of a favorable PLZ and appropriate aortic sizing for the most proximal TEVAR.
6. Discussion
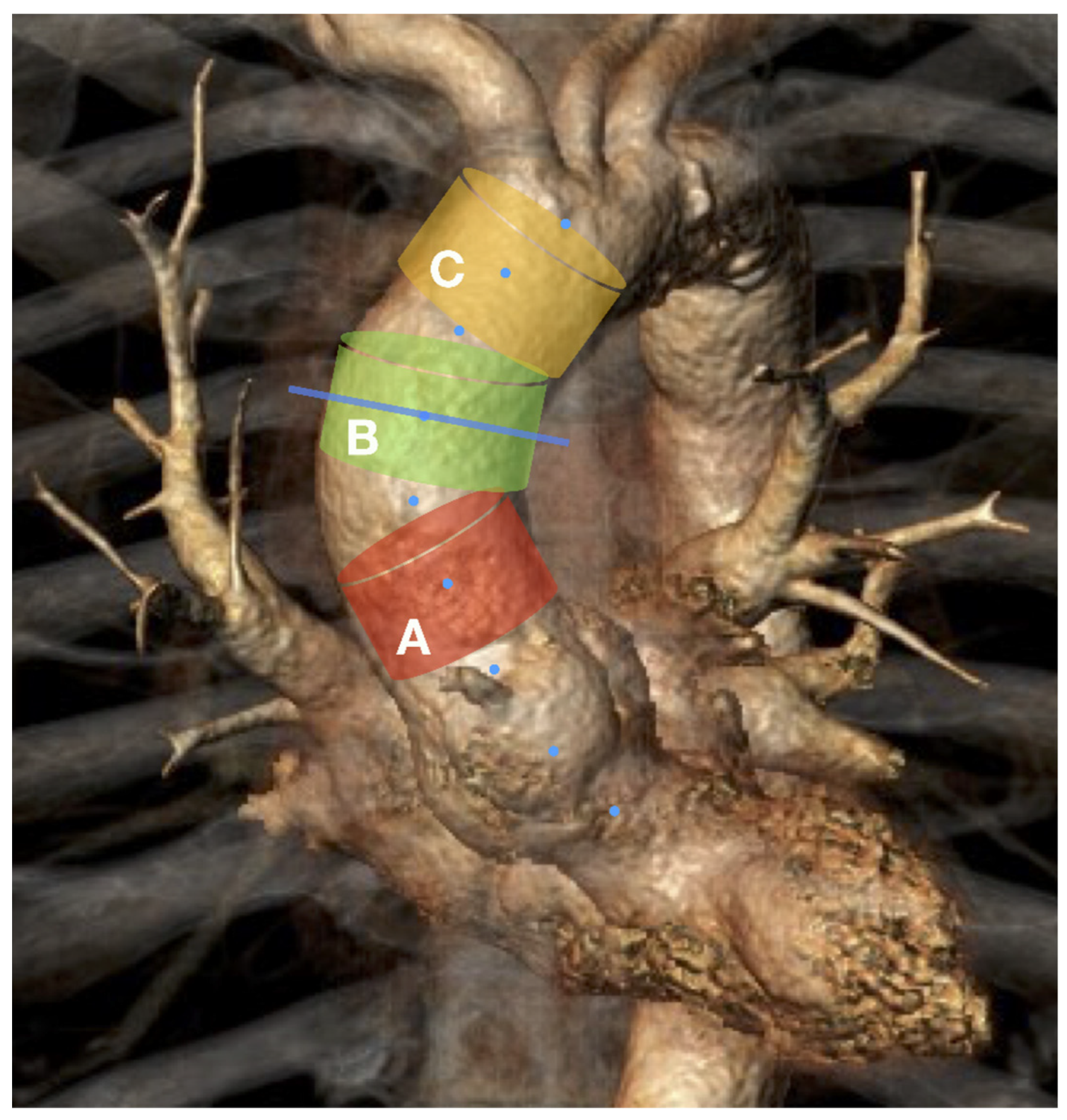
The current study shows a predominance of the systolic over diastolic diameter during the whole cardiac cycle at all levels of the ascending aorta. Each aortic plane demonstrated an oval-shaped 2D morphology with a 10% predominance of maximum plane diameters over small diameters. Furthermore, our analysis revealed a cylindrical form for the mid-ascending aortic segment, a slightly funneled form for the distal-ascending segments, and a pronounced conical form for most proximal segments of the AA.
The currently available reports in the literature highlight the pulsatility of some segments of the AA. De Heer et al. found that the aortic diameter at the sinotubular junction is larger in the systole phase than in the diastole phase (D
max systolic 32.4 ± 3.8 mm, D
max diastolic 31.5 ± 3.9 mm,
p < 0.001) [
17]. Jian-ping et al. reported significant changes in the aortic diameter of the distal AA during the cardiac cycle, with greater aortic size in the systole phase than in the diastole phase (3.26 ± 0.24 mm and 3.18 ± 0.27 mm, respectively,
p < 0.01) [
13]. Rengier et al. showed the prominent mid-ascending pulsatility of the AA in healthy volunteers, where the systolic aortic dimension was over 10% greater than the diastolic aortic dimension [
14]. In line with these prior reports, our current findings showed a wide range of variability in the cross-sectional dimensions of the aorta during the cardiac cycle, with clearly larger systolic dimensions than diastolic dimensions at all levels of the AA (
p ≤ 0.001).
Satriano et al. performed a 3D reconstruction of the ECG-CTA series and reported asymmetrical distension in the AA during the cardiac cycle, which was more prominent along the greater curvature of the AA, consistent with the jet flow direction during heart output [
21]. Other reports have described non-circular shapes for some aortic planes during the cardiac cycle [
11,
13,
22].
Liu et al. analyzed the precise sizing for TEVAR in the AA and reported that if the diameters differed by >5%, the real aortic diameter should be calculated as an average between the maximum and minimum diameters, to avoid retrograde aortic dissection [
22]. Our present findings confirmed the oval shape of the segmental planes at all levels of the AA, with a relative difference of approximately 10% between the maximum and minimum diameter throughout the cardiac cycle. Thus, it seems appropriate to use the average diameter for the precise measurement of the AA diameter.
In our current study, we observed increased aortic diameter in the systole phase compared with that in the diastole phase and showed an AA strain of up to 5%. Satriano et al. reported a 10.2 ± 6.0% peak principal strain amplitude for the entire AA [
21]. Redheuil et al. reported a similar AA strain (8 ± 4%) in patients over 70 years old and found an AA strain of up to 15 ± 8% in patients 40–49 years old [
23]. Thus, the published literature and the data from our current study support the use of a systolic CTA series for the most precise sizing of TEVAR in the AA, wherein 5–15% of the aortic diameter size may be balanced out compared with CTA in the diastolic phase, independent of the patient’s age.
In a recent systematic review, Muetterties et al. reported an 18.6% rate of early-term EL Ia after TEVAR in the AA [
4]. Similarly, a meta-analysis by Baikoussis et al. revealed a high pooled rate of late EL Ia (16.4%) after TEVAR in the AA [
2]. These results are most likely related to the inappropriate alignment of the endograft to the aortic wall [
4]. Accordingly, it is crucial to understand the 3D shape of the PLZ to improve outcomes.
A study by van Prehn et al. reported the dynamic plane morphology at the three AA levels and described the 3D motions of 2D aortic planes. However, the authors did not consider the 3D segmental morphology of AA, which is essential for understanding the volume geometry of a potential proximal landing zone [
24].
In the current study, we observed that the mid-ascending segment of the AA retained its cylindrical shape throughout the cardiac cycle; therefore, the common cylindrical design of the endograft seems appropriate in this setting. The distal AA segment showed a funnel form; however, the diameter size difference of 1.5 mm between the proximal and distal segmental planes does not seem to be relevant for practical sizing; therefore, a cylindrical endograft design could also be considered here. In contrast, most proximal AA segments showed a reversed funnel (conical) form, which is reportedly unfavorable for aortic endograft alignment [
25,
26,
27]. Moreover, the difference of >5 mm between the proximal (smaller) and distal (larger) diameters of segmental planes corresponds to an 18% (5.5/29.6 mm) systolic diameter difference between the proximal and distal segmental planes. Therefore, the conventional cylindrical endograft design may not be suitable for use in such cases.
7. Limitations
The present study has several limitations. First, we included patients with significant aortic stenosis, which may influence aortic asymmetry throughout the power and direction of jets during the cardiac cycle. However, previously published studies reported increased arterial stiffness for the whole arterial tree, including the AA, due to severe aortic stenosis; however, a reduced distensibility (a function of change in AA diameter and arterial pressure) of non-calcified AA was not observed compared with patients without several aortic stenoses if cardiac output and stroke volume were saved [
28,
29]. Furthermore, evenly distributed AA stiffness may introduce bias in terms of absolute diameter and area numbers. However, this is unlikely to result in any change in the aortic plane size ratios. Thus, the volumetric form of the AA segments would probably stay the same.
Second, our patient cohort included those with advanced age and atherosclerosis, which may influence aortic distensibility. One may speculate that AA compliance may be higher in younger subjects. Third, this study did not investigate the longitudinal motions, side deviations, or angulation of the AA during the cardiac cycle, which may be relevant for a complete description of 3D aortic geometry during the cardiac cycle.