Review of Basic Research about Ossification of the Spinal Ligaments Focusing on Animal Models
Abstract
1. Introduction
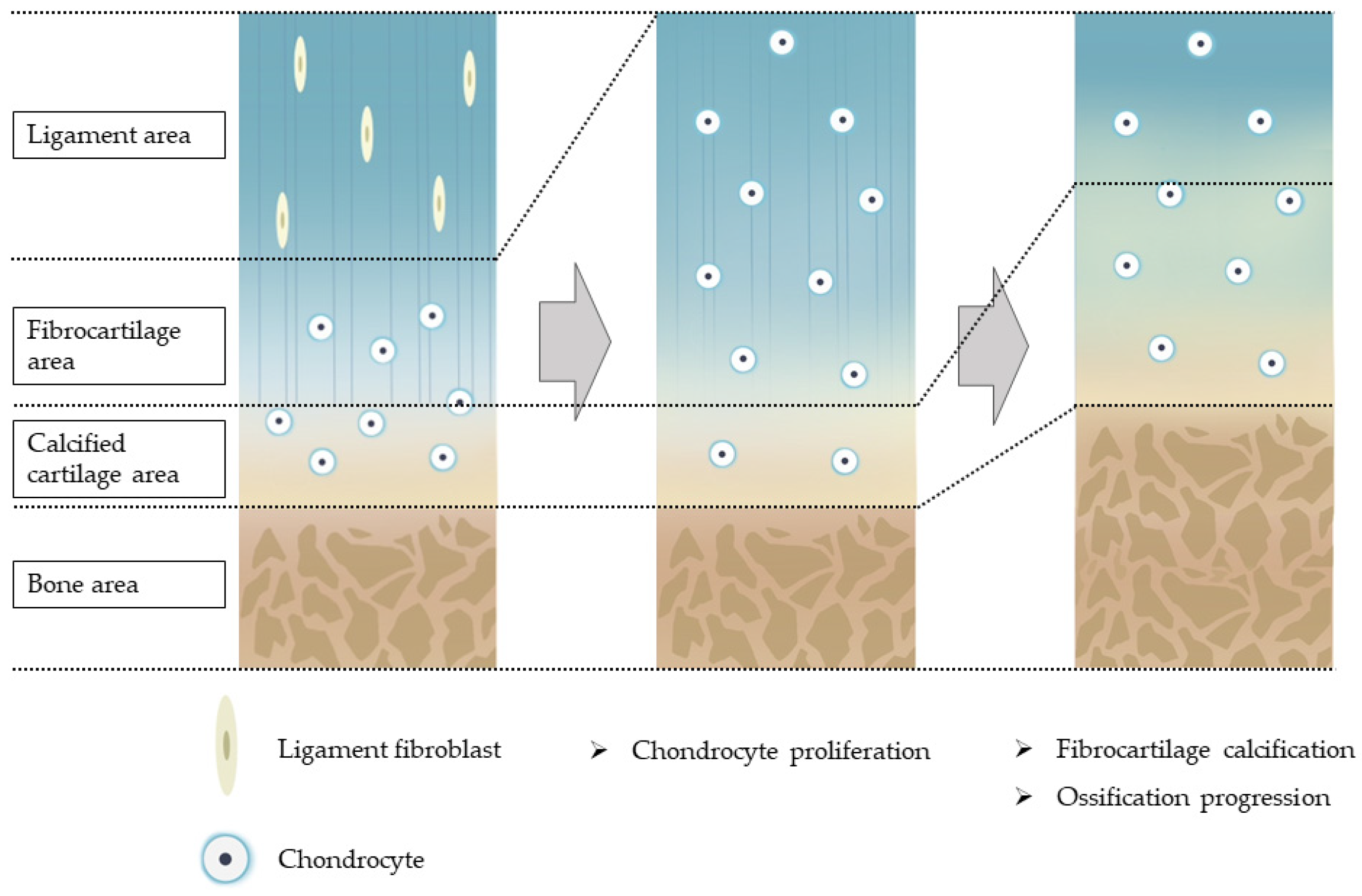
2. Histology of Human OSL and Hypothesis of Ossification Pathogenesis
2.1. Histology of Human OPLL
2.2. Histology of Human OLF
2.3. Hypothesis on the Pathogenesis of OSL
3. Tissue-Specific Progenitor Cells Involvement
4. ttw Mice
4.1. Overview
4.2. Causative Gene and Function
4.3. Histology and Ossification Process
4.4. Clinical Relevance
5. ZFR
5.1. Overview
5.2. Causative Gene and Function
5.3. Histology and Ossification Process
5.4. Clinical Relevance
6. Mechanical Stress-Induced Animal Models
6.1. Overview
6.2. Details of Each Animal Model
6.3. Pathogenesis
6.3.1. Cellular Mechanotransduction
6.3.2. Molecular Mechanisms Related to Spinal Ligament Cells
6.3.3. Inflammation Involvement
6.4. Clinical Relevance
7. New Approaches
7.1. Epigenetics
7.2. Genome-Wide Association Analysis
8. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujimori, T.; Le, H.; Hu, S.S.; Chin, C.; Pekmezci, M.; Schairer, W.; Tay, B.K.; Hamasaki, T.; Yoshikawa, H.; Iwasaki, M. Ossification of the Posterior Longitudinal Ligament of the Cervical Spine in 3161 Patients: A CT-Based Study. Spine 2015, 40, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, K.; Terayama, K.; Yanagihara, M.; Wada, K.; Kasuga, K.; Machida, T.; Matsushima, S. A Radiological Population Study on the Ossification of the Posterior Longitudinal Ligament in the Spine. Arch. Orthop. Trauma. Surg. Arch. Orthop. Unf.-Chir. 1987, 106, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, T.; Nakajima, N.; Sugiura, T.; Ikegami, D.; Sakaura, H.; Kaito, T.; Iwasaki, M. Epidemiology of Symptomatic Ossification of the Posterior Longitudinal Ligament: A Nationwide Registry Survey. J. Spine Surg. Hong Kong 2021, 7, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Yoshii, T.; Iwanami, A.; Takeuchi, K.; Mori, K.; Yamada, T.; Wada, K.; Koda, M.; Matsuyama, Y.; Takeshita, K.; et al. Prevalence and Distribution of Ossified Lesions in the Whole Spine of Patients with Cervical Ossification of the Posterior Longitudinal Ligament A Multicenter Study (JOSL CT Study). PLoS ONE 2016, 11, e0160117. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, T.; Watabe, T.; Iwamoto, Y.; Hamada, S.; Iwasaki, M.; Oda, T. Prevalence, Concomitance, and Distribution of Ossification of the Spinal Ligaments: Results of Whole Spine CT Scans in 1500 Japanese Patients. Spine 2016, 41, 1668–1676. [Google Scholar] [CrossRef]
- Nishimura, S.; Nagoshi, N.; Iwanami, A.; Takeuchi, A.; Hirai, T.; Yoshii, T.; Takeuchi, K.; Mori, K.; Yamada, T.; Seki, S.; et al. Prevalence and Distribution of Diffuse Idiopathic Skeletal Hyperostosis on Whole-Spine Computed Tomography in Patients With Cervical Ossification of the Posterior Longitudinal Ligament: A Multicenter Study. Clin. Spine Surg. 2018, 31, E460–E465. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Seki, S.; Hori, T.; Suzuki, K.; Makino, H.; Kimura, T. Characteristics of Ossification of the Spinal Ligament; Incidence of Ossification of the Ligamentum Flavum in Patients with Cervical Ossification of the Posterior Longitudinal Ligament—Analysis of the Whole Spine Using Multidetector CT. J. Orthop. Sci. 2016, 21, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Hirai, T.; Iwanami, A.; Nagoshi, N.; Takeuchi, K.; Mori, K.; Yamada, T.; Seki, S.; Tsuji, T.; Fujiyoshi, K.; et al. Co-Existence of Ossification of the Nuchal Ligament Is Associated with Severity of Ossification in the Whole Spine in Patients with Cervical Ossification of the Posterior Longitudinal Ligament—A Multi-Center CT Study. J. Orthop. Sci. 2019, 24, 35–41. [Google Scholar] [CrossRef]
- Mori, K.; Yoshii, T.; Hirai, T.; Iwanami, A.; Takeuchi, K.; Yamada, T.; Seki, S.; Tsuji, T.; Fujiyoshi, K.; Furukawa, M.; et al. Prevalence and Distribution of Ossification of the Supra/Interspinous Ligaments in Symptomatic Patients with Cervical Ossification of the Posterior Longitudinal Ligament of the Spine: A CT-Based Multicenter Cross-Sectional Study. BMC Musculoskelet. Disord. 2016, 17, 492. [Google Scholar] [CrossRef]
- Matsunaga, S.; Yamaguchi, M.; Hayashi, K.; Sakou, T. Genetic Analysis of Ossification of the Posterior Longitudinal Ligament. Spine 1999, 24, 937–938; discussion 939. [Google Scholar] [CrossRef]
- Terayama, K. Genetic Studies on Ossification of the Posterior Longitudinal Ligament of the Spine. Spine 1989, 14, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Imagama, S.; Kato, S.; Kaito, T.; Sakai, H.; Ikegawa, S.; Kawaguchi, Y.; Kanayama, M.; Hisada, Y.; Koike, Y.; et al. Association Between Vitamin A Intake and Disease Severity in Early-Onset Heterotopic Ossification of the Posterior Longitudinal Ligament of the Spine. Glob. Spine J. 2022, 12, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.-I. Current Topics in Pharmacological Research on Bone Metabolism: Molecular Basis of Ectopic Bone Formation Induced by Mechanical Stress. J. Pharmacol. Sci. 2006, 100, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, L.-S.; Dai, L.-Y. Hormones and Growth Factors in the Pathogenesis of Spinal Ligament Ossification. Eur. Spine J. 2007, 16, 1075–1084. [Google Scholar] [CrossRef]
- Moon, B.J.; Choi, S.K.; Shin, D.A.; Yi, S.; Kim, K.N.; Yoon, D.H.; Ha, Y. Prevalence, Incidence, Comorbidity, and Mortality Rates of Ossification of Posterior Longitudinal Ligament in the Cervical Spine: A Nested Case-Control Cohort Study. World Neurosurg. 2018, 117, e323–e328. [Google Scholar] [CrossRef]
- Okamoto, K.; Kobashi, G.; Washio, M.; Sasaki, S.; Yokoyama, T.; Miyake, Y.; Sakamoto, N.; Ohta, K.; Inaba, Y.; Tanaka, H.; et al. Dietary Habits and Risk of Ossification of the Posterior Longitudinal Ligaments of the Spine (OPLL); Findings from a Case-Control Study in Japan. J. Bone Miner. Metab. 2004, 22, 612–617. [Google Scholar] [CrossRef]
- Wang, P.N.; Chen, S.S.; Liu, H.C.; Fuh, J.L.; Kuo, B.I.; Wang, S.J. Ossification of the Posterior Longitudinal Ligament of the Spine. A Case-Control Risk Factor Study. Spine 1999, 24, 142–144; discussion 145. [Google Scholar] [CrossRef]
- Le, H.V.; Wick, J.B.; Van, B.W.; Klineberg, E.O. Ossification of the Posterior Longitudinal Ligament: Pathophysiology, Diagnosis, and Management. J. Am. Acad. Orthop. Surg. 2022, 30, 820–830. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Crane, J.; Zhen, G.; Li, F.; Yang, P.; Gao, M.; Deng, R.; Wang, Y.; Jia, X.; et al. Aberrant TGF-β Activation in Bone Tendon Insertion Induces Enthesopathy-like Disease. J. Clin. Investig. 2018, 128, 846–860. [Google Scholar] [CrossRef]
- Sato, R.; Uchida, K.; Kobayashi, S.; Yayama, T.; Kokubo, Y.; Nakajima, H.; Takamura, T.; Bangirana, A.; Itoh, H.; Baba, H. Ossification of the Posterior Longitudinal Ligament of the Cervical Spine: Histopathological Findings around the Calcification and Ossification Front. J. Neurosurg. Spine 2007, 7, 174–183. [Google Scholar] [CrossRef]
- Nakajima, H.; Watanabe, S.; Honjoh, K.; Okawa, A.; Matsumoto, M.; Matsumine, A. Expression Analysis of Susceptibility Genes for Ossification of the Posterior Longitudinal Ligament of the Cervical Spine in Human OPLL-Related Tissues and a Spinal Hyperostotic Mouse (Ttw/Ttw). Spine 2020, 45, E1460–E1468. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Yayama, T.; Cai, H.-X.; Nakajima, H.; Sugita, D.; Guerrero, A.R.; Kobayashi, S.; Yoshida, A.; Chen, K.-B.; Baba, H. Ossification Process Involving the Human Thoracic Ligamentum Flavum: Role of Transcription Factors. Arthritis Res. Ther. 2011, 13, R144. [Google Scholar] [CrossRef] [PubMed]
- Yayama, T.; Uchida, K.; Kobayashi, S.; Kokubo, Y.; Sato, R.; Nakajima, H.; Takamura, T.; Bangirana, A.; Itoh, H.; Baba, H. Thoracic Ossification of the Human Ligamentum Flavum: Histopathological and Immunohistochemical Findings around the Ossified Lesion. J. Neurosurg. Spine 2007, 7, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Mizuno, J.; Hashizume, Y.; Nakagawa, H. Immunohistochemistry of Symptomatic Hypertrophy of the Posterior Longitudinal Ligament with Special Reference to Ligamentous Ossification. Spinal Cord 2006, 44, 576–581. [Google Scholar] [CrossRef]
- Ono, K.; Yonenobu, K.; Miyamoto, S.; Okada, K. Pathology of Ossification of the Posterior Longitudinal Ligament and Ligamentum Flavum. Clin. Orthop. 1999, 359, 18–26. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]
- Segawa, Y.; Muneta, T.; Makino, H.; Nimura, A.; Mochizuki, T.; Ju, Y.-J.; Ezura, Y.; Umezawa, A.; Sekiya, I. Mesenchymal Stem Cells Derived from Synovium, Meniscus, Anterior Cruciate Ligament, and Articular Chondrocytes Share Similar Gene Expression Profiles. J. Orthop. Res. 2009, 27, 435–441. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Asari, T.; Furukawa, K.-I.; Tanaka, S.; Kudo, H.; Mizukami, H.; Ono, A.; Numasawa, T.; Kumagai, G.; Motomura, S.; Yagihashi, S.; et al. Mesenchymal Stem Cell Isolation and Characterization from Human Spinal Ligaments. Biochem. Biophys. Res. Commun. 2012, 417, 1193–1199. [Google Scholar] [CrossRef]
- Chen, W.C.W.; Park, T.S.; Murray, I.R.; Zimmerlin, L.; Lazzari, L.; Huard, J.; Péault, B. Cellular Kinetics of Perivascular MSC Precursors. Stem Cells Int. 2013, 2013, 983059. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.; Furukawa, K.-I.; Ono, A.; Asari, T.; Harada, Y.; Wada, K.; Tanaka, T.; Inaba, W.; Mizukami, H.; Motomura, S.; et al. Immunohistochemical Localization of Mesenchymal Stem Cells in Ossified Human Spinal Ligaments. Biochem. Biophys. Res. Commun. 2013, 436, 698–704. [Google Scholar] [CrossRef]
- Harada, Y.; Furukawa, K.-I.; Asari, T.; Chin, S.; Ono, A.; Tanaka, T.; Mizukami, H.; Murakami, M.; Yagihashi, S.; Motomura, S.; et al. Osteogenic Lineage Commitment of Mesenchymal Stem Cells from Patients with Ossification of the Posterior Longitudinal Ligament. Biochem. Biophys. Res. Commun. 2014, 443, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, Y.; Yoshimura, Y.; Higaki, S. A New Breed of Mouse Showing Multiple Osteochondral Lesions--Twy Mouse. Ryumachi Rheum. 1981, 21, 157–164. [Google Scholar]
- Okawa, A.; Nakamura, I.; Goto, S.; Moriya, H.; Nakamura, Y.; Ikegawa, S. Mutation in Npps in a Mouse Model of Ossification of the Posterior Longitudinal Ligament of the Spine. Nat. Genet. 1998, 19, 271–273. [Google Scholar] [CrossRef]
- Mackenzie, N.C.W.; Huesa, C.; Rutsch, F.; MacRae, V.E. New Insights into NPP1 Function: Lessons from Clinical and Animal Studies. Bone 2012, 51, 961–968. [Google Scholar] [CrossRef]
- Kato, K.; Nishimasu, H.; Okudaira, S.; Mihara, E.; Ishitani, R.; Takagi, J.; Aoki, J.; Nureki, O. Crystal Structure of Enpp1, an Extracellular Glycoprotein Involved in Bone Mineralization and Insulin Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 16876–16881. [Google Scholar] [CrossRef]
- Terkeltaub, R. Physiologic and Pathologic Functions of the NPP Nucleotide Pyrophosphatase/Phosphodiesterase Family Focusing on NPP1 in Calcification. Purinergic Signal. 2006, 2, 371–377. [Google Scholar] [CrossRef]
- Hirakawa, H.; Kusumi, T.; Nitobe, T.; Ueyama, K.; Tanaka, M.; Kudo, H.; Toh, S.; Harata, S. An Immunohistochemical Evaluation of Extracellular Matrix Components in the Spinal Posterior Longitudinal Ligament and Intervertebral Disc of the Tiptoe Walking Mouse. J. Orthop. Sci. 2004, 9, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, N.; Baba, H.; Imura, S.; Fukuda, M. Characteristics and Mechanism of the Ossification of Posterior Longitudinal Ligament in the Tip-Toe Walking Yoshimura (Twy) Mouse. Eur. J. Histochem. EJH 1996, 40, 199–210. [Google Scholar] [PubMed]
- Uchida, K.; Yayama, T.; Sugita, D.; Nakajima, H.; Rodriguez Guerrero, A.; Watanabe, S.; Roberts, S.; Johnson, W.E.; Baba, H. Initiation and Progression of Ossification of the Posterior Longitudinal Ligament of the Cervical Spine in the Hereditary Spinal Hyperostotic Mouse (Twy/Twy). Eur. Spine J. 2012, 21, 149–155. [Google Scholar] [CrossRef]
- Nitschke, Y.; Baujat, G.; Botschen, U.; Wittkampf, T.; du Moulin, M.; Stella, J.; Le Merrer, M.; Guest, G.; Lambot, K.; Tazarourte-Pinturier, M.-F.; et al. Generalized Arterial Calcification of Infancy and Pseudoxanthoma Elasticum Can Be Caused by Mutations in Either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012, 90, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Shimizu, Y.; Hori, M.; Taguchi, M.; Igarashi, T.; Fukumoto, S.; Fujitab, T. A Patient with Hypophosphatemic Rickets and Ossification of Posterior Longitudinal Ligament Caused by a Novel Homozygous Mutation in ENPP1 Gene. Bone 2011, 49, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Ferrer, A.; Kumar, R.; Singh, R.J.; Murthy, V.; Schultz-Rogers, L.; Zimmermann, M.; Lanpher, B.; Zimmerman, K.; Stabach, P.R.; et al. Clinical and Biochemical Phenotypes in a Family With ENPP1 Mutations. J. Bone Miner. Res. 2020, 35, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Mitchell, A.; Tysoe, C.; Caswell, R.; Owens, M.; Vincent, T. Novel Compound Heterozygous Mutations in ENPP1 Cause Hypophosphataemic Rickets with Anterior Spinal Ligament Ossification. Rheumatol. Oxf. Engl. 2012, 51, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ansh, A.J.; Lester, E.R.; Kinoshita, Y.; Hidaka, N.; Hoshino, Y.; Koga, M.; Taniguchi, Y.; Uchida, T.; Yamaguchi, H.; et al. Identification of ENPP1 Haploinsufficiency in Patients With Diffuse Idiopathic Skeletal Hyperostosis and Early-Onset Osteoporosis. J. Bone Miner. Res. 2022, 37, 1125–1135. [Google Scholar] [CrossRef]
- Nakamura, I.; Ikegawa, S.; Okawa, A.; Okuda, S.; Koshizuka, Y.; Kawaguchi, H.; Nakamura, K.; Koyama, T.; Goto, S.; Toguchida, J.; et al. Association of the Human NPPS Gene with Ossification of the Posterior Longitudinal Ligament of the Spine (OPLL). Hum. Genet. 1999, 104, 492–497. [Google Scholar] [CrossRef]
- Koshizuka, Y.; Kawaguchi, H.; Ogata, N.; Ikeda, T.; Mabuchi, A.; Seichi, A.; Nakamura, Y.; Nakamura, K.; Ikegawa, S. Nucleotide Pyrophosphatase Gene Polymorphism Associated with Ossification of the Posterior Longitudinal Ligament of the Spine. J. Bone Miner. Res. 2002, 17, 138–144. [Google Scholar] [CrossRef]
- Takaya, K.; Ogawa, Y.; Isse, N.; Okazaki, T.; Satoh, N.; Masuzaki, H.; Mori, K.; Tamura, N.; Hosoda, K.; Nakao, K. Molecular Cloning of Rat Leptin Receptor Isoform Complementary DNAs--Identification of a Missense Mutation in Zucker Fatty (Fa/Fa) Rats. Biochem. Biophys. Res. Commun. 1996, 225, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. The Zucker-Fatty Rat: A Review. Fed. Proc. 1977, 36, 148–153. [Google Scholar] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Philbrick, K.A.; Wong, C.P.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T. Leptin Stimulates Bone Formation in Ob/Ob Mice at Doses Having Minimal Impact on Energy Metabolism. J. Endocrinol. 2017, 232, 461–474. [Google Scholar] [CrossRef]
- Steppan, C.M.; Crawford, D.T.; Chidsey-Frink, K.L.; Ke, H.; Swick, A.G. Leptin Is a Potent Stimulator of Bone Growth in Ob/Ob Mice. Regul. Pept. 2000, 92, 73–78. [Google Scholar] [CrossRef]
- Zheng, B.; Jiang, J.; Luo, K.; Liu, L.; Lin, M.; Chen, Y.; Yan, F. Increased Osteogenesis in Osteoporotic Bone Marrow Stromal Cells by Overexpression of Leptin. Cell Tissue Res. 2015, 361, 845–856. [Google Scholar] [CrossRef]
- Fan, D.; Chen, Z.; Chen, Y.; Shang, Y. Mechanistic Roles of Leptin in Osteogenic Stimulation in Thoracic Ligament Flavum Cells. J. Biol. Chem. 2007, 282, 29958–29966. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, D.; Hinoi, E.; Ferron, M.; Kode, A.; Riley, K.J.; Zhou, B.; Guo, X.E.; Karsenty, G. Genetic Determination of the Cellular Basis of the Sympathetic Regulation of Bone Mass Accrual. J. Exp. Med. 2011, 208, 841–851. [Google Scholar] [CrossRef]
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.M.; Karsenty, G. Leptin Inhibits Bone Formation through a Hypothalamic Relay: A Central Control of Bone Mass. Cell 2000, 100, 197–207. [Google Scholar] [CrossRef]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin Regulates Bone Formation via the Sympathetic Nervous System. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Bartell, S.M.; Rayalam, S.; Ambati, S.; Gaddam, D.R.; Hartzell, D.L.; Hamrick, M.; She, J.-X.; Della-Fera, M.A.; Baile, C.A. Central (ICV) Leptin Injection Increases Bone Formation, Bone Mineral Density, Muscle Mass, Serum IGF-1, and the Expression of Osteogenic Genes in Leptin-Deficient Ob/Ob Mice. J. Bone Miner. Res. 2011, 26, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Kalra, S.P.; Wong, C.P.; Philbrick, K.A.; Lindenmaier, L.B.; Boghossian, S.; Iwaniec, U.T. Peripheral Leptin Regulates Bone Formation. J. Bone Miner. Res. 2013, 28, 22–34. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Zhou, B.O.; Shimada, I.S.; Zhao, Z.; Morrison, S.J. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 2016, 18, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Ishidou, Y.; Kato, M.; Imamura, T.; Yonemori, K.; Origuchi, N.; Matsunaga, S.; Yoshida, H.; ten Dijke, P.; Sakou, T. Orthotopic Ossification of the Spinal Ligaments of Zucker Fatty Rats: A Possible Animal Model for Ossification of the Human Posterior Longitudinal Ligament. J. Orthop. Res. 1997, 15, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal Splicing of the Leptin Receptor in Diabetic Mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef]
- Endo, T.; Takahata, M.; Koike, Y.; Iwasaki, N. Clinical Characteristics of Patients with Thoracic Myelopathy Caused by Ossification of the Posterior Longitudinal Ligament. J. Bone Miner. Metab. 2020, 38, 63–69. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nakajima, A.; Aiba, A.; Koda, M.; Okawa, A.; Takahashi, K.; Yamazaki, M. Association between Serum Leptin and Bone Metabolic Markers, and the Development of Heterotopic Ossification of the Spinal Ligament in Female Patients with Ossification of the Posterior Longitudinal Ligament. Eur. Spine J. 2011, 20, 1450–1458. [Google Scholar] [CrossRef]
- Tahara, M.; Aiba, A.; Yamazaki, M.; Ikeda, Y.; Goto, S.; Moriya, H.; Okawa, A. The Extent of Ossification of Posterior Longitudinal Ligament of the Spine Associated with Nucleotide Pyrophosphatase Gene and Leptin Receptor Gene Polymorphisms. Spine 2005, 30, 877–880; discussion 881. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Wangensteen, T.; Collins, S.; Kimber, W.; Matarese, G.; Keogh, J.M.; Lank, E.; Bottomley, B.; Lopez-Fernandez, J.; Ferraz-Amaro, I.; et al. Clinical and Molecular Genetic Spectrum of Congenital Deficiency of the Leptin Receptor. N. Engl. J. Med. 2007, 356, 237–247. [Google Scholar] [CrossRef]
- Wasim, M.; Awan, F.R.; Najam, S.S.; Khan, A.R.; Khan, H.N. Role of Leptin Deficiency, Inefficiency, and Leptin Receptors in Obesity. Biochem. Genet. 2016, 54, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, N.; Maeda, T.; Miura, H.; Jingushi, S.; Hosokawa, A.; Harimaya, K.; Higaki, H.; Kurata, K.; Iwamoto, Y. Repetitive Tensile Stress to Rat Caudal Vertebrae Inducing Cartilage Formation in the Spinal Ligaments: A Possible Role of Mechanical Stress in the Development of Ossification of the Spinal Ligaments. J. Neurosurg. Spine 2006, 5, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, B.; Cheng, L.; Zhou, S.; Tang, Y.; Zhang, Z.; Sun, Y.; Xu, Z.; Li, F.; Liao, X.; et al. Cyclic Tensile Stress to Rat Thoracolumbar Ligamentum Flavum Inducing the Ossification of Ligamentum Flavum: An In Vivo Experimental Study. Spine 2021, 46, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Suzuki, A.; Hayashi, K.; Ohyama, S.; Yabu, A.; Maruf, M.H.; Habibi, H.; Salimi, H.; Nakamura, H. Long-Term, Time-Course Evaluation of Ligamentum Flavum Hypertrophy Induced by Mechanical Stress: An Experimental Animal Study. Spine 2021, 46, E520–E527. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Suzuki, A.; Abdullah Ahmadi, S.; Terai, H.; Yamada, K.; Hoshino, M.; Toyoda, H.; Takahashi, S.; Tamai, K.; Ohyama, S.; et al. Mechanical Stress Induces Elastic Fibre Disruption and Cartilage Matrix Increase in Ligamentum Flavum. Sci. Rep. 2017, 7, 13092. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-Y.; Li, P.; Ao, X.; Qian, L.; Peng, Y.-X.; Chu, J.; Jiang, T.; Lian, Z.-N.; Zhang, Z.-M.; Wang, L. Characterization of a Novel Model of Lumbar Ligamentum Flavum Hypertrophy in Bipedal Standing Mice. Orthop. Surg. 2021, 13, 2457–2467. [Google Scholar] [CrossRef]
- Saito, T.; Yokota, K.; Kobayakawa, K.; Hara, M.; Kubota, K.; Harimaya, K.; Kawaguchi, K.; Hayashida, M.; Matsumoto, Y.; Doi, T.; et al. Experimental Mouse Model of Lumbar Ligamentum Flavum Hypertrophy. PloS ONE 2017, 12, e0169717. [Google Scholar] [CrossRef]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a Distance: Mechanically Coupling the Extracellular Matrix with the Nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in Vascular Physiology and Atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Chen, C.S.; Ingber, D.E. Demonstration of Mechanical Connections between Integrins, Cytoskeletal Filaments, and Nucleoplasm That Stabilize Nuclear Structure. Proc. Natl. Acad. Sci. USA 1997, 94, 849–854. [Google Scholar] [CrossRef]
- Fey, E.G.; Wan, K.M.; Penman, S. Epithelial Cytoskeletal Framework and Nuclear Matrix-Intermediate Filament Scaffold: Three-Dimensional Organization and Protein Composition. J. Cell Biol. 1984, 98, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid Signal Transduction in Living Cells Is a Unique Feature of Mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar] [CrossRef]
- Bryniarska, N.; Kubiak, A.; Łabędź-Masłowska, A.; Zuba-Surma, E. Impact of Developmental Origin, Niche Mechanics and Oxygen Availability on Osteogenic Differentiation Capacity of Mesenchymal Stem/Stromal Cells. Acta Biochim. Pol. 2019, 66, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sugita, D.; Nakajima, H.; Kokubo, Y.; Takeura, N.; Yayama, T.; Matsumine, A. Cyclic Tensile Strain Facilitates Ossification of the Cervical Posterior Longitudinal Ligament via Increased Indian Hedgehog Signaling. Sci. Rep. 2020, 10, 7231. [Google Scholar] [CrossRef] [PubMed]
- Sugita, D.; Yayama, T.; Uchida, K.; Kokubo, Y.; Nakajima, H.; Yamagishi, A.; Takeura, N.; Baba, H. Indian Hedgehog Signaling Promotes Chondrocyte Differentiation in Enchondral Ossification in Human Cervical Ossification of the Posterior Longitudinal Ligament. Spine 2013, 38, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, Y.; Yang, H.; Chen, D.; Zhang, X.; Fermandes, J.C.; Chen, Y. Connexin 43 Promotes Ossification of the Posterior Longitudinal Ligament through Activation of the ERK1/2 and P38 MAPK Pathways. Cell Tissue Res. 2016, 363, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, Y.; Li, T.; Shi, L.; Pan, M.; Chen, D. Role of Cx43-Mediated NFκB Signaling Pathway in Ossification of Posterior Longitudinal Ligament: An In Vivo and In Vitro Study. Spine 2017, 42, E1334–E1341. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.J.; Rashid, M.S.; Snelling, S.J.; Kirtley, S.; Carr, A.J.; Dakin, S.G. Trends in the Theory That Inflammation Plays a Causal Role in Tendinopathy: A Systematic Review and Quantitative Analysis of Published Reviews. BMJ Open Sport Exerc. Med. 2018, 4, e000332. [Google Scholar] [CrossRef]
- Qu, X.; Xu, G.; Hou, X.; Chen, G.; Fan, T.; Yang, X.; Chen, Z. M1 Macrophage-Derived Interleukin-6 Promotes the Osteogenic Differentiation of Ligamentum Flavum Cells. Spine 2022, 47, E527–E535. [Google Scholar] [CrossRef]
- Yayama, T.; Mori, K.; Saito, H.; Fujikawa, H.; Kitagawa, M.; Okumura, N.; Nishizawa, K.; Nakamura, A.; Kumagai, K.; Mimura, T.; et al. Cytokine Profile From the Ligamentum Flavum in Patients with Ossification of the Posterior Longitudinal Ligament in the Cervical Spine. Spine 2022, 47, 277–285. [Google Scholar] [CrossRef]
- Spiesz, E.M.; Thorpe, C.T.; Chaudhry, S.; Riley, G.P.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. Tendon Extracellular Matrix Damage, Degradation and Inflammation in Response to in Vitro Overload Exercise. J. Orthop. Res. 2015, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.B.; Li, Y.; Fung, D.T.; Majeska, R.J.; Schaffler, M.B.; Flatow, E.L. Coordinate Regulation of IL-1beta and MMP-13 in Rat Tendons Following Subrupture Fatigue Damage. Clin. Orthop. 2008, 466, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Yu, T.; An, S.; Deng, R.; Tan, X.; Crane, J.; Zhang, W.; Pan, D.; Wan, M.; et al. Inhibition of Integrin Avβ6 Activation of TGF-β Attenuates Tendinopathy. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, e2104469. [Google Scholar] [CrossRef]
- Vanden Bossche, L.; Vanderstraeten, G. Heterotopic Ossification: A Review. J. Rehabil. Med. 2005, 37, 129–136. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; et al. Inhibition of Overactive TGF-β Attenuates Progression of Heterotopic Ossification in Mice. Nat. Commun. 2018, 9, 551. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, W.; Tang, C.; Huang, J.; Fan, C.; Yin, Z.; Hu, Y.; Chen, W.; Ouyang, H.; Zhou, Y.; et al. Targeted Pathological Collagen Delivery of Sustained-Release Rapamycin to Prevent Heterotopic Ossification. Sci. Adv. 2020, 6, eaay9526. [Google Scholar] [CrossRef]
- Sorkin, M.; Huber, A.K.; Hwang, C.; Carson, W.F.; Menon, R.; Li, J.; Vasquez, K.; Pagani, C.; Patel, N.; Li, S.; et al. Regulation of Heterotopic Ossification by Monocytes in a Mouse Model of Aberrant Wound Healing. Nat. Commun. 2020, 11, 722. [Google Scholar] [CrossRef]
- Archambault, J.M.; Jelinsky, S.A.; Lake, S.P.; Hill, A.A.; Glaser, D.L.; Soslowsky, L.J. Rat Supraspinatus Tendon Expresses Cartilage Markers with Overuse. J. Orthop. Res. 2007, 25, 617–624. [Google Scholar] [CrossRef]
- Kato, K.; Yabuki, S.; Otani, K.; Nikaido, T.; Otoshi, K.-I.; Watanabe, K.; Kikuchi, S.-I.; Konno, S.-I. Ossification of the Ligamentum Flavum in the Thoracic Spine Mimicking Sciatica in a Young Baseball Pitcher:A Case Report. Fukushima J. Med. Sci. 2021, 67, 33–37. [Google Scholar] [CrossRef]
- Kato, K.; Otoshi, K.; Hakozaki, M.; Konno, S.-I. Progressive Enlargement of Thoracic Ossification of the Ligamentum Flavum in Professional Baseball Pitchers: A Report of Two Cases. J. Int. Med. Res. 2021, 49, 3000605211059465. [Google Scholar] [CrossRef]
- Kaneyama, S.; Doita, M.; Nishida, K.; Shimomura, T.; Maeno, K.; Tamura, Y.; Kurosaka, M.; Yonenobu, K. Thoracic Myelopathy Due to Ossification of the Yellow Ligament in Young Baseball Pitchers. J. Spinal Disord. Technol. 2008, 21, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Luk, K.D.K.; Karppinen, J.; Yang, H.; Cheung, K.M.C. Prevalence, Distribution, and Morphology of Ossification of the Ligamentum Flavum: A Population Study of One Thousand Seven Hundred Thirty-Six Magnetic Resonance Imaging Scans. Spine 2010, 35, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kasahara, T.; Mimura, T.; Nishizawa, K.; Murakami, Y.; Matsusue, Y.; Imai, S. Prevalence, Distribution, and Morphology of Thoracic Ossification of the Yellow Ligament in Japanese: Results of CT-Based Cross-Sectional Study. Spine 2013, 38, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, T.; Iwasaki, M.; Nagamoto, Y.; Ishii, T.; Kashii, M.; Murase, T.; Sugiura, T.; Matsuo, Y.; Sugamoto, K.; Yoshikawa, H. Kinematics of the Thoracic Spine in Trunk Rotation: In Vivo 3-Dimensional Analysis. Spine 2012, 37, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Wang, C.; Yuan, W. Rotational Stress: Role in Development of Ossification of Posterior Longitudinal Ligament and Ligamentum Flavum. Med. Hypotheses 2011, 76, 73–76. [Google Scholar] [CrossRef]
- Maigne, J.Y.; Ayral, X.; Guérin-Surville, H. Frequency and Size of Ossifications in the Caudal Attachments of the Ligamentum Flavum of the Thoracic Spine. Role of Rotatory Strains in Their Development. An Anatomic Study of 121 Spines. Surg. Radiol. Anat. SRA 1992, 14, 119–124. [Google Scholar] [CrossRef]
- Ota, M.; Furuya, T.; Maki, S.; Inada, T.; Kamiya, K.; Ijima, Y.; Saito, J.; Takahashi, K.; Yamazaki, M.; Aramomi, M.; et al. Addition of Instrumented Fusion after Posterior Decompression Surgery Suppresses Thickening of Ossification of the Posterior Longitudinal Ligament of the Cervical Spine. J. Clin. Neurosci. 2016, 34, 162–165. [Google Scholar] [CrossRef]
- Lee, C.-H.; Jahng, T.-A.; Hyun, S.-J.; Kim, K.-J.; Kim, H.-J. Expansive Laminoplasty Versus Laminectomy Alone Versus Laminectomy and Fusion for Cervical Ossification of the Posterior Longitudinal Ligament: Is There a Difference in the Clinical Outcome and Sagittal Alignment? Clin. Spine Surg. 2016, 29, 9–15. [Google Scholar] [CrossRef]
- Katsumi, K.; Izumi, T.; Ito, T.; Hirano, T.; Watanabe, K.; Ohashi, M. Posterior Instrumented Fusion Suppresses the Progression of Ossification of the Posterior Longitudinal Ligament: A Comparison of Laminoplasty with and without Instrumented Fusion by Three-Dimensional Analysis. Eur. Spine J. 2016, 25, 1634–1640. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Matsumoto, M.; Iwasaki, M.; Izumi, T.; Okawa, A.; Matsunaga, S.; Chiba, K.; Tsuji, T.; Yamazaki, M.; Fujimori, T.; et al. New Classification System for Ossification of the Posterior Longitudinal Ligament Using CT Images. J. Orthop. Sci. 2014, 19, 530–536. [Google Scholar] [CrossRef]
- Fujimori, T.; Iwasaki, M.; Nagamoto, Y.; Kashii, M.; Ishii, T.; Sakaura, H.; Sugamoto, K.; Yoshikawa, H. Three-Dimensional Measurement of Intervertebral Range of Motion in Ossification of the Posterior Longitudinal Ligament: Are There Mobile Segments in the Continuous Type? J. Neurosurg. Spine 2012, 17, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.J.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional Delivery of Viral MiRNAs via Exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, H.; Gu, W.; Wu, H.; Chen, Y.; Zhou, W.; Sun, B.; Shen, X.; Zhang, Z.; Wang, Y.; et al. The MicroRNA-10a/ID3/RUNX2 Axis Modulates the Development of Ossification of Posterior Longitudinal Ligament. Sci. Rep. 2018, 8, 9225. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Sun, Y.; Zeng, J.; Yuan, B.; Zhao, Y.; Geng, X.; Jia, L.; Zhou, S.; Chen, X. Exosomal MiR-140-5p Inhibits Osteogenesis by Targeting IGF1R and Regulating the MTOR Pathway in Ossification of the Posterior Longitudinal Ligament. J. Nanobiotechnology 2022, 20, 452. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Z.; Liu, N.; Li, L.; Zhong, H.; Wang, R.; Shi, Q.; Zhang, Z.; Wei, L.; Hu, B.; et al. Small Extracellular Vesicle-Mediated MiR-320e Transmission Promotes Osteogenesis in OPLL by Targeting TAK1. Nat. Commun. 2022, 13, 2467. [Google Scholar] [CrossRef]
- Nakajima, M.; Takahashi, A.; Tsuji, T.; Karasugi, T.; Baba, H.; Uchida, K.; Kawabata, S.; Okawa, A.; Shindo, S.; Takeuchi, K.; et al. A Genome-Wide Association Study Identifies Susceptibility Loci for Ossification of the Posterior Longitudinal Ligament of the Spine. Nat. Genet. 2014, 46, 1012–1016. [Google Scholar] [CrossRef]
- Nakajima, M.; Kou, I.; Ohashi, H.; Genetic Study Group of the Investigation Committee on the Ossification of Spinal Ligaments; Ikegawa, S. Identification and Functional Characterization of RSPO2 as a Susceptibility Gene for Ossification of the Posterior Longitudinal Ligament of the Spine. Am. J. Hum. Genet. 2016, 99, 202–207. [Google Scholar] [CrossRef]
- Tachibana, N.; Chijimatsu, R.; Okada, H.; Oichi, T.; Taniguchi, Y.; Maenohara, Y.; Miyahara, J.; Ishikura, H.; Iwanaga, Y.; Arino, Y.; et al. RSPO2 Defines a Distinct Undifferentiated Progenitor in the Tendon/Ligament and Suppresses Ectopic Ossification. Sci. Adv. 2022, 8, eabn2138. [Google Scholar] [CrossRef]
| Species | Region | Method | Result | Reference |
|---|---|---|---|---|
| Rat | Caudal spinal ligament | Cyclic tensile loading | Histological ossification | [72] |
| Rat | Thoracolumbar flavum | Cyclic tensile loading | Ossification detectable by microCT | [73] |
| Rabbit | Lumbar flavum | Adjacent vertebral fixation | Cartilage matrix production | [74,75] |
| Mouse | Lumbar flavum | Bipedal standing | Ligament hypertrophy | [76] |
| Mouse | Lumbar flavum | Consecutive flexion-extension stress | Ligament hypertrophy | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuta, M.; Kaito, T.; Fujimori, T.; Kitahara, T.; Furuichi, T.; Bun, M.; Hirai, H.; Ukon, Y.; Kanie, Y.; Takenaka, S.; et al. Review of Basic Research about Ossification of the Spinal Ligaments Focusing on Animal Models. J. Clin. Med. 2023, 12, 1958. https://doi.org/10.3390/jcm12051958
Ikuta M, Kaito T, Fujimori T, Kitahara T, Furuichi T, Bun M, Hirai H, Ukon Y, Kanie Y, Takenaka S, et al. Review of Basic Research about Ossification of the Spinal Ligaments Focusing on Animal Models. Journal of Clinical Medicine. 2023; 12(5):1958. https://doi.org/10.3390/jcm12051958
Chicago/Turabian StyleIkuta, Masato, Takashi Kaito, Takahito Fujimori, Takayuki Kitahara, Takuya Furuichi, Masayuki Bun, Hiromasa Hirai, Yuichiro Ukon, Yuya Kanie, Shota Takenaka, and et al. 2023. "Review of Basic Research about Ossification of the Spinal Ligaments Focusing on Animal Models" Journal of Clinical Medicine 12, no. 5: 1958. https://doi.org/10.3390/jcm12051958
APA StyleIkuta, M., Kaito, T., Fujimori, T., Kitahara, T., Furuichi, T., Bun, M., Hirai, H., Ukon, Y., Kanie, Y., Takenaka, S., & Okada, S. (2023). Review of Basic Research about Ossification of the Spinal Ligaments Focusing on Animal Models. Journal of Clinical Medicine, 12(5), 1958. https://doi.org/10.3390/jcm12051958






