Prevalence of Overweight and Obesity and Their Impact on Spirometry Parameters in Patients with Asthma: A Multicentre, Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
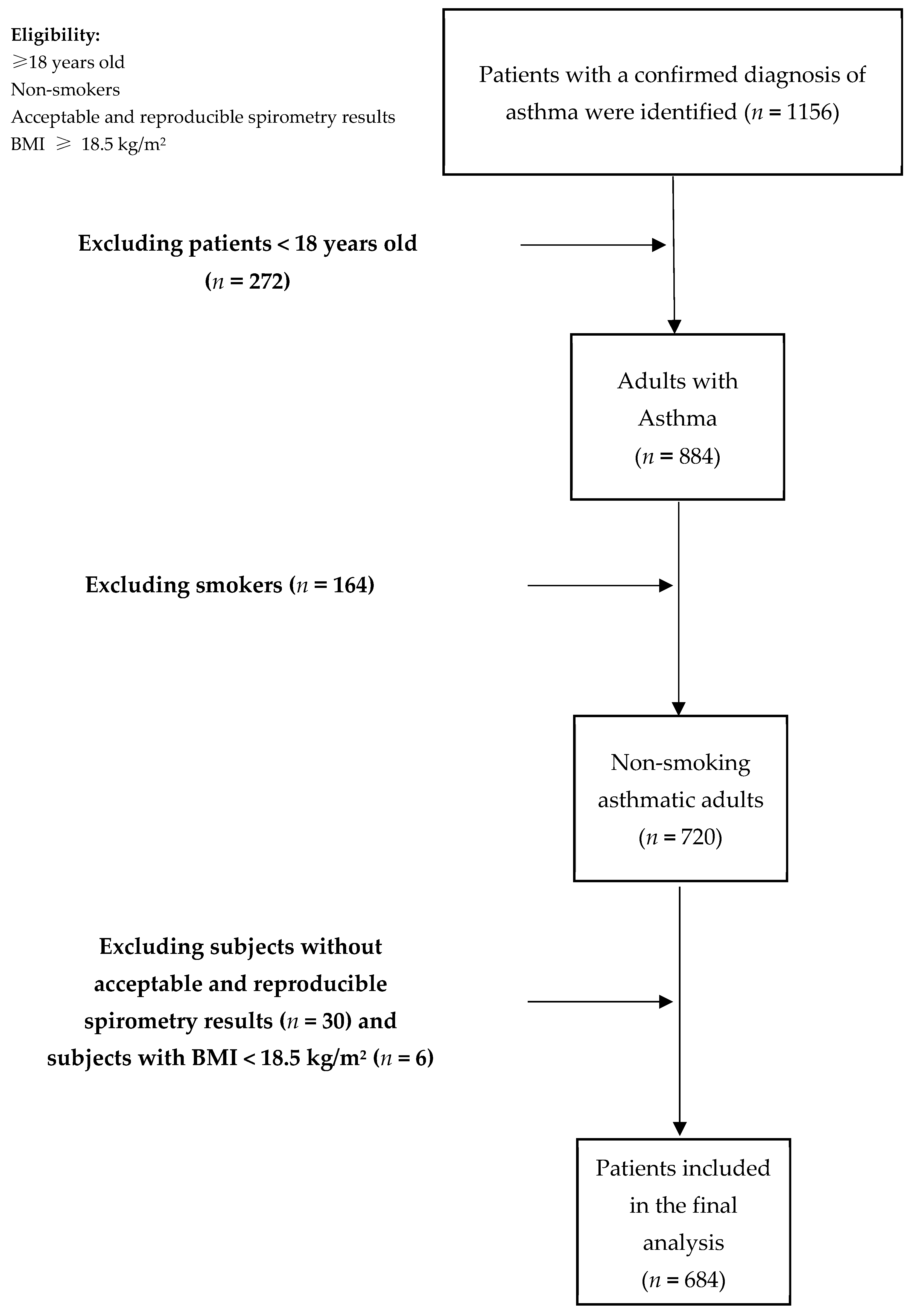
2.2. Study Population
2.3. Spirometry Parameters
2.4. Body Mass Index
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
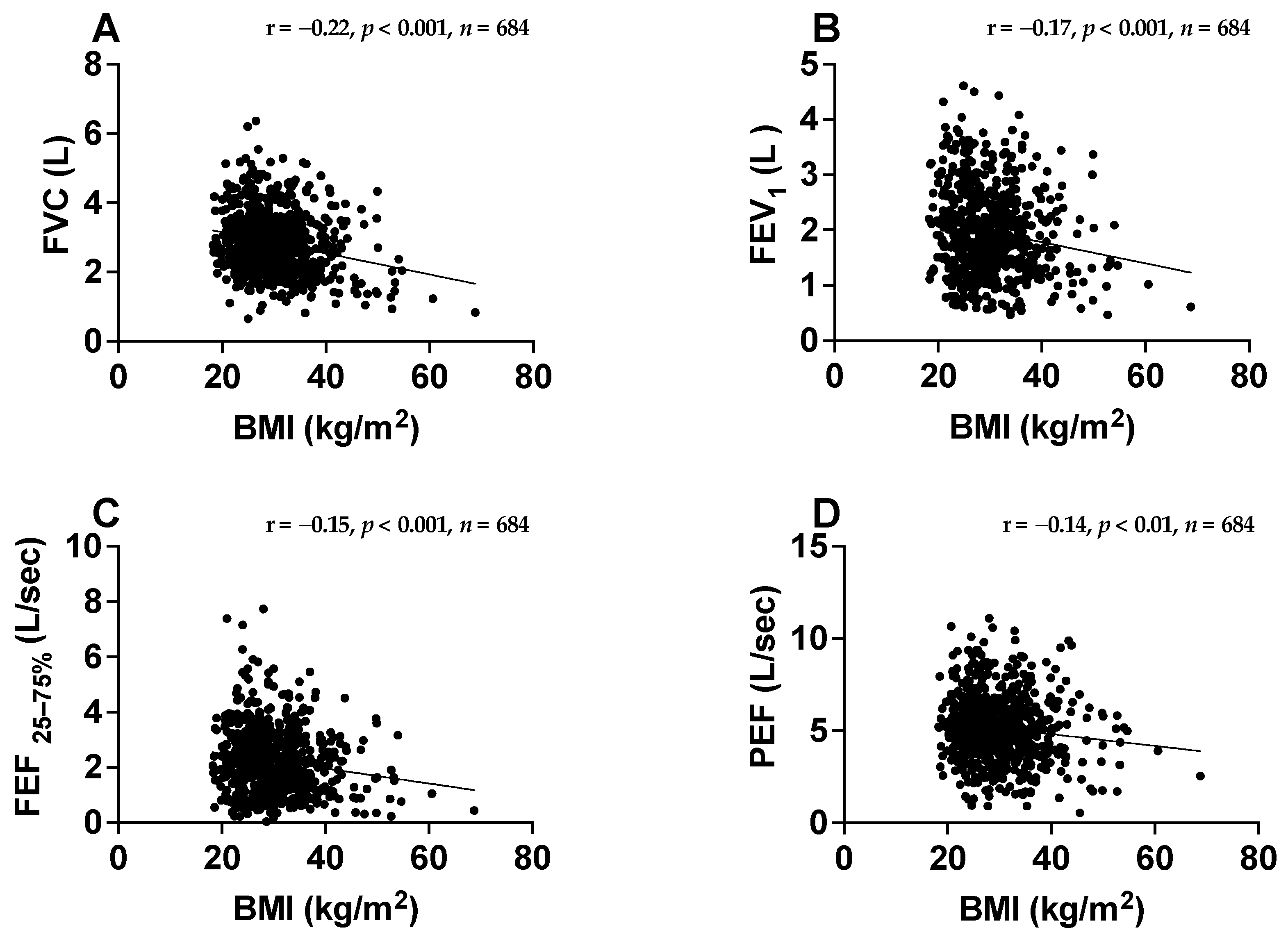
3.2. Associations between BMI and Spirometry Parameters
3.3. Impact of Overweight and Obesity on Spirometry Parameters
4. Discussion
4.1. Strengths
4.2. Limitations
4.3. Practical Implementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. Global Initiative for Asthma P. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021. Executive Summary and Rationale for Key Changes. Arch. Bronconeumol. 2022, 58, 35–51. [Google Scholar] [CrossRef]
- Lim, Y.; Boster, J. Obesity and Comorbid Conditions; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ghesmaty Sangachin, M.; Cavuoto, L.A.; Wang, Y. Use of various obesity measurement and classification methods in occupational safety and health research: A systematic review of the literature. BMC Obes. 2018, 5, 28. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 January 2023).
- Defining Adult Overweight & Obesity by Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/obesity/basics/adult-defining.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fobesity%2Fadult%2Findex.html#print (accessed on 13 January 2023).
- Sin, D.D.; Jones, R.L.; Man, S.F. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch. Intern. Med. 2002, 162, 1477–1481. [Google Scholar] [CrossRef]
- Kasteleyn, M.J.; Bonten, T.N.; de Mutsert, R.; Thijs, W.; Hiemstra, P.S.; le Cessie, S.; Rosendaal, F.R.; Chavannes, N.H.; Taube, C. Pulmonary function, exhaled nitric oxide and symptoms in asthma patients with obesity: A cross-sectional study. Respir. Res. 2017, 18, 205. [Google Scholar] [CrossRef]
- Klepaker, G.; Henneberger, P.K.; Hertel, J.K.; Holla, O.L.; Kongerud, J.; Fell, A.K.M. Influence of asthma and obesity on respiratory symptoms, work ability and lung function: Findings from a cross-sectional Norwegian population study. BMJ Open Respir. Res. 2021, 8, e000932. [Google Scholar] [CrossRef]
- Reddel, H.; Ware, S.; Marks, G.; Salome, C.; Jenkins, C.; Woolcock, A. Differences between asthma exacerbations and poor asthma control. Lancet 1999, 353, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.; Israel, E.; Jarjour, N.N.; et al. Obesity and asthma: An association modified by age of asthma onset. J. Allergy Clin. Immunol. 2011, 127, 1486–1493.e2. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Al-Moamary, M.S.; Alhaider, S.A.; Alangari, A.A.; Idrees, M.M.; Zeitouni, M.O.; Al Ghobain, M.O.; Alanazi, A.F.; Al-Harbi, A.S.; Yousef, A.A.; Alorainy, H.S.; et al. The Saudi Initiative for Asthma—2021 Update: Guidelines for the diagnosis and management of asthma in adults and children. Ann. Thorac. Med. 2021, 16, 4–56. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Chu, Y.C.; Huang, H.L.; Hwang, J.S.; Chan, T.C. The Effects of Asthma on the Association Between Pulmonary Function and Obesity: A 16-Year Longitudinal Study. J. Asthma Allergy 2021, 14, 347–359. [Google Scholar] [CrossRef]
- Althumiri, N.A.; Basyouni, M.H.; AlMousa, N.; AlJuwaysim, M.F.; Almubark, R.A.; BinDhim, N.F.; Alkhamaali, Z.; Alqahtani, S.A. Obesity in Saudi Arabia in 2020: Prevalence, Distribution, and Its Current Association with Various Health Conditions. Healthcare 2021, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Trudo, F.; Voorham, J.; Xu, X.; Kerkhof, M.; Jie, J.L.Z.; Tran, T.N. Adverse outcomes from initiation of systemic corticosteroids for asthma: Long-term observational study. J. Asthma Allergy 2018, 11, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Peerboom, S.; Graff, S.; Seidel, L.; Paulus, V.; Henket, M.; Sanchez, C.; Guissard, F.; Moermans, C.; Louis, R.; Schleich, F. Predictors of a good response to inhaled corticosteroids in obesity-associated asthma. Biochem. Pharmacol. 2020, 179, 113994. [Google Scholar] [CrossRef] [PubMed]
- Gibeon, D.; Batuwita, K.; Osmond, M.; Heaney, L.G.; Brightling, C.E.; Niven, R.; Mansur, A.; Chaudhuri, R.; Bucknall, C.E.; Rowe, A.; et al. Obesity-associated severe asthma represents a distinct clinical phenotype: Analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest 2013, 143, 406–414. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Lee, J.; Li, C.; Han, T.; Lee, S.B.; Lee, H.; Back, S.K.; Na, H.S. Juvenile obesity aggravates disease severity in a rat model of atopic dermatitis. Allergy Asthma Immunol. Res. 2015, 7, 69–75. [Google Scholar] [CrossRef]
- Neveu, W.A.; Allard, J.L.; Raymond, D.M.; Bourassa, L.M.; Burns, S.M.; Bunn, J.Y.; Irvin, C.G.; Kaminsky, D.A.; Rincon, M. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir. Res. 2010, 11, 28. [Google Scholar] [CrossRef]
- Dixon, A.E.; Shade, D.M.; Cohen, R.I.; Skloot, G.S.; Holbrook, J.T.; Smith, L.J.; Lima, J.J.; Allayee, H.; Irvin, C.G.; Wise, R.A. American Lung Association-Asthma Clinical Research C. Effect of obesity on clinical presentation and response to treatment in asthma. J. Asthma 2006, 43, 553–558. [Google Scholar] [CrossRef]
- Aaron, S.D.; Fergusson, D.; Dent, R.; Chen, Y.; Vandemheen, K.L.; Dales, R.E. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 2004, 125, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar]
- Mehari, A.; Afreen, S.; Ngwa, J.; Setse, R.; Thomas, A.N.; Poddar, V.; Davis, W.; Polk, O.D.; Hassan, S.; Thomas, A.V. Obesity and Pulmonary Function in African Americans. PLoS ONE 2015, 10, e0140610. [Google Scholar] [CrossRef] [PubMed]

| Variable | Lean (n = 157) | Overweight (n = 213) | Class I Obesity (n = 179) | Class II Obesity (n = 82) | Class III Obesity (n = 53) |
|---|---|---|---|---|---|
| Age (years) | 40 ± 16 | 47 ± 17 | 51 ± 15 | 51 ± 14 | 53 ± 14 |
| Height (cm) | 161 ± 8 | 163 ± 8 | 161 ± 10 | 157 ± 21 | 156 ± 9 |
| Weight (kg) | 59 ± 8 | 71 ± 8 | 83 ± 12 | 90 ± 18 | 111 ± 16 |
| BMI (kg/m2) | 22 ± 2 | 27 ± 1 | 32 ± 1 | 37 ± 1 | 46 ± 6 |
| Female, n (%) | 104 (66%) | 160 (75%) | 130 (73%) | 69 (84%) | 44 (83%) |
| Independent Variable | BMI | |
|---|---|---|
| β (95% CI; p-Value) | Adjusted β (95% CI; p-Value) | |
| FVC L | −0.03 (−0.04 to −0.02; p < 0.001) | −0.02 (−0.028 to −0.01; p < 0.001) 1 |
| FEV1 L | −0.02 (−0.028 to −0.01; p < 0.05) | −0.01 (−0.01 to −0.001; p < 0.05) 1 |
| FEF (25–75%) L/s | −0.03 (−0.04 to −0.014; p < 0.001) | −0.012 (−0.025 to 0.001; p = 0.058) 1 |
| PEF L/s | −0.03 (−0.05 to −0.01; p < 0.01) | −0.014 (−0.03 to 0.01; p = 0.179) 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, A.A.; Aldhahir, A.M.; Siraj, R.A.; Alqahtani, J.S.; Alshehri, H.H.; Alshamrani, A.M.; Namnqani, A.A.; Alsaidalani, L.N.; Tawhari, M.N.; Badr, O.I.; et al. Prevalence of Overweight and Obesity and Their Impact on Spirometry Parameters in Patients with Asthma: A Multicentre, Retrospective Study. J. Clin. Med. 2023, 12, 1843. https://doi.org/10.3390/jcm12051843
Alqarni AA, Aldhahir AM, Siraj RA, Alqahtani JS, Alshehri HH, Alshamrani AM, Namnqani AA, Alsaidalani LN, Tawhari MN, Badr OI, et al. Prevalence of Overweight and Obesity and Their Impact on Spirometry Parameters in Patients with Asthma: A Multicentre, Retrospective Study. Journal of Clinical Medicine. 2023; 12(5):1843. https://doi.org/10.3390/jcm12051843
Chicago/Turabian StyleAlqarni, Abdullah A., Abdulelah M. Aldhahir, Rayan A. Siraj, Jaber S. Alqahtani, Hams H. Alshehri, Amal M. Alshamrani, Ahlam A. Namnqani, Lama N. Alsaidalani, Mohammed N. Tawhari, Omaima I. Badr, and et al. 2023. "Prevalence of Overweight and Obesity and Their Impact on Spirometry Parameters in Patients with Asthma: A Multicentre, Retrospective Study" Journal of Clinical Medicine 12, no. 5: 1843. https://doi.org/10.3390/jcm12051843
APA StyleAlqarni, A. A., Aldhahir, A. M., Siraj, R. A., Alqahtani, J. S., Alshehri, H. H., Alshamrani, A. M., Namnqani, A. A., Alsaidalani, L. N., Tawhari, M. N., Badr, O. I., & Alwafi, H. (2023). Prevalence of Overweight and Obesity and Their Impact on Spirometry Parameters in Patients with Asthma: A Multicentre, Retrospective Study. Journal of Clinical Medicine, 12(5), 1843. https://doi.org/10.3390/jcm12051843






