Abstract
Background: Taxifolin (TXF) is a flavonoid found abundantly in citrus/onion. Encouraging results on its renoprotective effect have been reported in a limited number of drug-induced nephrotoxicity animal models. The present study aimed to evaluate for the first time the potential renoprotective effects of TXF in a paracetamol (PAR)-induced nephrotoxicity rat model. Methods: Rats were divided into three equal groups (n = 6 animals per group). Group 1 (PAR group, PARG) received PAR diluted in normal saline by gavage (1000 mg/kg). Group 2 (TXF group, TXFG) received TXF diluted in normal saline by gavage (50 mg/kg) one hour after PAR administration. Group 3 (control group, CG) received normal saline. Twenty-four hours after PAR administration, all animals were sacrificed using high-dose anesthesia. Blood samples were collected and kidneys were removed. Results: The serum blood urea nitrogen, creatinine levels and serum malondialdehyde levels were significantly increased in the PARG. The serum glutathione peroxidase, glutathione reductase and total glutathione levels were significantly higher in the TXFG. At the same time, the kidneys of the PARG animals demonstrated tubular epithelium swelling, distension and severe vacuolar degeneration. The kidneys of the TXFG animals showed mildly dilated/congested blood vessels. Conclusions: The TXF renoprotective effects are promising in preventing PAR-induced nephrotoxicity, mainly through antioxidant activity, and warrant further testing in future studies.
1. Introduction
Paracetamol (4′-hydroxyacetanilide, N-acetyl-p-aminophenol, acetaminophen, PAR) is an analgesic-antipyretic drug sold worldwide without prescription in most countries [1]. It is an effective and useful drug at therapeutic doses; however, severe side effects have been reported at high doses [2]. Both isolated renal failure and renal failure combined with liver failure have been reported in association with PAR overdosing [3]. Existing guidelines still encourage treatment based mostly on the dose calculated in the 1970s for the reported studies of acetylcysteine in PAR toxicity [4]. A massive PAR overdose causing nephrotoxicity could occur after extrapolated 4 h plasma PAR concentration (about >250 mg/L) or the intravenous administration of a 150 mg/kg bolus dose [5]. While PAR has been administered by gavage at 0.5–2.5 g/kg to create hepatotoxicity and nephrotoxicity in rats, exact known toxic doses remain inconclusive in the international literature [6].
Acute tubular necrosis and acute renal failure due to PAR toxicity may develop [7]. The elevation of serum creatinine (sCr) and blood urea nitrogen (BUN) levels may be indicators of PAR-induced acute tubular necrosis. Approximately 1–2% of patients exposed to PAR overdose develop renal failure [8,9]. Paracetamol induces liver injury due to its metabolic conversion to the highly reactive intermediate N-acetyl p-benzoquinonimine (NAPQI) by cytochrome P-450 mediated oxidases [10]. This mechanism has been accepted for PAR-induced nephrotoxicity too [11]. Renal toxicity is mediated through NAPQI formation after PAR conjugation through glucuronidation and sulphation is saturated [12]. A PAR overdose causes systemic toxicity by disrupting the balance between NAPQI and basal glutathione levels [2,13]. Accumulated toxic metabolites cause subendothelial damage and acute tubular necrosis. Apart from direct nephrotoxicity, oxidative stress and an insufficient amount of renal glutathione contribute to renal injury and progressing renal failure [14,15,16].
Nowadays, natural antioxidants, rather than chemical drugs, are used as protective agents against organ damage in many diseases [17]. Polyphenols are herbal agents abundant in marine pine bark and contain some antioxidant flavonoids [18,19]. Despite the fact that data regarding the biological functions of polyphenols are abundant, evidence is still inadequate to support the clear beneficial effects on human chronic diseases [20]. Nevertheless, from these antioxidant flavonoids, for example, the protective effect of proanthocyanin and silymarin against renal damage has previously been reported [21,22]. Flavonoids exhibit antioxidant activity by inhibiting the lipid peroxidation and enzymatic reactions responsible for the formation of free radicals. Taxifolin (3, 5, 7, 3, 4-pentahydroxy flavanone or dihydroquercetin, TXF) is a flavonoid found abundantly in citrus and onion [23]. Recently, the protective effects of TXF on hepatotoxicity-induced liver injury have been demonstrated [24]. Encouraging results on the renoprotective effect of TXF have been reported in a limited number of drug-induced nephrotoxicity animal models [25,26,27]. The present study aimed to evaluate for the first time the potential renoprotective effects of TXF in a PAR-induced nephrotoxicity rat model.
2. Materials and Methods
2.1. Animals
Eighteen adults male Wistar rats (aged 8–10 weeks) weighing 255 ± 15 g were obtained from Atatürk University Medical Experimental Practice and Research Center (Erzurum, Turkey). They were bred and housed in ventilated rooms at a temperature of 24 ± 2 °C, with a 12 h light/dark cycle and a humidity of 60 ± 4%. Thiopental sodium (Pental Sodium, IE Ulagay Drug Co., Istanbul, Turkey), TXF (Evalar, Hertz, Moscow, Russia) and PAR (Parol, Atabay Drug Co., Istanbul, Turkey) were purchased. Rats were randomly divided into three equal groups (n = 6 animals in each group). Group 1 (PAR group, PARG) received PAR diluted in normal saline by gavage (1000 mg/kg suspension) at a single dose. Group 2 (TXF group, TXFG) received TXF diluted in normal saline by gavage (50 mg/kg suspension) one hour after PAR administration. Group 3 (Control group, CG) received normal saline only. Twenty-four hours after PAR administration, all animals were sacrificed using high-dose anesthesia (50 mg/kg thiopental), blood samples were collected and kidneys were removed. Prerequisites for the experimental process were in accordance with the Guide for the Care and Use of Laboratory Animals of Atatürk University. The Ethical Committee approved the study (Protocol; 2018/8812460-00.99-EE.45082).
2.2. Blood Analyses and Histopathological Evaluation
Serum creatinine (sCr) and blood urea nitrogen (BUN) levels were determined using COBAS Integra 800 analyzer (Roche, Schaffhausen, Switzerland). Oxidative stress biomarkers were measured in the animals’ renal tissues as previously described using renal tissue homogenate (diluted 1:2) [28]. Total plasma protein was determined using a Bradford reagent (Sigm, Rockville, USA). More specifically, malondialdehyde (MDA) was measured in 532 nm wavelength and the pink complex formed at a high temperature (95 °C) based on spectrophotometric measurement [29]. The total glutathione (tGlu) assay (yellow color) was measured at 412 nm wavelength [30]. Glutathione peroxidase (GPO) and Glutathione reductase (GR) activity were determined at 340 nm wavelength (Lawrence and Burk for GPO and Carlberg and Mannervik for GR were used) [31,32]. Rat kidneys were removed immediately after sacrifice. They were fixed in neutral formalin 10% (Sigma, Rockville, USA) solution for 24 h, then embedded in paraffin wax and sectioned (4 µm thickness) for histopathological assessment. Renal tissue sections were stained with hematoxylin and eosin (H and E stain) using a standard protocol and were evaluated under light microscopy [33,34,35].
2.3. Statistical Analysis
Statistical analysis was performed using the SPSS software (SPSS Inc., Version 25.0. Chicago, IL, USA). Groups were compared using one-way analysis of variance (ANOVA), following normality/homoscedasticity checks with Shapiro–Wilk test/Leven’s test, respectively. Post hoc comparisons were performed using Tukey/Tamhane’s test, as appropriate. Bivariate correlation analysis was used to find correlations among the biochemical markers monitored both in serum and tissue. The chi-square (χ2) test was used for the analysis of associations between histopathological findings and levels of oxidative stress markers in tissue samples and sCr. p value ≤ 0.05 was considered significant.
3. Results
The results of the biochemical analysis in the PARG, TXFG and CG animals, in serum and tissue samples, are summarized in Table 1. Serum creatinine and BUN levels increased following PAR administration compared to the CG; this increase was statistically significant in the PARG and reached over 600% for sCr and over 300% for BUN levels, while in the TXFG the increase in sCr was 11% (nearly significant, p = 0.076) and for BUN was insignificant and less than 10%. Oxidative stress markers in renal tissue samples were severely altered after PAR administration. Malondialdehyde, one of the final products of polyunsaturated fatty acid peroxidation in the cells, was measured at more than 2 times higher in the PARG animals compared to the CG. Taxifolin administration almost restored MDA levels compared to the CG (16% increase). Similarly, GSH levels diminished by 63% in the PARG compared to the CG, which points to elevated cellular vulnerability towards oxidative stress and was restored by 83% in the TXFG. The same pattern was observed for GPO and GRx. A non-consistent and weak pattern of bivariate correlations between the biochemical markers monitored was observed within the experimental groups of the present study. In the PARG animals, the renal tissue levels of MDA were positively correlated with GPO (r = 0.890, p = 0.046), while GPO kidney levels were negatively correlated with GRx levels in the TXFG animals (r = −0.868, p = 0.025). Serum creatine and BUN did not correlate with any tissue oxidative stress markers monitored in all groups, with the exception of sCr; its mildly elevated levels in the TXFG animals were negatively correlated with GPO (r = 0.920, p = 0.013).

Table 1.
Levels of Malondialdehyde (MDA), total Glutathione (tGSH), Glutathione peroxidase (GPO) and Glutathione reductase (GRx) monitored in renal tissues samples, serum Creatinine (sCr) and blood urea nitrogen (BUN) from the Paracetamol group (PARG), the Taxifolin group (TXFG) and the Control (CG).
Kidney specimens demonstrated tubular epithelium swelling, distension and severe vacuolar degeneration in the PARG. Kidney specimens showed mildly dilated and congested blood vessels in the TXFG. No abnormal morphological changes were observed under light microscopy in the kidney specimens of the CG, which received normal saline only. The histopathological results are presented in Figure 1 and their rating is summarized in Table 2.
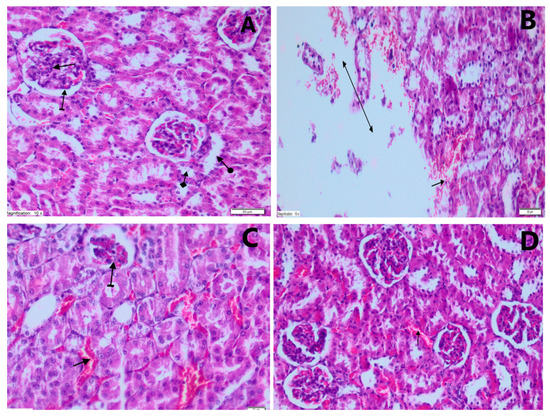
Figure 1.
Results of the histopathological analysis. (A) Histological status in the control group (CG) animals, without any signs of kidney damage (H&EX200): glomerular structure (straight arrow), bowman capsule (straight arrow), proximal tubule (check arrow) and distal tubule (round arrow). (B) Histopathological changes of damaged kidney tissue in the paracetamol group (PARG) (H&EX200): hemorrhage in the parenchyma (straight arrow), destruction and edema (double-sided arrow). (C) Histopathological appearance of damaged kidney tissue in the PARG (H&EX200): mild atrophic glomerulus (striated arrow), dilated and congested blood vessels (straight arrow). (D) Histopathological changes (H&EX200) in the kidneys of animals treated with Taxifolin after administration of high dose PAR (TXFG): mildly dilated and congested blood vessels (straight arrow).

Table 2.
Summary of the histopathological scores based on H and E staining results for kidney tissues.
Serum creatine increase was associated with the severity of glomerular damage (PARG: p = 0.015; TXFG: p = 0.022). On the other hand, only in the PARG was the MDA increase associated both with the extent of parenchyma destruction and edema (p = 0.01) and glomerular damage (p = 0.02). Hyperemia in the PARG animals was nearly significantly associated with the decreased GSH levels (p = 0.057).
4. Discussion
The present study aimed to evaluate for the first time any potential renoprotective effects of TXF in a PAR-induced nephrotoxicity rat model. Potential protective agents previously studied in experimental nephrotoxicity models using PAR high-dosing schemes follow different modes of action and the results remain controversial [36,37,38]. Acetaminophen-related kidney dysfunction has been defined in clinical practice based on the laboratory examination of sCr, BUN, the glomerular filtration rate and albumin sCr ratio [39,40]. In our study, after PAR administration, sCr and BUN levels severely increased; TXF administration counteracted this increase to a large extent for both markers of renal function. Previous studies reported a protective effect of rhein, silymarin and grape seed pro-anthocyanidin on renal function in PAR toxicity and ischemia-reperfusion injury [26]. There are also reports that high sCr, BUN and MDA levels due to nephropathy were significantly decreased after flavonoid administration. In this relation, Baponva et al. (2022), showed that the use of the aqueous extract of the African plant Amblygonocarpus andongensis’s stem bark helped recovery from hepatic and renal failure caused by PAR toxicity [9,41].
After orally receiving PAR, nearly 63% of PAR is metabolized through glucuronidation and 34% via sulphation mainly in the liver [42,43]. N-acetyl p-benzoquinonimine plays a role as a reactive intermediate, when the oxidization of 55% of PAR occurs by the microsomal P-450 enzyme system. Taking into consideration PAR metabolism and excretion, changes and the aggravation of the oxidative stress status are expected, not only regarding circulating oxidative stress, but also on a tissue level. N-acetyl p-benzoquinonimine is detoxified by intracellular GSH, when receiving PAR in therapeutic doses. Therefore, serum tGSH levels during nephrotoxicity following PAR overdosing have been recognized as a biochemical marker indicating the degree of kidney injury in animal models. There are also reports of PAR renal toxicity without liver toxicity [42,43]. In our study, although the mean tGSH in the TXFG still remained slightly lower than the CG, the alleviating effect of TXF is evident, similar to other reports on flavonoid administration (Nigella Sativa, Rhein) in order to treat PAR toxicity [44,45].
In the case of kidney damage caused by chemicals and drugs, the inability to eliminate free radicals after oxidative stress results in cellular destruction [46]. Therefore, the local changes in the oxidative status observed could also entail kidney histopathological alterations. In the current study, histopathology showed the presence of hemorrhage, destruction and edema of the renal parenchyma as previously described [42]. Moreover, a mild atrophic glomerulus and dilated/congested blood vessels in kidney specimens of the PARG were found. The subendothelial damage shown—acute tubular necrosis caused by PAR overdose—was documented through elevated sCr and BUN levels. Taxifolin administration restored to a large extent even the histopathological alterations due to PAR toxicity.
Injury to the nephrocellular membrane causes lipid peroxidation, leading to the release of inflammatory mediators or free radicals. Malondialdehyde has been widely accepted as an indicator of the degree of lipid peroxidation [47,48,49]. In our study, mean renal tissue MDA levels were significantly higher in the PARG compared to the CG and the TXFG. Tissue MDA levels after TXF administration returned to CG levels. Our work was consistent with previous studies [50].
Free radicals oxidize biomolecules including proteins, lipids and DNA. The enzyme GPO is a selenium-dependent enzyme and its main effect is the removal of H2O2, and also it inhibits the formation of highly reactive hydroxyl radicals [29,51]. Glutathione reductase is also a crucial enzyme and serves to restore cellular glutathione levels by reducing oxidized disulfide-glutathione [31]. The mechanism of nephrotoxicity is closely related to the depletion of the antioxidant defense system [52,53,54]. In the present study, GRx and GPO activities were monitored and our findings further support the results of previous experiments demonstrating the protective antioxidant activity of flavonoids, such as TFX, in the PAR nephrotoxicity experimental model [55,56,57].
The limitation of the current study is the use of a single and not escalating dosing scheme for TFX after PAR nephrotoxicity is established. Furthermore, different time intervals for administering TXF could be studied, such as pre-conditioning with TXF [39] and simultaneous administration with PAR, in order to mimic hospitalization treatment conditions. Finally, a comparative study of other antioxidants with TFX in the said model could further demonstrate the nephroprotective effectiveness of TXF.
5. Conclusions
Taxifolin has been accepted as an essential component of some dietary supplements and antioxidant-rich functional foods. Moreover, the antioxidant activity of TXF has been previously demonstrated by the ferric thiocyanate method [24,27,28,31,43]. The present study, for the first time, evaluated the potential renoprotective effects of TXF in a PAR-induced nephrotoxicity rat model. Oxidative damage was proven as a component of PAR nephrotoxicity. Our results provide evidence that TXF may have the potential to prevent PAR-induced nephrotoxicity through its antioxidant activity and warrants further testing in future studies and clinical trials.
Author Contributions
Conceptualization, I.T., T.C., İ.M., A.T. (Aristidis Tsatsakis), K.T., C.T. and A.T. (Ali Taghizadehghalehjoughi); methodology, I.T., M.Y.Ö., T.C., İ.M., A.H., C.M., A.T. (Aristidis Tsatsakis), K.T., C.T. and A.T. (Ali Taghizadehghalehjoughi); validation, I.T., M.Y.Ö. and İ.M.; formal analysis, M.Y.Ö., A.H., A.T. (Aristidis Tsatsakis) and A.T. (Ali Taghizadehghalehjoughi); investigation, M.Y.Ö., A.H., C.M., A.T. (Aristidis Tsatsakis), K.T. and A.T. (Ali Taghizadehghalehjoughi); resources, I.T., M.Y.Ö., T.C. and İ.M.; data curation, I.T., M.Y.Ö. and A.H.; writing—original draft, M.Y.Ö. and T.C.; writing—review and editing, C.M., A.T. (Aristidis Tsatsakis), K.T., C.T. and A.T. (Ali Taghizadehghalehjoughi); visualization, M.Y.Ö., İ.M., A.T. (Aristidis Tsatsakis) and A.T. (Ali Taghizadehghalehjoughi); supervision, M.Y.Ö., A.T. (Aristidis Tsatsakis) and C.T.; project administration, I.T., M.Y.Ö. and İ.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Care and Use of Laboratory Animals was performed according to the Guide for of the Atatürk University. The Ethical Committee approved the study (Protocol; 2018/8812460-00.99-EE.45082).
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data is available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patterson, T.; Turner, J.; Gnjidic, D.; Mintzes, B.; Bennett, C.; Bywaters, L.; Clavisi, O.; Baysari, M.; Ferreira, M.; Beckenkamp, P.; et al. (C)onsumer focused (E)ducation on p(A)racetamol (S)ide (E)ffects, i(N)adequate (O)utcomes and (W)eaning (CEASE NOW) for individuals with low back pain: Results of a feasibility study. BMJ Open 2022, 12, e068164. [Google Scholar] [CrossRef]
- Prescott, L. Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmacol. 1980, 10, 291S–298S. [Google Scholar] [CrossRef] [PubMed]
- Chiew, A.L.; Isbister, G.K.; Duffull, S.B.; Buckley, N.A. Evidence for the changing regimens of acetylcysteine. Br. J. Clin. Pharmacol. 2016, 81, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.; Illingworth, R.; Critchley, J.; Stewart, M.; Adam, R.; Proudfoot, A. Intravenous N-acetylcystine: The treatment of choice for paracetamol poisoning. Br. Med. J. 1979, 2, 1097–1100. [Google Scholar] [CrossRef]
- Marks, D.J.; Dargan, P.I.; Archer, J.R.; Davies, C.L.; Dines, A.M.; Wood, D.M.; Greene, S.L. Outcomes from massive paracetamol overdose: A retrospective observational study. Br. J. Clin. Pharmacol. 2017, 83, 1263–1272. [Google Scholar] [CrossRef]
- Bektur, N.E.; Sahin, E.; Baycu, C.; Unver, G. Protective effects of silymarin against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice. Toxicol. Ind. Health 2016, 32, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Makhdoom, S.; Maqbool, A.; Muhammad, H.; Makhdoom, M.; Ashraf, H.; Khan, W.A.; Irfan, M. Nephrocurative Effect of Parthenium hysterophorus (Carrot Grass) in Paracetamol Induced Nephrotoxicity in Rabbits. Cell Mol. Biol. (Noisy-Le-Grand) 2022, 68, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Zhou, G.-D.; Yang, H.-B.; Wang, J.-B.; Shan, L.-M.; Li, R.-s.; Xiao, X.-H. Rhein protects against acetaminophen-induced hepatic and renal toxicity. Food Chem. Toxicol. 2011, 49, 1705–1710. [Google Scholar] [CrossRef]
- Georgiadis, G.; Mavridis, C.; Belantis, C.; Zisis, I.E.; Skamagkas, I.; Fragkiadoulaki, I.; Heretis, I.; Tzortzis, V.; Psathakis, K.; Tsatsakis, A.; et al. Nephrotoxicity issues of organophosphates. Toxicology 2018, 406–407, 129–136. [Google Scholar] [CrossRef]
- Commandeur, J.; Vermeulen, N. Cytotoxicity and cytoprotective activities of natural compounds. The case of curcumin. Xenobiotica 1996, 26, 667–680. [Google Scholar] [CrossRef]
- Stamper, B.D. Acetyl-p-Aminophenol and Acetyl-m-Aminophenol: Using Structure-Toxicity Relationships to Identify Biomarkers for Hepatocellular Injury; University of Washington: Washington, DC, USA, 2010. [Google Scholar]
- Richie Jr, J.P.; Lang, C.A.; Chen, T.S. Acetaminophen-induced depletion of glutathione and cysteine in the aging mouse kidney. Biochem. Pharmacol. 1992, 44, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Devalia, J.L.; Ogilvie, R.C.; McLean, A.E. Dissociation of cell death from covalent binding of paracetamol by flavones in a hepatocyte system. Biochem. Pharmacol. 1982, 31, 3745–3749. [Google Scholar] [CrossRef] [PubMed]
- Manyike, P.T.; Kharasch, E.D.; Kalhorn, T.F.; Slattery, J.T. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin. Pharmacol. Ther. 2000, 67, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Blantz, R.C. Acetaminophen: Acute and chronic effects on renal function. Am. J. Kidney Dis. 1996, 28, S3–S6. [Google Scholar] [CrossRef]
- Iordache, A.M.; Docea, A.O.; Buga, A.M.; Zlatian, O.; Ciurea, M.E.; Rogoveanu, O.C.; Burada, F.; Sosoi, S.; Mitrut, R.; Mamoulakis, C.; et al. Sildenafil and tadalafil reduce the risk of contrast-induced nephropathy by modulating the oxidant/antioxidant balance in a murine model. Food Chem. Toxicol. 2020, 135, 111038. [Google Scholar] [CrossRef]
- Cicek, B.; Genc, S.; Yeni, Y.; Kuzucu, M.; Cetin, A.; Yildirim, S.; Bolat, I.; Kantarci, M.; Hacimuftuoglu, A.; Lazopoulos, G. Artichoke (Cynara Scolymus) Methanolic Leaf Extract Alleviates Diethylnitrosamine-Induced Toxicity in BALB/c Mouse Brain: Involvement of Oxidative Stress and Apoptotically Related Klotho/PPARγ Signaling. J. Pers. Med. 2022, 12, 2012. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Alhammad, A.M.; Al Aamer, K.; Alshehri, S.; Alhuwahmel, A.; Kharbosh, A.; Alshehri, A.; Alshareef, H.; Al Sulaihim, I.; et al. The potential role of adjunctive ascorbic acid in the prevention of colistin-induced nephrotoxicity in critically ill patients: A retrospective study. Saudi Pharm. J. 2022, 30, 1748–1754. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Hassan, S.M.; Sayed, A.M.; Abo-Youssef, A.M. Carvacrol mitigates vancomycin-induced nephrotoxicity via regulation of IkBalpha/p38MAPK and Keap1/Nrf2 signaling pathways: An experimental study with in silico evidence. Eur. Rev. Med. Pharm. Sci. 2022, 26, 8738–8755. [Google Scholar] [CrossRef]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Boozari, M.; Hosseinzadeh, H. Natural medicines for acute renal failure: A review. Phytother. Res. 2017, 31, 1824–1835. [Google Scholar] [CrossRef]
- Yanarates, O.; Guven, A.; Sizlan, A.; Uysal, B.; Akgul, O.; Atim, A.; Ozcan, A.; Korkmaz, A.; Kurt, E. Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Ren. Fail. 2008, 30, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Wei, R.; Ding, R.; Tang, L.; Wang, Y. Grape seed proanthocyanidin extract reduces renal ischemia/reperfusion injuries in rats. Am. J. Med. Sci. 2012, 343, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, A.C.; Oladejo, C.O.; Josiah, S.S.; Famusiwa, C.D.; Ojo, O.B.; Olaleye, M.T. Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: Relevance for therapy in pesticide-induced liver toxicity? Pathophysiology 2018, 25, 365–371. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Wei, Y.; Zheng, X.; Feng, W. Taxifolin improves disorders of glucose metabolism and water-salt metabolism in kidney via PI3K/AKT signaling pathway in metabolic syndrome rats. Life Sci. 2020, 263, 118713. [Google Scholar] [CrossRef]
- Bedir, F.; Kocatürk, H.; Yapanoğlu, T.; Gürsul, C.; Arslan, R.; Mammadov, R.; Çoban, A.; Altuner, D.; Suleyman, H. Protective effect of taxifolin against prooxidant and proinflammatory kidney damage associated with acrylamide in rats. Biomed. Pharmacother. 2021, 139, 111660. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 484–490. [Google Scholar]
- Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Cetin, M.; Ugur, A.B.; Galateanu, B.; Mezhuev, Y.; Okkay, U.; Taspinar, N.; Taspinar, M.; Uyanik, A. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: In vitro and in vivo studies. Nanomedicine 2018, 13, 1595–1606. [Google Scholar] [CrossRef]
- Çomaklı, S.; Sevim, Ç.; Kontadakis, G.; Doğan, E.; Taghizadehghalehjoughi, A.; Özkaraca, M.; Aschner, M.; Nikolouzakis, T.K.; Tsatsakis, A. Acute glufosinate-based herbicide treatment in rats leads to increased ocular interleukin-1β and c-Fos protein levels, as well as intraocular pressure. Toxicol. Rep. 2019, 6, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, H.; Taghizadehghalehjoughi, A.; Yildirim, S.; Ozkaraca, M.; Genc, S.; Yeni, Y.; Mokresh, M.Y.; Hacimuftuoglu, A.; Tsatsakis, A.; Tsarouhas, K. Deteriorated Vascular Homeostasis in Hypertension: Experimental Evidence from Aorta, Brain, and Pancreatic Vasculature. J. Pers. Med. 2022, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, E.; Caglar, O.; Firinci, B.; Ahiskalioglu, A.; Aydin, M.D.; Kocak, M.N.; Taghizadehghalehjoughi, A.; Yigiter, M.; Sipal, S.; Gundogdu, B. Predeterminative role of Onuf’s nucleus ischemia on mesenteric artery vasospasm in spinal subarachnoid hemorrhage: A preliminary experimental study. Asian J. Surg. 2019, 42, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pickering, G. Paracetamol metabolism and related genetic differences. Drug Metab. Rev. 2011, 43, 41–52. [Google Scholar] [CrossRef]
- Mamoulakis, C.; Fragkiadoulaki, I.; Karkala, P.; Georgiadis, G.; Zisis, I.E.; Stivaktakis, P.; Kalogeraki, A.; Tsiaoussis, I.; Burykina, T.; Lazopoulos, G.; et al. Contrast-induced nephropathy in an animal model: Evaluation of novel biomarkers in blood and tissue samples. Toxicol. Rep. 2019, 6, 395–400. [Google Scholar] [CrossRef]
- Mamoulakis, C.; Tsarouhas, K.; Fragkiadoulaki, I.; Heretis, I.; Wilks, M.F.; Spandidos, D.A.; Tsitsimpikou, C.; Tsatsakis, A. Contrast-induced nephropathy: Basic concepts, pathophysiological implications and prevention strategies. Pharmacol. Ther. 2017, 180, 99–112. [Google Scholar] [CrossRef]
- Ndetan, H.; Evans, M.W., Jr.; Singal, A.K.; Brunner, L.J.; Calhoun, K.; Singh, K.P. Light to moderate drinking and therapeutic doses of acetaminophen: An assessment of risks for renal dysfunction. Prev. Med. Rep. 2018, 12, 253–258. [Google Scholar] [CrossRef]
- Baponwa, O.; Amang, A.P.; Mezui, C.; Koubala, B.B.; Siwe, G.T.; Vandi, V.L.; Tan, P.V. Antioxidant Mechanism of Renal and Hepatic Failure Prevention Related to Paracetamol Overdose by the Aqueous Extract of Amblygonocarpus andongensis Stem Bark. Biomed. Res. Int. 2022, 2022, 1846558. [Google Scholar] [CrossRef]
- Zisis, I.E.; Georgiadis, G.; Docea, A.O.; Calina, D.; Cercelaru, L.; Tsiaoussis, J.; Lazopoulos, G.; Sofikitis, N.; Tsatsakis, A.; Mamoulakis, C. Renoprotective Effect of Vardenafil and Avanafil in Contrast-Induced Nephropathy: Emerging Evidence from an Animal Model. J. Pers. Med. 2022, 12, 670. [Google Scholar] [CrossRef]
- Ramachandran, V.; Saravanan, R.; Raja, B. Attenuation of oxidative stress by syringic acid on acetaminophen-induced nephrotoxic rats. Comp. Clin. Pathol. 2012, 21, 1559–1564. [Google Scholar] [CrossRef]
- Tsarouhas, K.; Tsitsimpikou, C.; Papantoni, X.; Lazaridou, D.; Koutouzis, M.; Mazzaris, S.; Rezaee, R.; Mamoulakis, C.; Georgoulias, P.; Nepka, C.; et al. Oxidative stress and kidney injury in trans-radial catheterization. Biomed. Rep. 2018, 8, 417–425. [Google Scholar] [CrossRef]
- Canayakin, D.; Bayir, Y.; Kilic Baygutalp, N.; Sezen Karaoglan, E.; Atmaca, H.T.; Kocak Ozgeris, F.B.; Keles, M.S.; Halici, Z. Paracetamol-induced nephrotoxicity and oxidative stress in rats: The protective role of Nigella sativa. Pharm. Biol. 2016, 54, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Şener, G.; Sehirli, Ö.; Cetinel, Ş.; Yeğen, B.G.; Gedik, N.; Ayanoğlu-Dülger, G. Protective effects of MESNA (2-mercaptoethane sulphonate) against acetaminophen-induced hepatorenal oxidative damage in mice. J. Appl. Toxicol. Int. J. 2005, 25, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Iordache, A.M.; Buga, A.M.; Albulescu, D.; Vasile, R.C.; Mitrut, R.; Georgiadis, G.; Zisis, I.E.; Mamoulakis, C.; Tsatsakis, A.; Docea, A.O.; et al. Phosphodiesterase-5 inhibitors ameliorate structural kidney damage in a rat model of contrast-induced nephropathy. Food Chem. Toxicol. 2020, 143, 111535. [Google Scholar] [CrossRef]
- Knight, J.A. Free radicals: Their history and current status in aging and disease. Ann. Clin. Lab. Sci. 1998, 28, 331–346. [Google Scholar]
- Fujita, T.; Fujimoto, Y. Formation and removal of active oxygen species and lipid peroxides in biological systems. Nihon Yakurigaku Zasshi. Folia Pharmacol. Jpn. 1992, 99, 381–389. [Google Scholar] [CrossRef]
- Vageli, D.P.; Doukas, S.G.; Doukas, P.G.; Judson, B.L. Bile reflux and hypopharyngeal cancer (Review). Oncol. Rep. 2021, 46, 224. [Google Scholar] [CrossRef]
- Vageli, D.P.; Doukas, P.G.; Doukas, S.G.; Tsatsakis, A.; Judson, B.L. Noxious Combination of Tobacco Smoke Nitrosamines with Bile, Deoxycholic Acid, Promotes Hypopharyngeal Squamous Cell Carcinoma, via NFkappaB, In Vivo. Cancer Prev. Res. 2022, 15, 297–308. [Google Scholar] [CrossRef]
- Vageli, D.P.; Doukas, P.G.; Siametis, A.; Judson, B.L. Targeting STAT3 prevents bile reflux-induced oncogenic molecular events linked to hypopharyngeal carcinogenesis. J. Cell Mol. Med. 2022, 26, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Mamoulakis, C.; Tsarouhas, K.; Georgiadis, G.; Lazopoulos, G.; Tsatsakis, A.; Shojaei Asrami, E.; Rezaee, R. Crocin: A fighter against inflammation and pain. Food Chem. Toxicol. 2020, 143, 111521. [Google Scholar] [CrossRef] [PubMed]
- Vageli, D.P.; Prasad, M.L.; Sasaki, C.T. Gastro-duodenal fluid induced Nuclear Factor-κappaB activation and early pre-malignant alterations in murine hypopharyngeal mucosa. Oncotarget 2016, 7, 5892. [Google Scholar] [CrossRef] [PubMed]
- Halka, J.; Spaleniak, S.; Kade, G.; Antosiewicz, S.; Sigorski, D. The Nephrotoxicity of Drugs Used in Causal Oncological Therapies. Curr. Oncol. 2022, 29, 9681–9694. [Google Scholar] [CrossRef] [PubMed]
- Metin, T.O.; Bayrak, G.; Yaman, S.; Doganer, A.; Yoldas, A.; Eser, N.; Aykan, D.A.; Yilmaz, B.C.; Kurt, A.H.; Ayaz, L.; et al. Expression of ER stress markers (GRP78 and PERK) in experimental nephrotoxicity induced by cisplatin and gentamicin: Roles of inflammatory response and oxidative stress. Naunyn Schmiedebergs Arch. Pharm. 2022, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).