Treatment of Reticular Oral Lichen Planus with Photodynamic Therapy: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Therapeutic Procedure
2.2. Examination
- Size Group 0—no apparent lesion;
- Size Group I—lesions < 3 cm2;
- Size Group II—lesions in the range of 3 to 6 cm2;
- Size Group III—lesions > 6 cm2.
2.3. Statistical Analysis
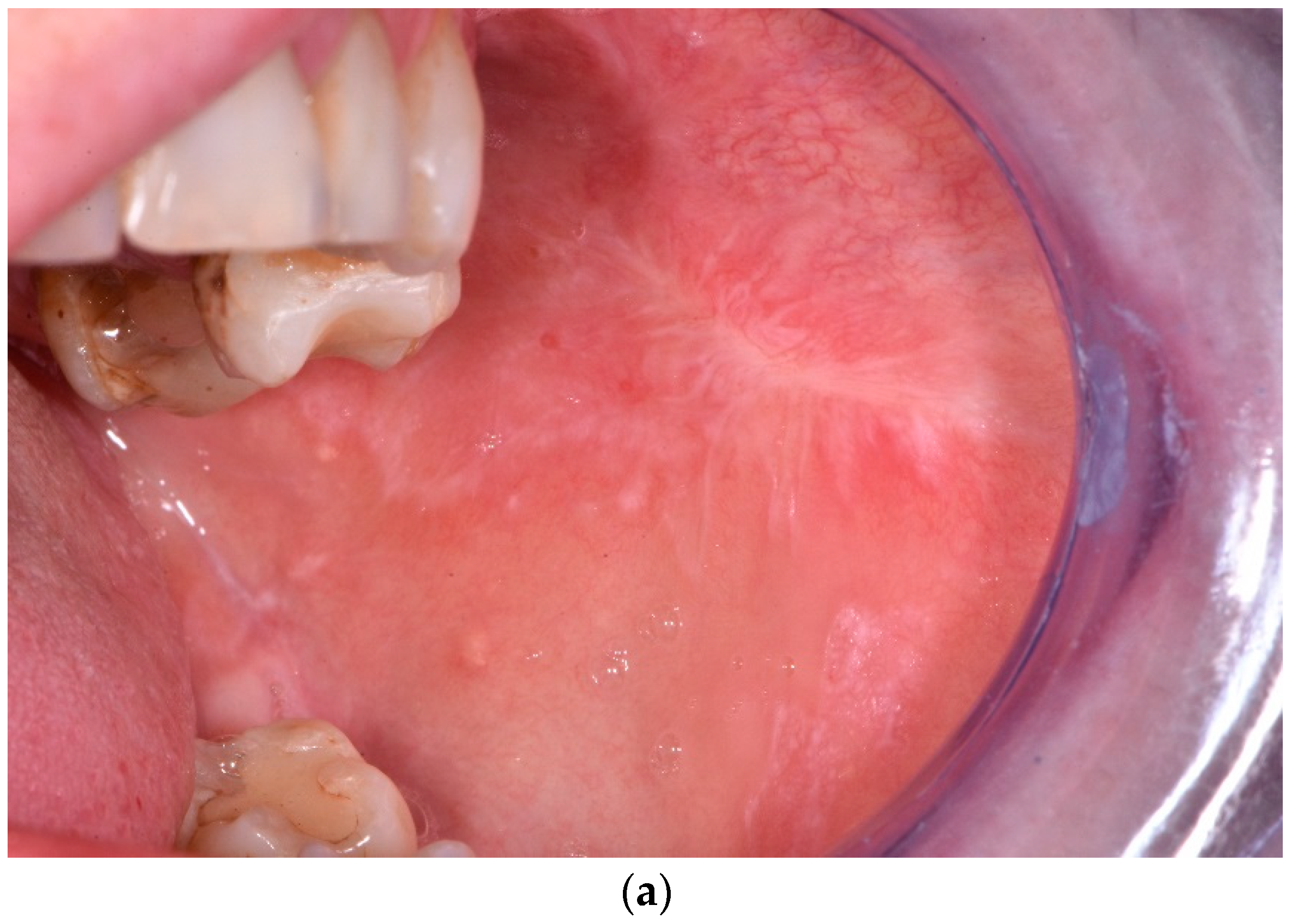
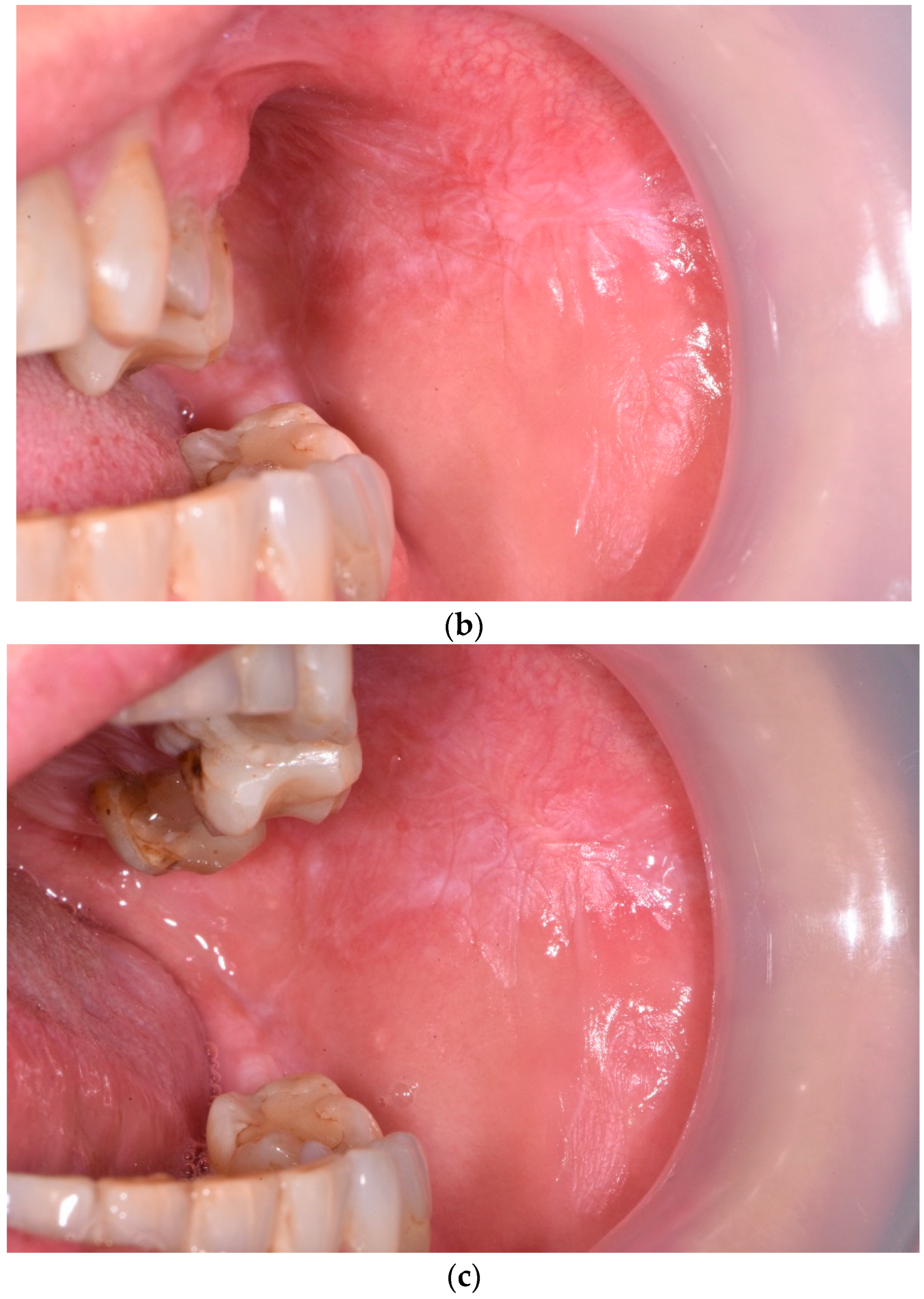
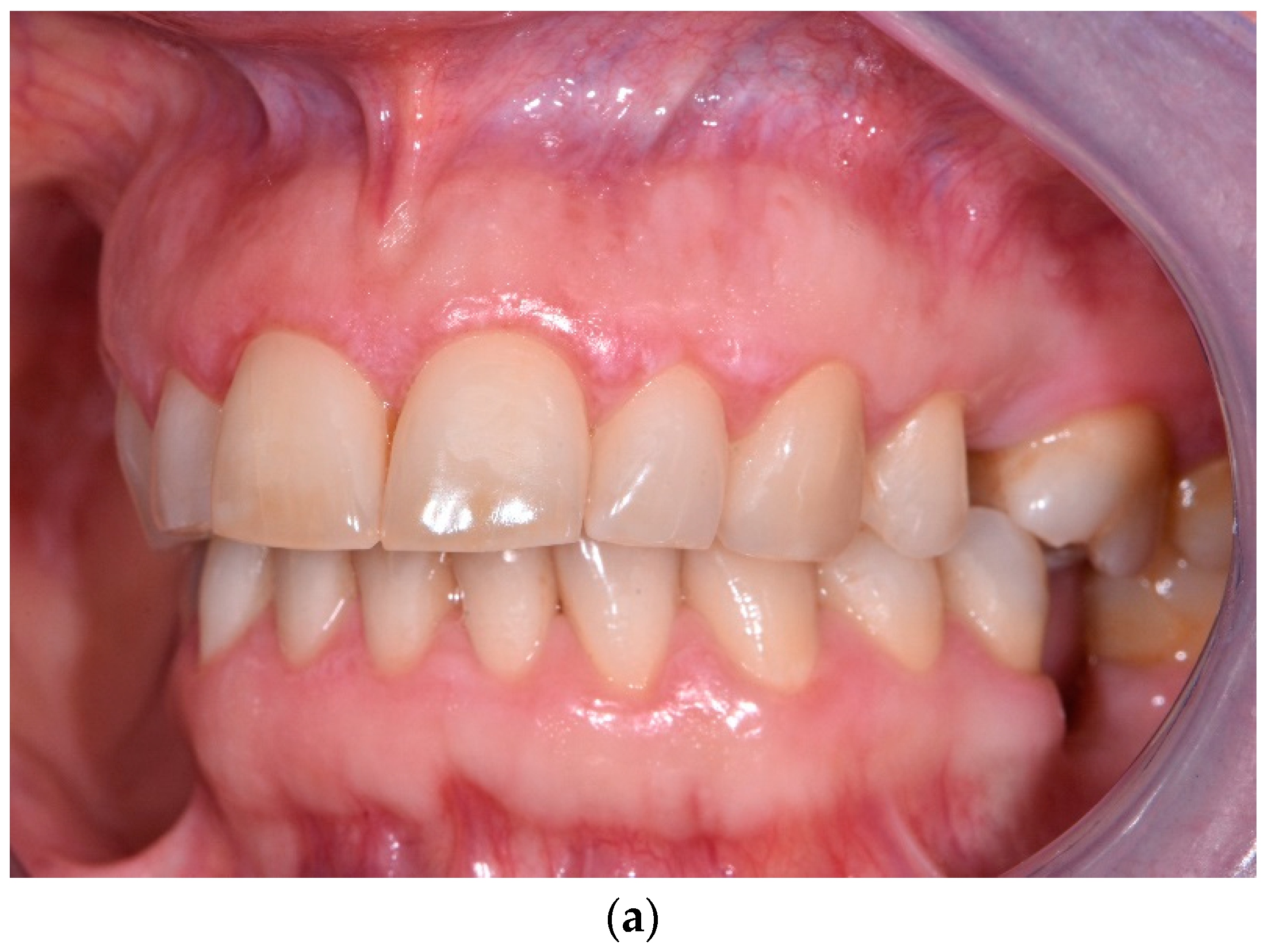
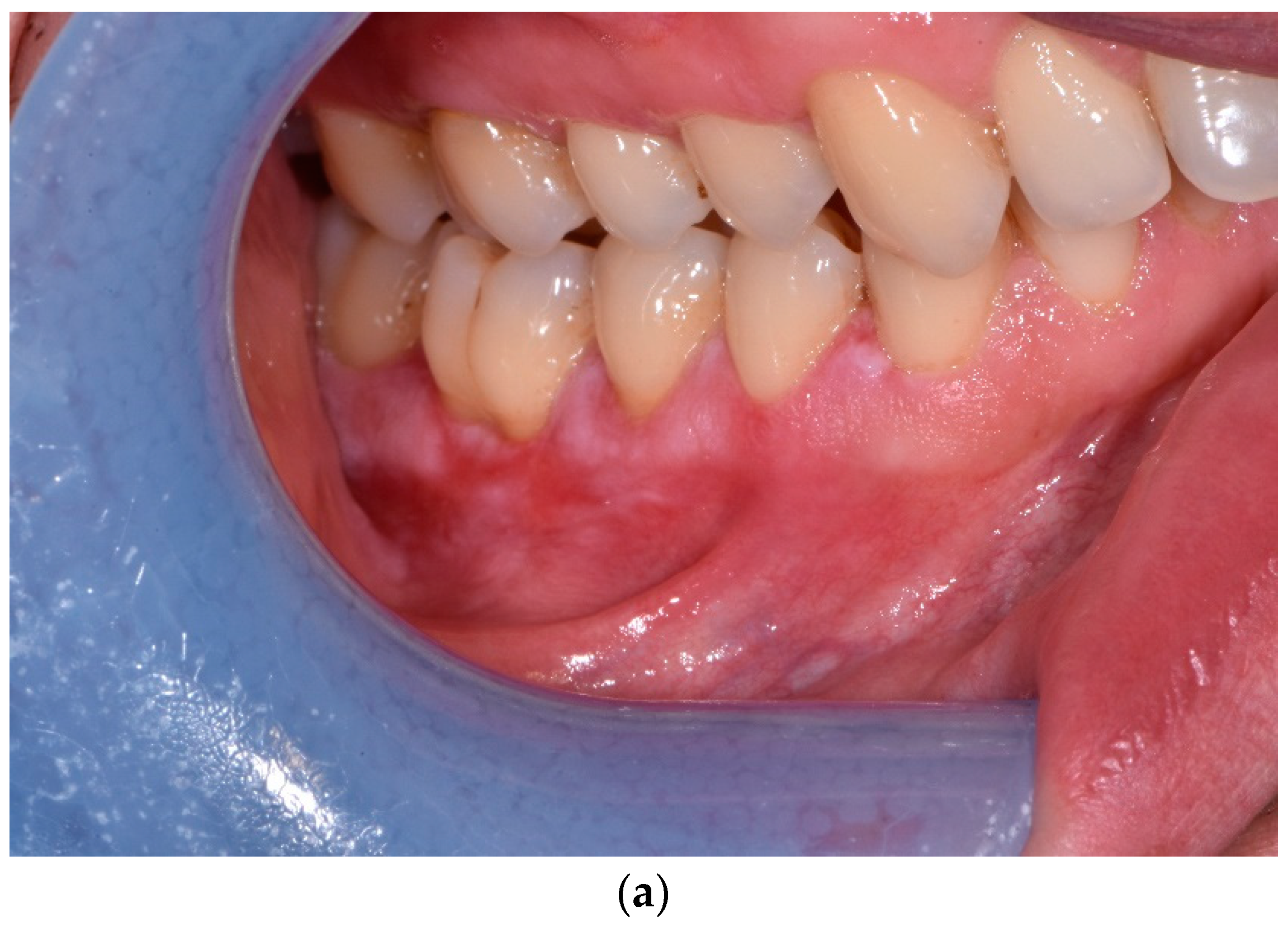
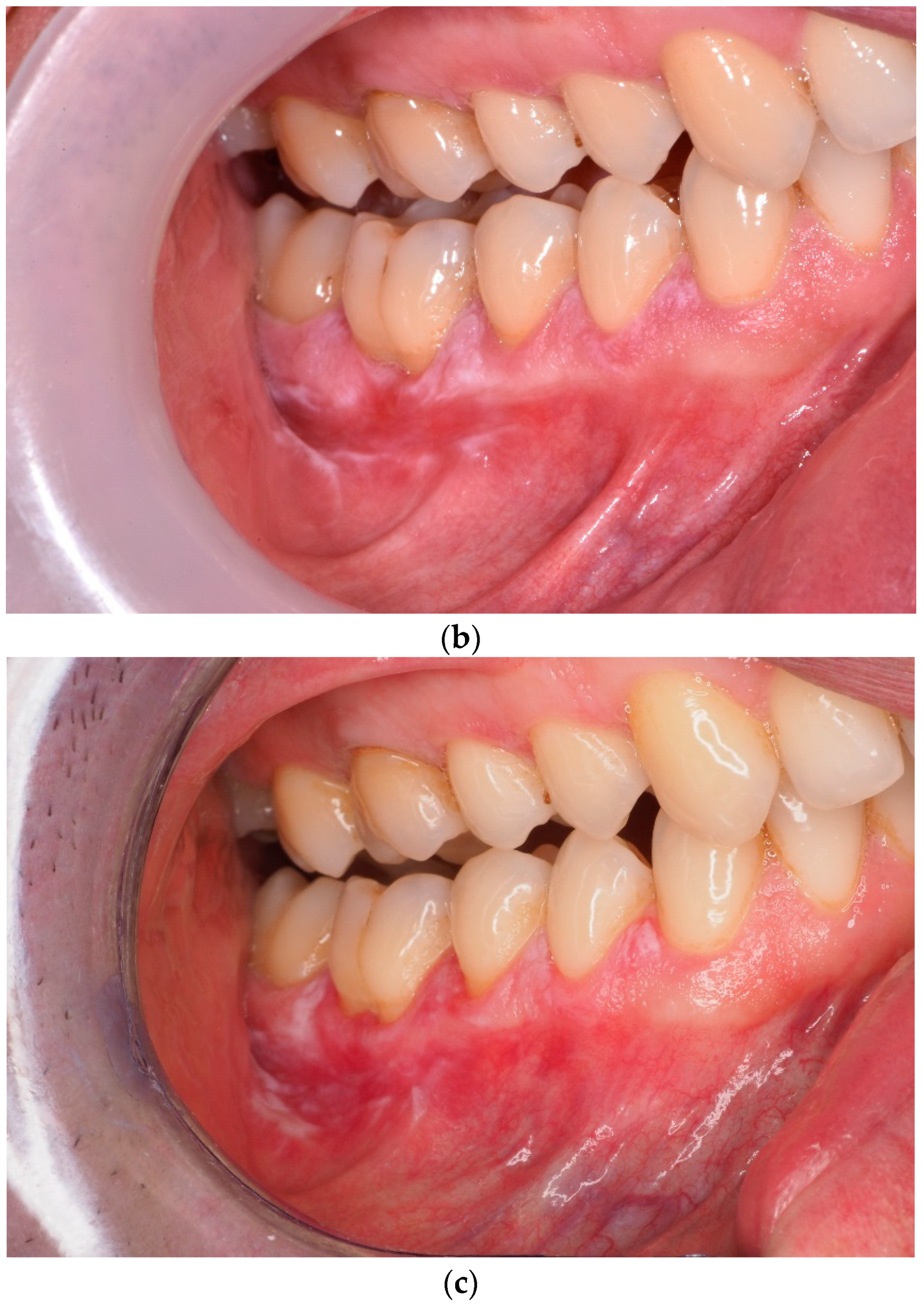
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aghahosseini, F.; Mirzaii-Dizgah, I.; Abdollahi, M.; Akbari-Gillani, N. Efficacy of IMOD in the treatment of oral lichen planus—Efficacy of IMOD in oral lichen planus. Open J. Stomatol. 2011, 1, 13–17. [Google Scholar] [CrossRef]
- Davari, P.; Hsiao, H.H.; Fazel, N. Mucosal lichen planus: An evidence-based treatment update. Am. J. Clin. Dermatol. 2014, 15, 181–395. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.; Carrozzo, M.; Sebastian, J.V.B.; Thongprasom, K. Number V Oral lichen planus: Clinical features and management. Oral Dis. 2005, 11, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Córdova, P.; Rubio, A.; Echeverría, P. Oral lichen planus: A look from diagnosis to treatment. J. Oral Res. 2014, 3, 62–67. [Google Scholar] [CrossRef]
- Mostafa, D.; Tarakji, B. Photodynamic therapy in treatment of oral lichen planus. J. Clin. Med. Res. 2015, 7, 393–399. [Google Scholar] [CrossRef]
- Mollaoglu, N. Oral lichen planus: A review. Br. J. Oral Maxillofac. Surg. 2000, 38, 370–377. [Google Scholar] [CrossRef]
- Lavanya, N.; Jayanthi, P.; Umadevi, K.; Rao, U.K.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef]
- Thongprasom, K.; Dhanuthai, K. Steriods in the treatment of lichen planus: A review. J. Oral Sci. 2008, 50, 377–385. [Google Scholar] [CrossRef]
- Aghahosseini, F.; Arbabi-Kalati, F.; Fashtami, L.A.; Djavid, G.E.; Fateh, M.; Beitollahi, J.M. Methylene blue-mediated photodynamic therapy: A possible alternative treatment for oral lichen planus. Lasers Surg. Med. 2006, 38, 33–38. [Google Scholar] [CrossRef]
- Koneru, J.; Mahalakshmi, M.; Naik, R. Management of oral lichen planus—A review. Indian J. Appl. Res. 2013, 3, 289–290. [Google Scholar] [CrossRef]
- Horch, H.H.; Gerlach, K.L.; Schaefer, H.E. CO2 laser surgery of oral premalignant lesions. Int. J. Oral Maxillofac Surg. 1986, 15, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Poswilo, D.E. Cryosurgery of the oral mucous membranes. Proc. R Soc. Med. 1975, 68, 608–609. [Google Scholar]
- Yeh, C.J. Simple cryosurgical treatment for oral lesions. Int. J. Oral Maxillofac Surg. 2000, 29, 212–216. [Google Scholar] [CrossRef] [PubMed]
- White, J.M.; Chaudhry, S.I.; Kudler, J.J.; Sekandari, N.; Schoelch, M.L.; Silverman, S., Jr. Nd: YAG and CO2 laser therapy of oral mucosal lesions. J. Clin. Laser Med. Surg. 1998, 16, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Kuusilehto, A.; Lehtinen, R.; Happonen, R.P.; Heikinheimo, K.; Lehtimäki, K.; Jansén, C.T. An open clinical trial of a new mouth-PUVA variant in the treatment of oral lichenoid lesions, Oral Surg, Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Mishra, M.K. A comparative study of PUVASOL therapy in lichen planus. Indian J. Dermatol. Venereol. Leprol. 2003, 69, 212–213. [Google Scholar]
- Kassem, R.; Yarom, N.; Scope, A.; Babaev, M.; Traun, H.; Pavlotzky, F. Treatment of erosive oral lichen planus with local ultraviolet B phototherapy. J. Am. Acad. Dermatol. 2012, 66, 761–766. [Google Scholar] [CrossRef]
- Pavlic, V.; Vujic-Aleksic, V. Phototherapy approaches in treatment of oral lichen planus. Photodermatol. Photoimmunol. Photomed. 2014, 30, 15–24. [Google Scholar] [CrossRef]
- Lundquist, G.; Forsgren, H.; Gajecki, M.; Emtestam, L. Photochemotherapy of oral lichen planus. A controlled study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 554–558. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Van Duijnhoven, F.H.; Aalbers, R.I.; Rovers, J.P.; Terpstra, O.T.; Kuppen, P. The immunological consequences of photodynamic treatment of cancer, a literature review. Immunobiology 2003, 207, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; van der Waal, I. Disease scoring systems for oral lichen planus; a critical appraisal. Med. Oral Patol. Oral Cir. Buccal 2015, 20, e199–e204. [Google Scholar] [CrossRef] [PubMed]
- Sulewska, M.; Duraj, E.; Sobaniec, S.; Graczyk, A.; Milewski, R.; Wroblewska, M.; Pietruski, J.; Pietruska, M. A clinical evaluation of efficacy of photodynamic therapy in treatment of reticular oral lichen planus: A case series. Photodiagn. Photodyn. Ther. 2019, 25, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sulewska, M.; Duraj, E.; Sobaniec, S.; Graczyk, A.; Milewski, R.; Wróblewska, M.; Pietruski, J.; Pietruska, M. A clinical evaluation of the efficacy of photodynamic therapy in the treatment of erosive oral lichen planus: A case series. Photodiagn. Photodyn. Ther. 2017, 18, 12–19. [Google Scholar] [CrossRef]
- Maloth, K.N.; Velpula, N.; Kodangal, S.; Sangmesh, M.; Vellamchetla, K.; Ugrappa, S.; Meka, N. Photodynamic therapy—A non-invasive treatment modality for precancerous lesions. J. Lasers Med. Sci. 2016, 7, 30–36. [Google Scholar] [CrossRef]
- Kvaal, S.I.; Angell-Petersen, E.; Warloe, T. Photodynamic treatment of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 62–70. [Google Scholar] [CrossRef]
- Aghahosseini, F.; Arbabi-Kalati, F.; Fashtam, L.A.; Fatch, M.; Djavid, G.E. Treatment of oral lichen planus with photodynamic therapy mediated methylene blue: A case report. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E126–E129. [Google Scholar]
- Sadaksharam, J.; Nayaki, K.T.; Panneer Selvam, N. Treatment of oral lichen planus with methylene blue mediated photodynamic therapy—A clinical study. Photodermatol. Photoimmunol. Photomed. 2012, 28, 97–101. [Google Scholar] [CrossRef]
- Prasanna, S.W.; Ingle, E.; Aruna, P.R.; Pravada, C.; Koteeswaran, D.; Ganesan, S. Photodynamic therapy of oral leukoplakia and oral lichen planus using methylene blue: A pilot study. J. Innov. Opt. Health Sci. 2015, 8, 1540005–1540011. [Google Scholar] [CrossRef]
- Sobaniec, S.; Bernaczyk, P.; Pietruski, J.; Cholewa, M.; Skurska, A.; Dolińska, E.; Duraj, E.; Tokajuk, G.; Paniczko, A.; Olszewska, E.; et al. Clinical assessment of the efficacy of photodynamic therapy in the treatment of oral lichen planus. Lasers Med. Sci. 2013, 28, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hesse, J.; Schmalfuss, A.; Kvaal, S.I. Photodynamic therapy of oral lichen planus. Photochem. Photobiol. Sci. 2020, 19, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Tocut, S.M.; Mitran, M.I.; Mitran, C.I.; Tampa, M.; Sarbu, M.I.; Popa, G.L.; Georgescu, S.R. Photodynamic therapy as a new therapeutic approach of oral lichen planus. J. Mind Med. Sci. 2019, 6, 64–71. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Ashraf, S.; Kalakonda, B.; Halboub, E.; Petro, W.; AlAizari, N.A. Efficacy of photodynamic therapy in the treatment of symptomatic oral lichen planus: A systematic review. J. Oral Pathol. Med. 2018, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Javed, F.; Hosein, M.; Al-Qahtani, M.A.; Alshehri, F.; Alzahrani, A.I.; Vohra, F. Photodynamic therapy in the treatment of symptomatic oral lichen planus: A systematic review. Photodermatol. Photoimmunol. Photomed. 2018, 34, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.; El Hart, K. The Photodynamic Treatment of Oral Lichen Planus: A Literature Review. Integr. J. Med. Sci. 2021, 8, 30–35. [Google Scholar] [CrossRef]
- Mostafa, D.; Moussa, E.; Alnouaem, M. Evaluation of photodynamic therapy in treatment of oral erosive lichen planus in comparison with topically applied corticosteroids. Photodiagn. Photodyn. Ther. 2017, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, S.; Azari-Marhabi, S.; Mojahedi, S.M.; Namdari, M.; Rankohi, Z.E.; Jafari, S. Comparing clinical effects of photodynamic therapy as a novel method with topical corticosteroid for treatment of Oral Lichen Planus. Photodiagn. Photodyn. Ther. 2017, 20, 159–164. [Google Scholar] [CrossRef]
- He, Y.; Deng, J.; Zhao, Y.; Tao, H.; Dan, H.; Xu, H.; Chen, Q. Efficacy evaluation of photodynamic therapy for oral lichen planus: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 302. [Google Scholar] [CrossRef]
- Lavaee, F.; Shadmanpour, M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019, 25, 1954–1963. [Google Scholar] [CrossRef]
- Saleh, W.E.; Khashaba, O.H.; El Nagdy, S.Y.; Moustafa, M.D. Photodynamic therapy of oral erosive lichen planus in diabetic and hypertensive patients. Mansoura J. Dent. 2014, 1, 119–123. [Google Scholar]
- Saleh, W.E.; Tageldin, S.; Khashaba, E.; Darwish, M.; Elnagdy, S.; Khashaba, O. Could photodynamic therapy be utilized as a treatment modality for oral lichen planus? Photodiagnosis Photodyn. Ther. 2020, 30, 101677. [Google Scholar] [CrossRef] [PubMed]
- Jajarm, H.H.; Falaki, F.; Sanatkhani, M.; Ahmadzadeh, M.; Ahrari, F.; Shafaee, H. A comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: A randomized clinical controlled trial. Lasers Med. Sci. 2015, 30, 1475–1480. [Google Scholar] [CrossRef]
- Jajarm, H.H.; Asadi, R.; Bardideh, E.; Shafaee, H.; Khazaei, Y.; Emadzadeh, M. The effects of photodynamic and low-level laser therapy for treatment of oral lichen planus—A systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2018, 23, 254–260. [Google Scholar] [CrossRef]
- Gorouhi, F.; Solhpour, A.; Beitollahi, J.M.; Afshar, S.; Davari, P.; Hashemi, P.; Nassiri Kashani, M.; Firooz, A. Randomized trial of pimecrolimus cream versus triamcinolone acetonide paste in the treatment of oral lichen planus. J. Am. Acad. Dermatol. 2007, 57, 806–813. [Google Scholar] [CrossRef]
- Amanat, D.; Ebrahimi, H.; Zahedani, M.Z.; Zeini, N.; Pourshahidi, S.; Ranjbar, Z. Comparing the effects of cryotherapy with nitrous oxide gas versus topical corticosteroids in the treatment of oral lichen planus. Indian J. Dent. Res. 2014, 25, 711–716. [Google Scholar]
- Patil, S.; Khandelwal, S.; Sinha, N.; Kaswan, S.; Rahman, F.; Tipu, S. Treatment modalities of oral lichen planus: An update. J. Oral Diag. 2016, 1, e3. [Google Scholar] [CrossRef]
- Kini, R.; Nagaratna, D.V.; Saha, A. Therapeutic management of oral lichen planus: A review for the clinicians. World J. Dent. 2011, 2, 249–253. [Google Scholar] [CrossRef]
- Chamani, G.; Rad, M.; Zarei, M.R.; Lotfi, S.; Sadeghi, M.; Ahmadi, Z. Efficacy of tacrolimus and clobetasol in the treatment of oral lichen planus: A systematic review and meta-analysis. Int. J. Dermatol. 2015, 54, 996–1004. [Google Scholar] [CrossRef]
- Carbone, M.; Goss, E.; Carrozzo, M.; Castellano, S.; Conrotto, D.; Broccoletti, R.; Gandolfo, S. Systemic and topical corticosteroid treatment of oral lichen planus: A comparative study with long-term follow-up. J. Oral Pathol. Med. 2003, 32, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Decani, S.; Federighi, V.; Baruzzi, E.; Sardella, A.; Lodi, G. Iatrogenic Cushing’s syndrome and topical steroid therapy: Case series and review of the literature. J. Dermatolog. Treat. 2014, 25, 495–500. [Google Scholar] [CrossRef]
- Rakesh, N.; Clint, J.B.; Reddy, S.S.; Nagi, R.; Chauhan, P.; Sharma, S.; Sharma, P.; Kaur, A.; Shetty, B.; Ashwini, S. Clinical evaluation of photodynamic therapy for the treatment of refractory oral Lichen planus—A case series. Photodiagn. Photodyn. Ther. 2018, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Rehman, N.; Alrahlah, A.; Alamri, W.R.; Vohra, F. Efficacy of photodynamic therapy or low level laser therapy against steroid therapy in the treatment of erosive-atrophic oral lichen planus. Photodiagn. Photodyn. Ther. 2018, 21, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Zborowski, J.; Kida, D.; Szarwaryn, A.; Nartowski, K.; Rak, P.; Jurczyszyn, K.; Konopka, T. A Comparison of Clinical Efficiency of Photodynamic Therapy and Topical Corticosteroid in Treatment of Oral Lichen Planus: A Split-Mouth Randomised Controlled Study. J. Clin. Med. 2021, 10, 3673. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, R.; Pollmann, R.; Sharif, J.; Schmidt, T.; Stein, R.; Bodea, A.; Auschill, T.; Sculean, A.; Eming, R.; Greene, B.; et al. Photodynamic therapy in oral lichen planus: A prospective case-controlled pilot study. Sci. Rep. 2020, 10, 1667. [Google Scholar] [CrossRef]



| Number of Patients | Location of Lesions | Number of Lesions | Mean Size (cm2) and Standard Deviation | Mean Size Reduction | p-Level of Significance | ||||
|---|---|---|---|---|---|---|---|---|---|
| At Baseline | On Therapy Completion | 12 Months after Therapy | On Therapy Completion | 12 Months after Therapy | On Therapy Completion | 12 Months after Therapy | |||
| 20 | Oral lichen planus | 40 | 2.74 ± 3.03 | 1.20 ± 1.40 | 0.88 ± 1.30 | 56.20% | 67.88% | 0.003449 * | 0.000092 * |
| 13 | Men | 26 | 2.32 ± 2.61 | 1.15 ± 1.52 | 0.82 ± 1.29 | 50.43% | 64.66% | 0.000018 * | 0.000027 * |
| 7 | Women | 14 | 3.52 ± 3.66 | 1.30 ± 1.18 | 0.99 ± 1.35 | 63.07% | 71.87% | 0.003511 * | 0.006947 * |
| 15 | Buccal and sublingual | 30 | 2.97 ± 3.40 | 1.02 ± 1.40 | 0.75 ± 1.27 | 65.66% | 74.75% | 0.000006 * | 0.000017 * |
| 9 | Women | 18 | 2.35 ± 2.97 | 0.85 ± 1.48 | 0.61 ± 1.18 | 63.83% | 74.04% | 0.000196 * | 0.000293 * |
| 6 | Men | 12 | 3.79 ± 3.88 | 1.25 ± 1.24 | 0.95 ± 1.38 | 67.02% | 74.93% | 0.004742 * | 0.013472 * |
| 5 | Gingival and lingual | 10 | 2.02 ± 1.32 | 1.74 ± 1.32 | 1.27 ± 1.37 | 13.86% | 37.13% | 0.035693 * | 0.011719 * |
| 4 | Women | 8 | 2.22 ± 1.39 | 1.95 ± 1.44 | 1.40 ± 1.50 | 12.16% | 36.94% | 0.027709 * | 0.027709 * |
| 1 | Men | 2 | 1.86 ± 1.61 | 1.65 ± 1.06 | 1.26 ± 1.61 | 11.29% | 32.26% | - | - |
| Location of Lesions | Number of Lesions | Mean Size Reduction (cm2) at Baseline and on Completion (Mean ± Std.dev.) | Mean Diff. (95% Cl) between Baseline and Completion | Mean Size Reduction (cm2) at Baseline and 12 Months Post-Therapy (Mean ± Std.dev.) | Mean Diff. (95% Cl) between Baseline and 12 Months Post-Therapy |
|---|---|---|---|---|---|
| Oral lichen planus | 40 | 1.53 ± 2.62 | 1.63 (0.72–2.53) | 1.86 ± 2.83 | 1.96 (0.98–2.73) |
| Women | 26 | 1.17 ± 2.37 | 2.37 (1.46–3.27) | 1.50 ± 2.42 | 2.42 (1.49–3.35) |
| Men | 14 | 0.97 ± 1.35 | 1.32 (0.64–1.99) | 2.52 ± 3.48 | 3.39 (1.65–5.13) |
| Buccal and sublingual | 30 | 1.95 ± 1.04 | 2.91 (1.87–3.95) | 2.22 ± 3.16 | 3.17 (2.03–4.30) |
| Women | 18 | 1.55 ± 2.77 | 2.72 (1.71–3.84) | 1.81 ± 2.81 | 2.86 (1.83–3.89) |
| Men | 12 | 0.95 ± 1.38 | 0.70 (0.23–1.17) | 2.85 ± 3.67 | 2.35 (0.92–3.77) |
| Gingival and lingual | 10 | 0.74 ± 0.91 | 0.74 (0.11–1.36) | 0.86 ± 0.81 | 0.86 (0.29–1.42) |
| Women | 8 | 0.60 ± 0.76 | 0.60 (0.07–1.13) | 0.92 ± 0.91 | 0.92 (0.29–1.55) |
| Men | 2 | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulewska, M.E.; Tomaszuk, J.; Sajewicz, E.; Pietruski, J.; Starzyńska, A.; Pietruska, M. Treatment of Reticular Oral Lichen Planus with Photodynamic Therapy: A Case Series. J. Clin. Med. 2023, 12, 875. https://doi.org/10.3390/jcm12030875
Sulewska ME, Tomaszuk J, Sajewicz E, Pietruski J, Starzyńska A, Pietruska M. Treatment of Reticular Oral Lichen Planus with Photodynamic Therapy: A Case Series. Journal of Clinical Medicine. 2023; 12(3):875. https://doi.org/10.3390/jcm12030875
Chicago/Turabian StyleSulewska, Magdalena Ewa, Jagoda Tomaszuk, Eugeniusz Sajewicz, Jan Pietruski, Anna Starzyńska, and Małgorzata Pietruska. 2023. "Treatment of Reticular Oral Lichen Planus with Photodynamic Therapy: A Case Series" Journal of Clinical Medicine 12, no. 3: 875. https://doi.org/10.3390/jcm12030875
APA StyleSulewska, M. E., Tomaszuk, J., Sajewicz, E., Pietruski, J., Starzyńska, A., & Pietruska, M. (2023). Treatment of Reticular Oral Lichen Planus with Photodynamic Therapy: A Case Series. Journal of Clinical Medicine, 12(3), 875. https://doi.org/10.3390/jcm12030875









