Usefulness of the Cognitive Composition Test as an Early Discriminator of Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome Measures
2.2.1. MoCA
2.2.2. MMSE
2.2.3. TMT-A, -B
2.2.4. CCT-A, -B, -C
2.3. Statistical Analysis
3. Results
3.1. Concurrent Validity of MoCA and Each Neuropsychological Test
3.2. Comparison of Each Neuropsychological Test between the HC and MCI Groups
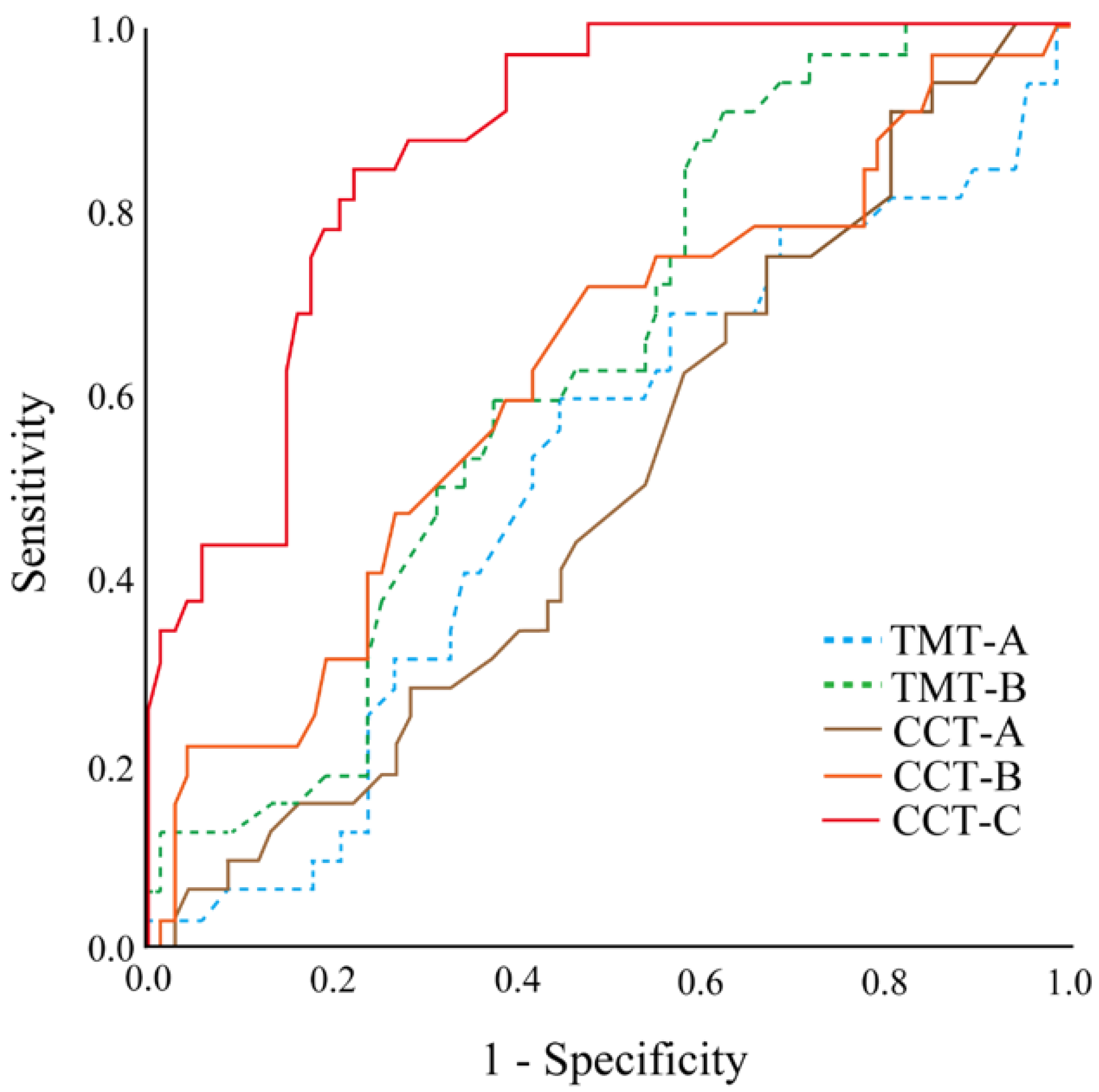
3.3. Discrimination Sensitivity and Cutoff Value of CCT-C
4. Discussion
4.1. Effectiveness of CCT
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsuchiya, Y.; Shida, N.; Tsujiuchi, T.; Machida, K. Healthy aging and concerns among elderly Japanese: A text analysis approach. Int. Q. Community Health Educ. 2015, 35, 215–226. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Lee, S.; Lee, S. Conversion and reversion rates in Japanese older people with mild cognitive impairment. J. Am. Med. Dir. Assoc. 2017, 18, 808.e1–808.e6. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Manly, J.J.; Tang, M.X.; Schupf, N.; Stern, Y.; Vonsattel, J.P.; Mayeux, R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann. Neurol. 2008, 63, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Leifer, B.P. Early diagnosis of Alzheimer’s disease: Clinical and economic benefits. J. Am. Geriatr. Soc. 2003, 51, S281–S288. [Google Scholar] [CrossRef]
- Nakata, E.; Kasai, M.; Kasuya, M.; Akanuma, K.; Meguro, M.; Ishii, H.; Yamaguchi, S.; Meguro, K. Combined memory and executive function tests can screen mild cognitive impairment and converters to dementia in a community: The Osaki-Tajiri project. Neuroepidemiology 2009, 33, 103–110. [Google Scholar] [CrossRef]
- Perry, R.J.; Hodges, J.R. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain 1999, 122 Pt 3, 383–404. [Google Scholar] [CrossRef]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef]
- Saunders, N.L.; Summers, M.J. Longitudinal deficits to attention, executive, and working memory in subtypes of mild cognitive impairment. Neuropsychology 2011, 25, 237–248. [Google Scholar] [CrossRef]
- Ashendorf, L.; Jefferson, A.L.; O’Connor, M.K.; Chaisson, C.; Green, R.C.; Stern, R.A. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch. Clin. Neuropsychol. 2008, 23, 129–137. [Google Scholar] [CrossRef]
- Lehrner, J.; Krakhofer, H.; Lamm, C.; Macher, S.; Moser, D.; Klug, S.; Dal-Bianco, P.; Pirker, W.; Auff, E.; Pusswald, G. Visuo-constructional functions in patients with mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. Neuropsychiatrie 2015, 29, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Roalf, D.R.; Rupert, P.; Mechanic-Hamilton, D.; Brennan, L.; Duda, J.E.; Weintraub, D.; Trojanowski, J.Q.; Wolk, D.; Moberg, P.J. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J. Neurol. 2018, 265, 1365–1375. [Google Scholar] [CrossRef]
- Tamaru, Y.; Tanaka, H.; Ueda, M.; Sumino, H.; Imaoka, M.; Matsugi, A.; Nishikawa, T.; Ishii, R.; Naito, Y. Effect of Alzheimer’s disease severity on upper limb function. Psychogeriatrics 2020, 20, 802–804. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Wilson, R.S.; Beck, T.L.; Bienias, J.L.; Bennett, D.A. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch. Neurol. 2006, 63, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Oyama, A.; Takeda, S.; Ito, Y.; Nakajima, T.; Takami, Y.; Takeya, Y.; Yamamoto, K.; Sugimoto, K.; Shimizu, H.; Shimamura, M.; et al. Novel Method for Rapid Assessment of Cognitive Impairment Using High-Performance Eye-Tracking Technology. Sci. Rep. 2019, 9, 12932. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.H.; Tumosa, N.; Chibnall, J.T.; Perry, M.H., 3rd; Morley, J.E. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—A pilot study. Am. J. Geriatr. Psychiatry 2006, 14, 900–910. [Google Scholar] [CrossRef]
- Saxton, J.; Morrow, L.; Eschman, A.; Archer, G.; Luther, J.; Zuccolotto, A. Computer assessment of mild cognitive impairment. Postgrad. Med. 2009, 121, 177–185. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Williams, C.S.; Braaten, A.J.; Gill, K.; Zimmerman, S.; Sloane, P.D. Cognitive screening for dementia and mild cognitive impairment in assisted living: Comparison of 3 tests. J. Am. Med. Dir. Assoc. 2008, 9, 586–593. [Google Scholar] [CrossRef]
- Woodford, H.J.; George, J. Cognitive assessment in the elderly: A review of clinical methods. QJM Int. J. Med. 2007, 100, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Suzuki, H.; Yasunaga, M.; Sugiyama, M.; Ijuin, M.; Sakuma, N.; Inagaki, H.; Iwasa, H.; Ura, C.; Yatomi, N.; et al. Brief screening tool for mild cognitive impairment in older Japanese: Validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr. Gerontol. Int. 2010, 10, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.; Chan, J.Y.; Hirai, H.W.; Wong, S.Y.; Kwok, T.C. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Hopp, G.A.; Dixon, R.A.; Grut, M.; Bäckman, L. Longitudinal and psychometric profiles of two cognitive status tests in very old adults. J. Clin. Psychol. 1997, 53, 673–686. [Google Scholar] [CrossRef]
- Tamaru, Y.; Naito, Y.; Nishikawa, T. Earlier and greater hand pre-shaping in the elderly: A study based on kinematic analysis of reaching movements to grasp objects. Psychogeriatrics 2017, 17, 382–388. [Google Scholar] [CrossRef]
- Xu, X.; Chong, E.; Hilal, S.; Ikram, M.K.; Venketasubramanian, N.; Chen, C. Beyond Screening: Can the Mini-Mental State Examination be Used as an Exclusion Tool in a Memory Clinic? Diagnostics 2015, 5, 475–486. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sakuma, N.; Kobayashi, M.; Ogawa, S.; Inagaki, H.; Edahiro, A.; Ura, C.; Sugiyama, M.; Miyamae, F.; Watanabe, Y.; et al. Normative Data of the Trail Making Test Among Urban Community-Dwelling Older Adults in Japan. Front. Aging Neurosci. 2022, 14, 832158. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnott, K.; Frank, J. Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 2000, 22, 518–528. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Suzuki, H.; Kuraoka, M.; Yasunaga, M.; Nonaka, K.; Sakurai, R.; Takeuchi, R.; Murayama, Y.; Ohba, H.; Fujiwara, Y. Cognitive intervention through a training program for picture book reading in community-dwelling older adults: A randomized controlled trial. BMC Geriatr. 2014, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yamashiro, D.; Ogawa, S.; Kobayashi, M.; Cho, D.; Iizuka, A.; Tsukamoto-Yasui, M.; Takada, M.; Isokawa, M.; Nagao, K.; et al. Intake of Seven Essential Amino Acids Improves Cognitive Function and Psychological and Social Function in Middle-Aged and Older Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Front. Nutr. 2020, 7, 586166. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Crowe, S.F. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J. Clin. Psychol. 1998, 54, 585–591. [Google Scholar] [CrossRef]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Adrover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef]
- Reitan, R.M. Trail making test results for normal and brain-damaged children. Percept. Mot. Ski. 1971, 33, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, Y.; Sumino, H.; Matsugi, A. “DATASET_CCT”, Mendeley Data, V1. Available online: https://data.mendeley.com/datasets/gx75cp3g4z/1 (accessed on 20 December 2022).
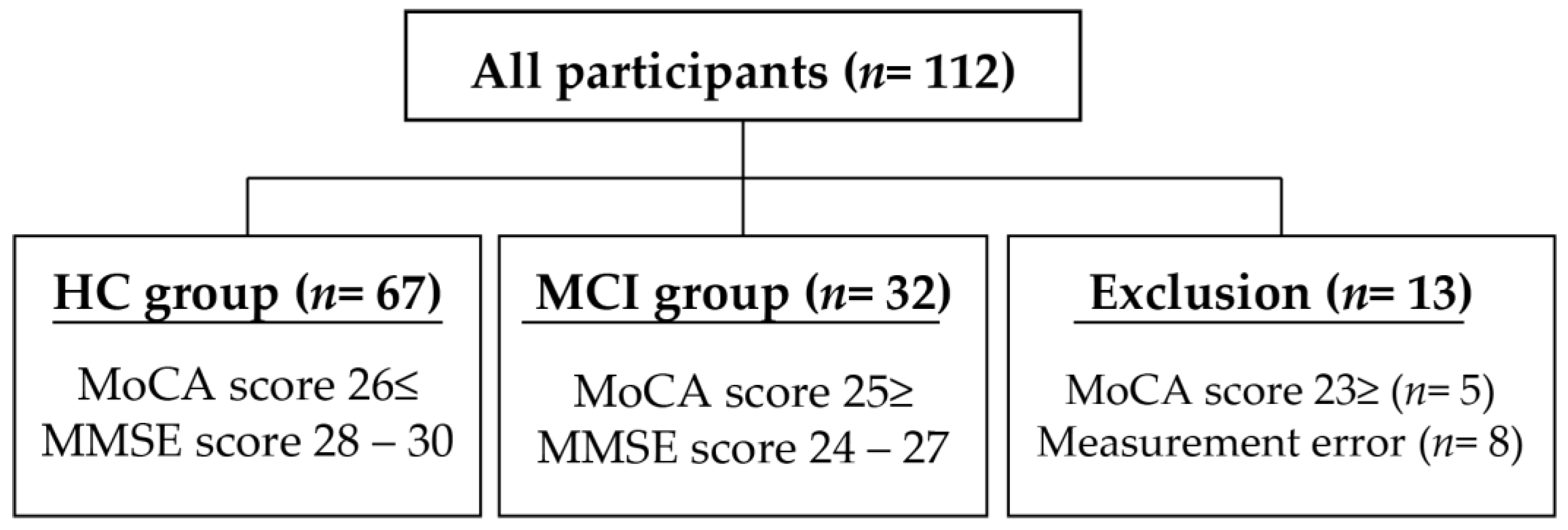
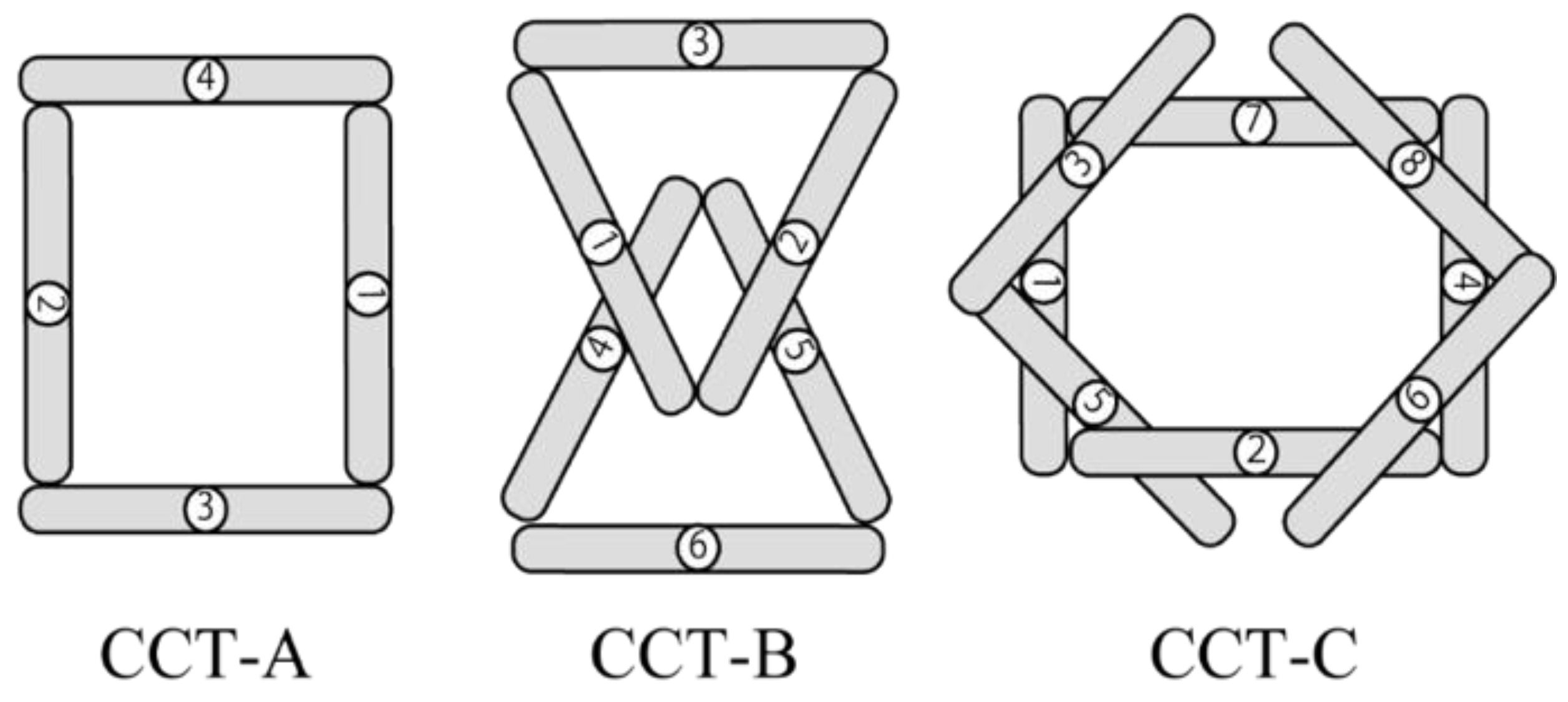


| HC (Mean ± SD) | MCI (Mean ± SD) | p-Value | Cohen’s d | |
|---|---|---|---|---|
| Age | 72.7 ± 4.8 | 73.9 ± 4.7 | 0.22 | 0.216 |
| Sex (male, female) | M: 27 F:40 | M: 14 F: 18 | - | - |
| Dominant hand 1 | R: 61 L: 6 | R: 30 L: 2 | - | - |
| MoCA | 27.4 ± 1.2 | 23.2 ± 1.42 | 0.001 | 0.359 |
| MMSE | 28.6 ± 0.8 | 25.5 ± 1.19 | 0.001 | 0.365 |
| AUC | p-Value | Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| TMT-A | 0.513 | 0.840 | 35.70 | 59.4% | 55.2% |
| TMT-B | 0.630 | 0.037 | 73.55 | 90.6% | 37.3% |
| CCT-A | 0.500 | 0.994 | 13.05 | 90.6% | 19.4% |
| CCT-B | 0.621 | 0.052 | 35.55 | 71.9% | 52.2% |
| CCT-C | 0.867 | 0.000 | 65.75 | 84.4% | 77.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaru, Y.; Sumino, H.; Matsugi, A. Usefulness of the Cognitive Composition Test as an Early Discriminator of Mild Cognitive Impairment. J. Clin. Med. 2023, 12, 1203. https://doi.org/10.3390/jcm12031203
Tamaru Y, Sumino H, Matsugi A. Usefulness of the Cognitive Composition Test as an Early Discriminator of Mild Cognitive Impairment. Journal of Clinical Medicine. 2023; 12(3):1203. https://doi.org/10.3390/jcm12031203
Chicago/Turabian StyleTamaru, Yoshiki, Hiroyuki Sumino, and Akiyoshi Matsugi. 2023. "Usefulness of the Cognitive Composition Test as an Early Discriminator of Mild Cognitive Impairment" Journal of Clinical Medicine 12, no. 3: 1203. https://doi.org/10.3390/jcm12031203
APA StyleTamaru, Y., Sumino, H., & Matsugi, A. (2023). Usefulness of the Cognitive Composition Test as an Early Discriminator of Mild Cognitive Impairment. Journal of Clinical Medicine, 12(3), 1203. https://doi.org/10.3390/jcm12031203






