Predictors of Long-COVID and Chronic Impairment of Exercise Tolerance in Spiroergometry in Patients after 15 Months of COVID-19 Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Basic Characteristics
- Heart failure diagnosis or typical symptomatic heart failure;
- Previous myocardial infarction;
- Uncontrolled arterial hypertension;
- Unstable angina;
- Acute pericarditis or myocarditis;
- Active endocarditis;
- Acute pulmonary embolism;
- History of stroke, transient ischemic attack, or intracerebral haemorrhage;
- Unstable heart rhythm disorders;
- Advanced atrioventricular block;
- Cardiomyopathy diagnosis (dilated, hypertrophic, postpartum, restrictive, tachyarrhythmic);
- Active systemic infection;
- Carrier of Hepatitis B virus (HBV), Hepatitis C virus (HCV), or human immunodeficiency virus (HIV), or testing positive for hepatitis B surface antigen (HBsAg) or antibodies to HCV;
- Drug and alcohol abuse;
- Chronic kidney disease (stage IV and V according to the National Kidney Foundation) and dialysis treatment;
- Severe hypothyroidism and hyperthyroidism;
- Active autoimmune disease;
- Use of cytostatic drugs, immunosuppressants, glucocorticosteroids, or antiretroviral drugs;
- Documented neoplastic process;
- History of bone marrow transplantation or other organ transplantation, as well as treatment with blood products within the last 6 months;
- Underwent surgery or a significant injury in the last month;
- Pregnancy or lactation;
- A physical limitation that hinders the completion of a spiroergometric test;
- Patient’s incapacity to cooperate and/or provide informed consent to participate in research;
- Individuals who did not provide their informed consent to take part in the study [7].
2.2. Echocardiography
2.3. Spiroergometry
2.4. Body Mass Analysis
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Basic Characteristics
3.2. Evaluation of Laboratory Tests
3.3. Evaluation of Echocardiography
3.4. Evaluation of Spiroergometry
3.5. Evaluation of Body Mass Analysis
3.6. Multivariate Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization, The Impact of the COVID-19 Pandemic on Noncommunicable Disease Resources and Services: Results of a Rapid Assessment; World Health Organization: Geneva, Switzerland, 2020.
- Tsioufis, K.; Chrysohoou, C.; Kariori, M. The mystery of ‘missing’ visits in an emergency cardiology department, in the era of COVID-19, a time-series analysis in a tertiary Greek General Hospital. Clin. Res. Cardiol. 2020, 109, 1483–1489. [Google Scholar] [CrossRef]
- Leontsinis, I.; Papademetriou, V.; Chrysohoou, C.; Kariori, M.; Dalakouras, I.; Tolis, P.; Katsiki, N.; Fragoulis, C.; Kalos, T.; Tatakis, F.-P.; et al. Hypertensive urgencies during the first wave of the COVID-19 pandemic in a tertiary hospital setting: A U-shaped alarming curve. Arch. Med. Sci. 2022, 18, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Lewek, J.; Jatczak-Pawlik, I.; Maciejewski, M.; Jankowski, P.; Banach, M. COVID-19 and cardiovascular complications-preliminary results of the LATE-COVID study. Arch. Med. Sci. 2021, 17, 818–822. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; TLC Study Group; Sapey, E.; Calvert, M.J.; Nirantharakumar, K.; et al. Symptoms, complications and management of long-COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Gryglewska-Wawrzak, K.; Sakowicz, A.; Banach, M.; Maciejewski, M.; Bielecka-Dabrowa, A. Factors of Persistent Limited Exercise Tolerance in Patients after COVID-19 with Normal Left Ventricular Ejection Fraction. Biomedicines 2022, 10, 3257. [Google Scholar] [CrossRef] [PubMed]
- Gryglewska-Wawrzak, K.; Sakowicz, A.; Banach, M.; Bytyçi, I.; Bielecka-Dabrowa, A. Diagnostic Usefulness of Spiroergometry and Risk Factors of Long-COVID in Patients with Normal Left Ventricular Ejection Fraction. J. Clin. Med. 2023, 12, 4160. [Google Scholar] [CrossRef]
- Aparisi, Á.; Ladrón, R.; Ybarra-Falcón, C.; Tobar, J.; San Román, J.A. Exercise Intolerance in Post-Acute Sequelae of COVID-19 and the Value of Cardiopulmonary Exercise Testing—A Mini-Review. Front. Med. 2022, 9, 924819. [Google Scholar] [CrossRef]
- Wade, D.T. Rehabilitation after COVID-19, an evidence-based approach. Clin. Med. 2020, 20, 359–365. [Google Scholar] [CrossRef]
- Gryglewska-Wawrzak, K.; Cienkowski, K.; Cienkowska, A.; Banach, M.; Bielecka-Dabrowa, A. The Role of Multidisciplinary Approaches in the Treatment of Patients with Heart Failure and Coagulopathy of COVID-19. J. Cardiovasc. Dev. Dis. 2023, 10, 245. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Riegler, L.; Rucco, M.A.; Cocchia, R.; Scarafile, R.; Salerno, G.; Martone, F.; Vriz, O.; Caso, P.; Calabrò, R.; et al. Left atrial volume index in healthy subjects: Clinical and echocardiographic correlates. Echocardiography 2013, 30, 1001–1007. [Google Scholar] [CrossRef]
- Daskalov, I.R.; Petrovsky, P.D.; Demirevska, L.D. Mitral annular systolic velocity as a marker of preclinical systolic dysfunction among patients with arterial hypertension. Cardiovasc. Ultrasound. 2012, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Hoogslag, G.E.; Abou, R.; Joyce, E.; Boden, H.; Kamperidis, V.; Regeer, M.V.; van Rosendael, P.J.; Schalij, M.J.; Bax, J.J.; Marsan, N.A.; et al. Comparison of Changes in Global Longitudinal Peak Systolic Strain After ST-Segment Elevation Myocardial Infarction in Patients with Versus Without Diabetes Mellitus. Am. J. Cardiol. 2015, 116, 1334–1339. [Google Scholar] [CrossRef]
- Tousignant, C.; Kim, H.; Papa, F.; Mazer, C.D. Evaluation of TAPSE as a measure of right ventricular output. Can. J. Anaesth. 2012, 59, 376–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Datta, D.; Normandin, E.; ZuWallack, R. Cardiopulmonary exercise testing in the assessment of exertional dyspnea. Ann. Thorac. Med. 2015, 10, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, 70–88. [Google Scholar] [CrossRef]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Postgrad. Med. J. 2007, 83, 675–682. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Jaafar, Z.A.; Kreidieh, D.; Itani, L.; Tannir, H.; El Masri, D.; El Ghoch, M. Cross-validation of prediction equations for estimating the body fat percentage in adults with obesity. Clin. Nutr. ESPEN 2021, 41, 346–350. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Nakano, C.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; et al. Extracellular water to total body water ratio in viral liver diseases: A study using bioimpedance analysis. Nutrients 2018, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Askin, L.; Tanriverdi, O.; Turkmen, S. Clinical importance of high- sensitivity troponin T in patients without coronary artery disease. North. Clin. Istanb. 2020, 7, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.Y.; Zhu, X.F.; Yang, Y.; Ye, P. High-sensitive cardiac troponin T. J. Geriatr. Cardiol. 2013, 10, 102–109. [Google Scholar] [PubMed]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Joy, G.; Artico, J.; Kurdi, H.; Seraphim, A.; Lau, C.; Thornton, G.D.; Oliveira, M.F.; Adam, R.D.; Aziminia, N.; Menacho, K.; et al. COVIDsortium Investigators. Prospective Case-Control Study of Cardiovascular Abnormalities 6 Months Following Mild COVID-19 in Healthcare Workers. JACC Cardiovasc. Imaging 2021, 14, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, D.R.; Leddy, J.J.; Venkatraman, J.T. A perspective on fat intake in athletes. J. Am. Coll. Nutr. 2000, 19, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Simonson, D.C.; DeFronzo, R.A. Indirect calorimetry: Methodological and interpretative problems. Am. J. Physiol. 1990, 258, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Joris, M.; Pincemail, J.; Colson, C.; Joris, J.; Calmes, D.; Cavalier, E.; Misset, B.; Guiot, J.; Minguet, G.; Rousseau, A.-F. Exercise Limitation after Critical Versus Mild COVID-19 Infection: A Metabolic Perspective. J. Clin. Med. 2022, 11, 4322. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Guo, S.S.; Zeller, C.M.; Reo, N.V.; Baumgartner, R.N.; Garry, P.J.; Siervogel, R.M.; Heymsfield, S.B.; Pierson, R.N., Jr.; Wang, J. Total body water reference values and prediction equations for adults. Kidney Int. 2001, 59, 2250–2258. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Guo, S.S.; Zeller, C.M.; Reo, N.V.; Siervogel, R.M. Total body water data for white adults 18 to 64 years of age: The Fels Longitudinal Study. Kidney Int. 1999, 56, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Vegas-Aguilar, I.M.; Lukaski, H.; Talluri, A.; Bellido-Guerrero, D.; Tinahones, F.J.; García-Almeida, J.M. Overhydration Assessed Using Bioelectrical Impedance Vector Analysis Adversely Affects 90-Day Clinical Outcome among SARS-CoV2 Patients: A New Approach. Nutrients 2022, 14, 2726. [Google Scholar] [CrossRef] [PubMed]
- Ranu, H.; Wilde, M.; Madden, B. Pulmonary function tests. Ulster Med. J. 2011, 80, 84–90. [Google Scholar] [PubMed]
- Gold, W.M.; Koth, L.L. Pulmonary Function Testing. Murray Nadel’s Textb. Respir. Med. 2016, 1, 407–435. [Google Scholar]
- You, J.; Zhang, L.; Ni-Jia-Ti, M.; Zhang, J.; Hu, F.; Chen, L. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J. Infect. 2020, 81, e150–e152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.-Q.; Xie, H.; Xu, Q.-F.; Jia, J.-L.; Li, L.-M.; Mao, H.-L.; Zhou, X.-M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Kou, S.; Luo, P.; Zhao, M.; Yu, K. Lung ventilation function characteristics of survivors from severe COVID-19, a prospective study. Crit. Care 2020, 24, 300. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Saigal, A.; Karia, N.; Patel, R.K.; Razvi, Y.; Constantinou, N.; Knight, D.S.; Muthurangu, V.; Goldring, J.; Fontana, M.; et al. Ongoing exercise intolerance following COVID-19, A magnetic resonance-augmented cardiopulmonary exercise test study. J. Am. Heart Assoc. 2022, 11, e024207. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Friedman, J.M. Obesity: Causes and control of excess body fat. Nature 2009, 459, 340–342. [Google Scholar] [CrossRef]
- Mondal, H.; Mishra, S.P. Effect of BMI, Body Fat Percentage and Fat Free Mass on Maximal Oxygen Consumption in Healthy Young Adults. J. Clin. Diagn. Res. 2017, 11, CC17–CC20. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.; Fields, D.A.; Hunter, G.R.; Herd, S.L.; Weinsier, R.L. Total body fat does not influence maximal aerobic capacity. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 841–848. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Patients Hospitalised Three to Six Months after COVID-19 Diagnosis n = 82 | Patients Hospitalised in One-Year Follow-Up n = 82 | p |
|---|---|---|---|
| Clinical characteristics | |||
| BMI (kg/m2) | (23.24–30.42), 26.79 * | (24.03–30.25), 26.28 * | 0.32 |
| BSA (m2) | (1.75–2.03), 1.86 * | (1.76–2.05), 1.88 * | 0.23 |
| SBP (mmHg) | (125.00–140.00), 130.00 * | (125.00–145.00), 132.00 * | 0.10 |
| DBP (mmHg) | (71.00–86.00), 80.00 * | (75.00–90.00), 82.00 * | 0.052 |
| Hypertension | 57% | 61% | 0.63 |
| Diabetes mellitus | 20% | 21% | 0.85 |
| Dyslipidaemia | 54% | 57% | 0.69 |
| Obesity | 24% | 29% | 0.48 |
| Alcohol | 4% | 2% | 0.68 |
| Smoking | 11% | 16% | 0.36 |
| Parameter | Patients Hospitalised Three to Six Months after COVID-19 Diagnosis n = 82 | Patients Hospitalised in One-Year Follow-Up n = 82 | p |
|---|---|---|---|
| Laboratory tests | |||
| hs-cTnT (pg/mL) (<14.00) | (3.20–8.40), 4.90 * | (3.90–9.30), 5.80 * | 0.03 |
| NT-proBNP (pg/mL) (<125.00) | (42.00–125.00), 87.00 * | (51.00–120.00), 82.00 * | 0.80 |
| RBC (106/μL) (women—3.80–5.80, men—4.50–6.50) | (4.20–4.90), 4.50 * | (4.20–4.90), 4.50 * | 0.37 |
| Haemoglobin (g/dL) (women—12.00–15.00; men—13.00–18.00) | (12.80–14.60), 13.70 * | (12.60–14.80), 13.40 * | 0.32 |
| PLT (103/μL) (150.00–400.00) | (165.00–224.00), 205.00 * | (172.00–238.00), 210.00 * | 0.70 |
| Creatinine (mg/dL) (0.55–1.02) | (0.67–0.91), 0.79 * | (0.65–0.93), 0.77 * | 0.97 |
| GFR (ml/min/1.73 m2) (>90.00) | (79.10–104.10), 92.20 * | (79.60–103.80), 91.50 * | 0.26 |
| Urea (mg/dL) (17.00–43.00) | (27.00–40.00), 32.00 * | (27.00–38.00), 41.00 * | 0.59 |
| Glucose (mg/dL) (60.00–99.00) | (85.00–96.00), 90.00 * | (84.00–96.00), 91.00 * | 0.66 |
| HDL cholesterol (mg/dL) (>40.00) | (39.00–59.00), 49.50 * | (40.00–57.00), 47.50 * | 0.58 |
| LDL cholesterol (mg/dL) (<115.00) | (70.00–110.00), 94.00 * | (62.00–106.00), 85.00 * | 0.27 |
| Triglycerides (mg/dL) (<150.00) | (83.00–148.00), 103.50 * | (78.00–130.00), 100.00 * | 0.07 |
| Total cholesterol (mg/dL) (<200.00) | (135.00–188.00), 169.00 * | (130.00–188.00), 161.00 * | 0.33 |
| ALT (U/L) (<50.00) | (17.00–29.00), 22.00 * | (17.00–30.00), 22.00 * | 0.59 |
| AST (U/L) (<50.00) | (25.00–31.00), 27.00 * | (25.00–32.00), 28.00 * | 0.73 |
| CRP (mg/dL) (<0.50) | (0.50–0.50), 0.50 * | (0.50–0.50), 0.50 * | 0.22 |
| D-dimer (ng/mL) (<500.00) | (200.50–398.50), 286.00 * | (167.00–326.00), 224.00 * | 0.19 |
| K (mmol/L) (3.50–5.10) | (4.20–4.60), 4.40 * | (4.20–4.60), 4.40 * | 0.78 |
| Na (mmol/L) (135.00–145.00) | (138.00–141.00), 139.00 * | (138.00–141.00), 140.00 * | 0.29 |
| Parameter | Patients Hospitalised Three to Six Months after COVID-19 Diagnosis n = 82 | Patients Hospitalised in One-Year Follow-Up n = 82 | p |
|---|---|---|---|
| Echocardiography | |||
| EF (%) | (55.00–65.00), 62.00 * | (58.00–65.00), 63.00 * | 0.27 |
| EDV (cm3) | (75.00–105.00), 92.00 * | (77.00–117.00), 99.00 * | 0.055 |
| ESV (cm3) | (26.00–48.00), 37.00 * | (28.00–48.00), 37.00 * | 0.26 |
| LA (mm) | (34.00–43.00), 36.00 * | (34.00–43.00), 38.00 * | 0.03 |
| LAVi (ml/m2) | (26.00–41.00), 34.00 * | (27.00–42.00), 33.00 * | 0.45 |
| E (cm/s) | (62.00–87.00), 75.00 * | (63.00–96.00), 78.00 * | 0.43 |
| A (cm/s) | (54.00–80.00), 67.00 * | (54.00–75.00), 65.00 * | 0.85 |
| E/A | (0.87–1.44), 1.12 * | (0.92–1.49), 1.14 * | 0.25 |
| GLPS (%) | (18.80–20.80), 20.00 * | (18.10–20.10), 19.10 * | 0.94 |
| TAPSE (mm) | (20.00–26.00), 23.00 * | (20.00–25.00), 22.00 * | 0.63 |
| TDE S’ (cm/s) | (12.00–15.00), 13.00 * | (11.00–15.00), 13.00 * | 0.06 |
| Parameter | Patients Hospitalised Three to Six Months after COVID-19 Diagnosis n = 82 | Patients Hospitalised in One-Year Follow-Up n = 82 | p |
|---|---|---|---|
| Spiroergometry | |||
| Exercise time (s) | (402.00–696.00), 518.00 * | (420.00–756.00), 609.00 * | 0.30 |
| HR max | (122.00–164.00), 146.00 * | (124.00–166.00), 142.00 * | 0.59 |
| Peripheral SBP max (mmHg) | (140.00–200.00), 160.00 * | (150.00–190.00), 165.00 * | 0.63 |
| Peripheral DBP max (mmHg) | (70.00–90.00), 80 * | (80.00–90.00), 80.00 * | 0.02 |
| FEV1 (l) | (2.55–3.56), 2.99 * | (2.45–3.53), 3.04 * | 0.07 |
| FVC (l) | (3.18–4.44), 3.79 * | (2.88–4.27), 3.71 * | 0.02 |
| FVC% | (95.00–117.00), 105.00 * | (91.00–112.00), 101.00 * | 0.001 |
| FEV1/FVC | (76.00–85.00), 82.00 * | (77.00–88.00), 82.00 * | 0.16 |
| FEV1/FVC% | (96.00–109.00), 103.00 * | (97.00–111.00), 103.00 * | 0.16 |
| FEF 25–75 (l/s) | (1.88–3.35), 2.72 * | (2.04–3.74), 3.02 * | 0.34 |
| RER | (1.02–1.12), 1.09 * | (1.05–1.13), 1.10 * | 0.008 |
| VO2max (ml/min/kg) | (17.00–26.00), 21.00 * | (18.00–26.00), 22.00 * | 0.12 |
| VO2max pred (%) | (71.00–104.00), 81.00 * | (71.00–98.00), 85.00 * | 0.53 |
| VO2AT (ml/min/kg) | (11.00–18.00), 14.00 * | (11.00–16.00), 13.00 * | 0.38 |
| Peak VO2max (l) | (1.25–1.98), 1.65 * | (1.32–1.98), 1.63 * | 0.56 |
| VE/VCO2 slope | (25.60–32.70), 29.60 * | (26.50–33.30), 29.10 * | 0.67 |
| Parameter | Patients Hospitalised Three to Six Months after COVID-19 Diagnosis n = 82 | Patients Hospitalised in One-Year Follow-Up n = 82 | p |
|---|---|---|---|
| Body mass analysis | |||
| Fat (%) | (22.30–34.10), 29.20 * | (23.80–33.40), 27.90 * | 0.96 |
| Fat (kg) | (16.00–29.20), 23.60 * | (17.70–28.90), 21.60 * | 0.45 |
| FFM (kg) | (48.00–62.50), 55.50 * | (48.80–64.50), 55.70 * | 0.67 |
| TBW (kg) | (34.30–44.70), 39.50 * | (35.10–48.10), 41.80 * | 0.07 |
| TBW (%) | (47.80–56.00), 49.50 * | (49.30–57.90), 53.10 * | <0.0001 |
| ECW (kg) | (15.30–19.60), 17.20 * | (15.20–20.90), 17.70 * | 0.06 |
| ICW (kg) | (19.40–25.50), 22.40 * | (20.50–27.30), 24.10 * | 0.17 |
| ECW/TBW × 100% | (40.80–45.30), 43.30 * | (40.70–45.50), 43.40 * | 0.93 |
| Variable | OR | 95% CI for OR | p | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
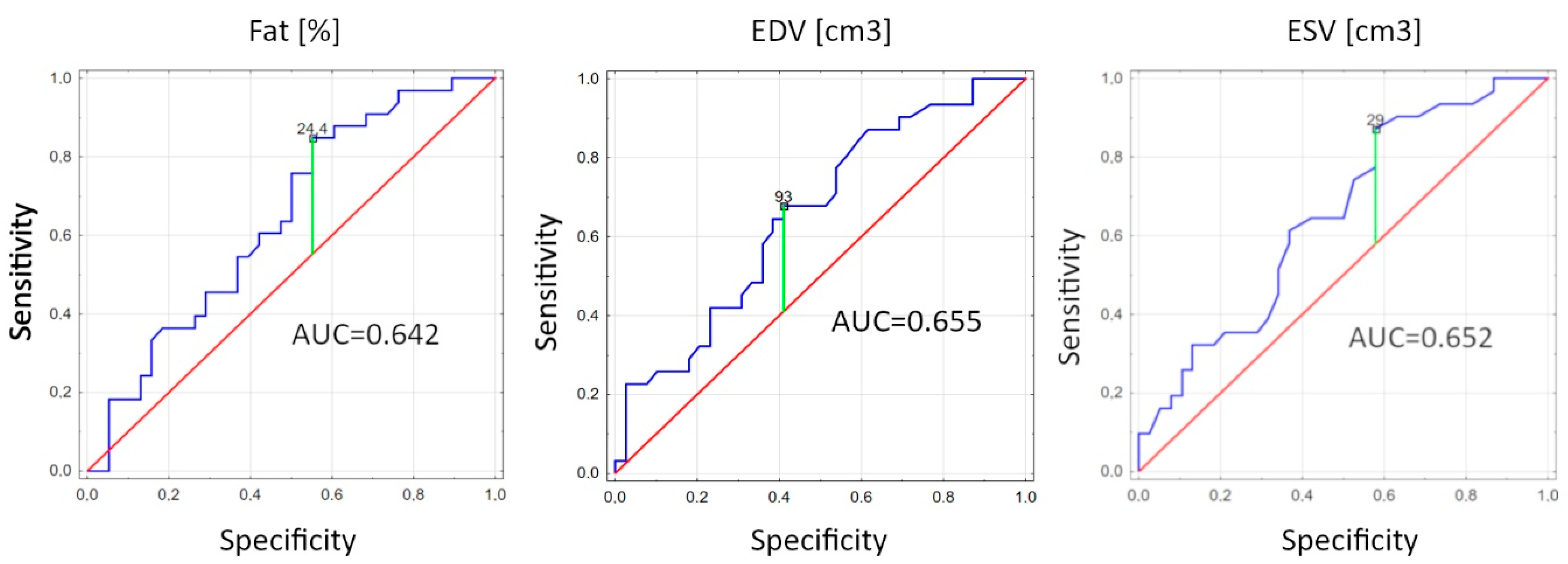
| Fat (%) | 2.16 | 0.51 | 0.77 | 0.03 |
| EDV (cm3) | 2.38 | 0.53 | 0.78 | 0.02 |
| ESV (cm3) | 2.30 | 0.52 | 0.78 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryglewska-Wawrzak, K.; Sakowicz, A.; Banach, M.; Bielecka-Dabrowa, A. Predictors of Long-COVID and Chronic Impairment of Exercise Tolerance in Spiroergometry in Patients after 15 Months of COVID-19 Recovery. J. Clin. Med. 2023, 12, 7689. https://doi.org/10.3390/jcm12247689
Gryglewska-Wawrzak K, Sakowicz A, Banach M, Bielecka-Dabrowa A. Predictors of Long-COVID and Chronic Impairment of Exercise Tolerance in Spiroergometry in Patients after 15 Months of COVID-19 Recovery. Journal of Clinical Medicine. 2023; 12(24):7689. https://doi.org/10.3390/jcm12247689
Chicago/Turabian StyleGryglewska-Wawrzak, Katarzyna, Agata Sakowicz, Maciej Banach, and Agata Bielecka-Dabrowa. 2023. "Predictors of Long-COVID and Chronic Impairment of Exercise Tolerance in Spiroergometry in Patients after 15 Months of COVID-19 Recovery" Journal of Clinical Medicine 12, no. 24: 7689. https://doi.org/10.3390/jcm12247689
APA StyleGryglewska-Wawrzak, K., Sakowicz, A., Banach, M., & Bielecka-Dabrowa, A. (2023). Predictors of Long-COVID and Chronic Impairment of Exercise Tolerance in Spiroergometry in Patients after 15 Months of COVID-19 Recovery. Journal of Clinical Medicine, 12(24), 7689. https://doi.org/10.3390/jcm12247689







