Neurosensory Deficits of the Mandibular Nerve Following Extraction of Impacted Lower Third Molars—A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
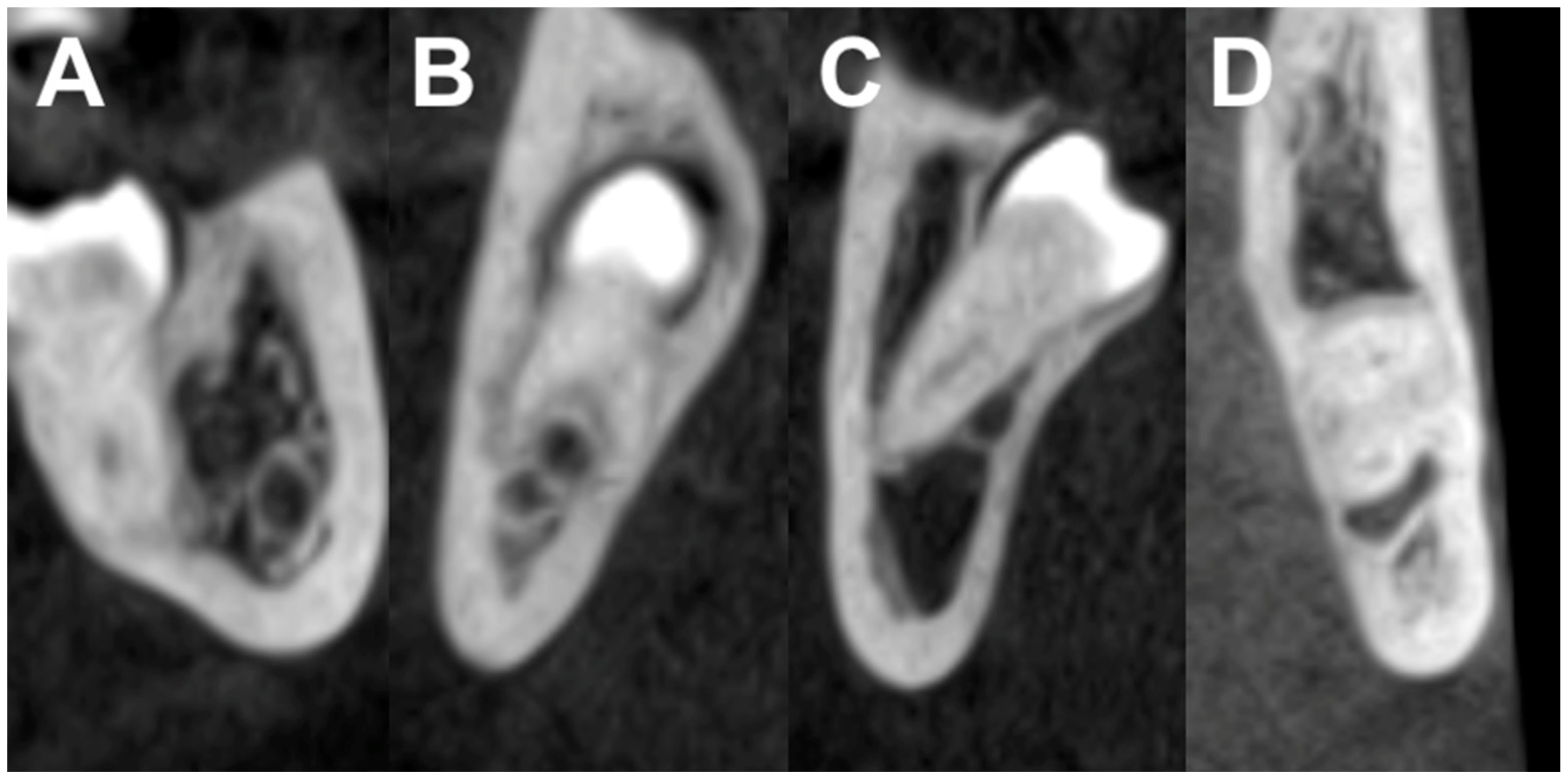
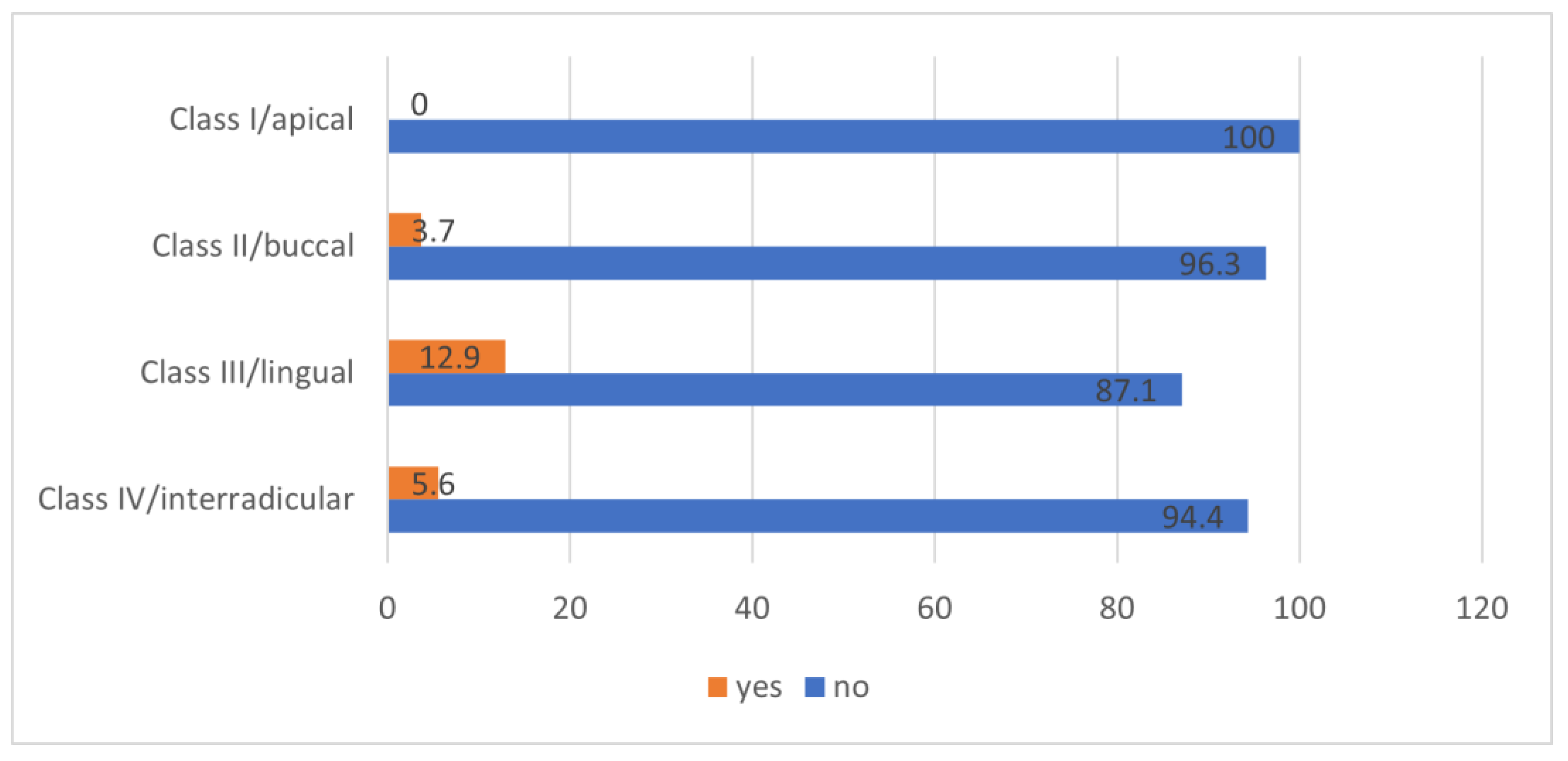
- Class I: the mandibular canal is located on the apical side (apical position).
- Class II: the mandibular canal is located on the buccal side (buccal position).
- Class III: the mandibular canal is located on the lingual side (lingual position).
- Class IV: the mandibular canal is located between the roots (interradicular position).
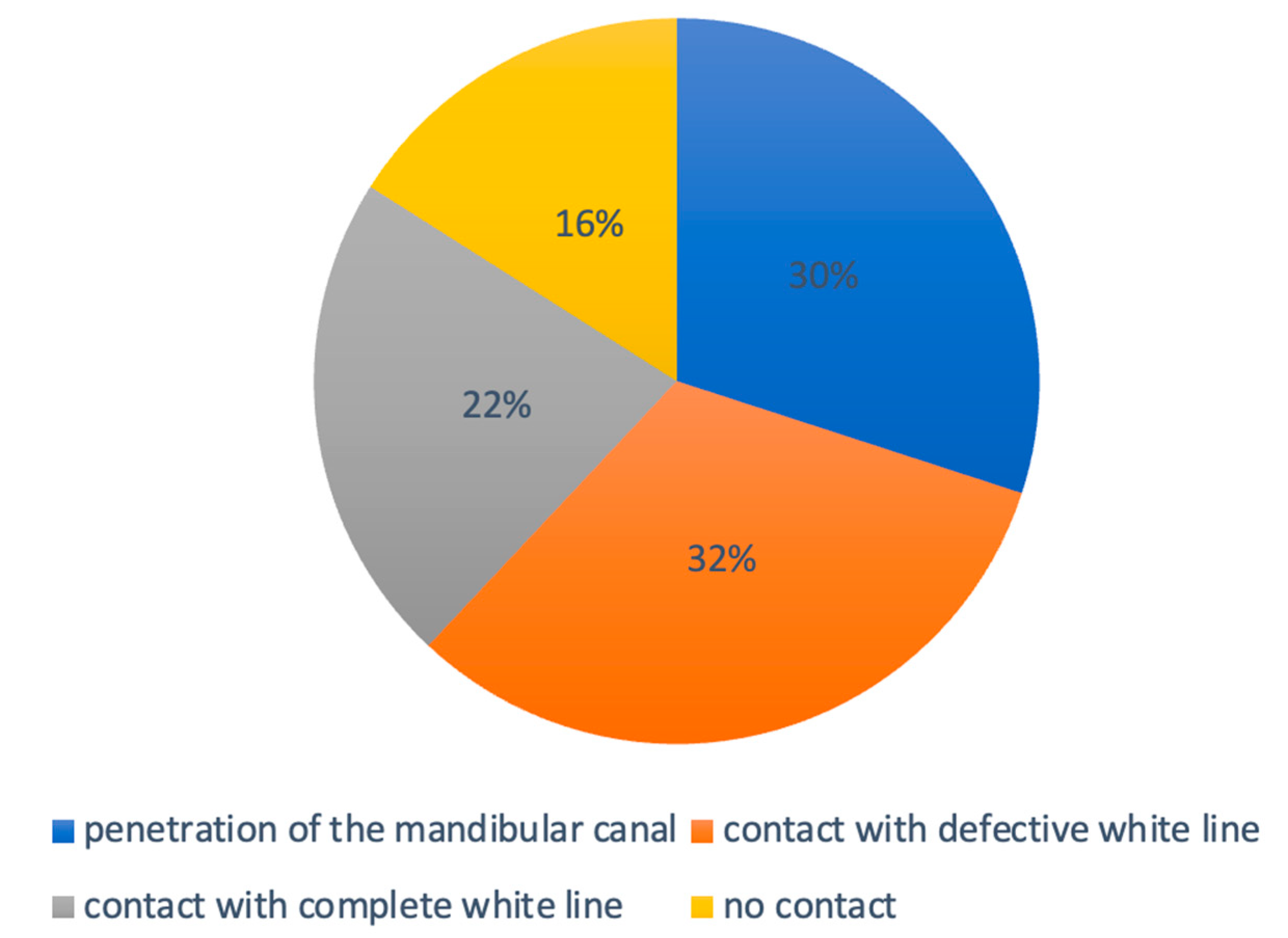
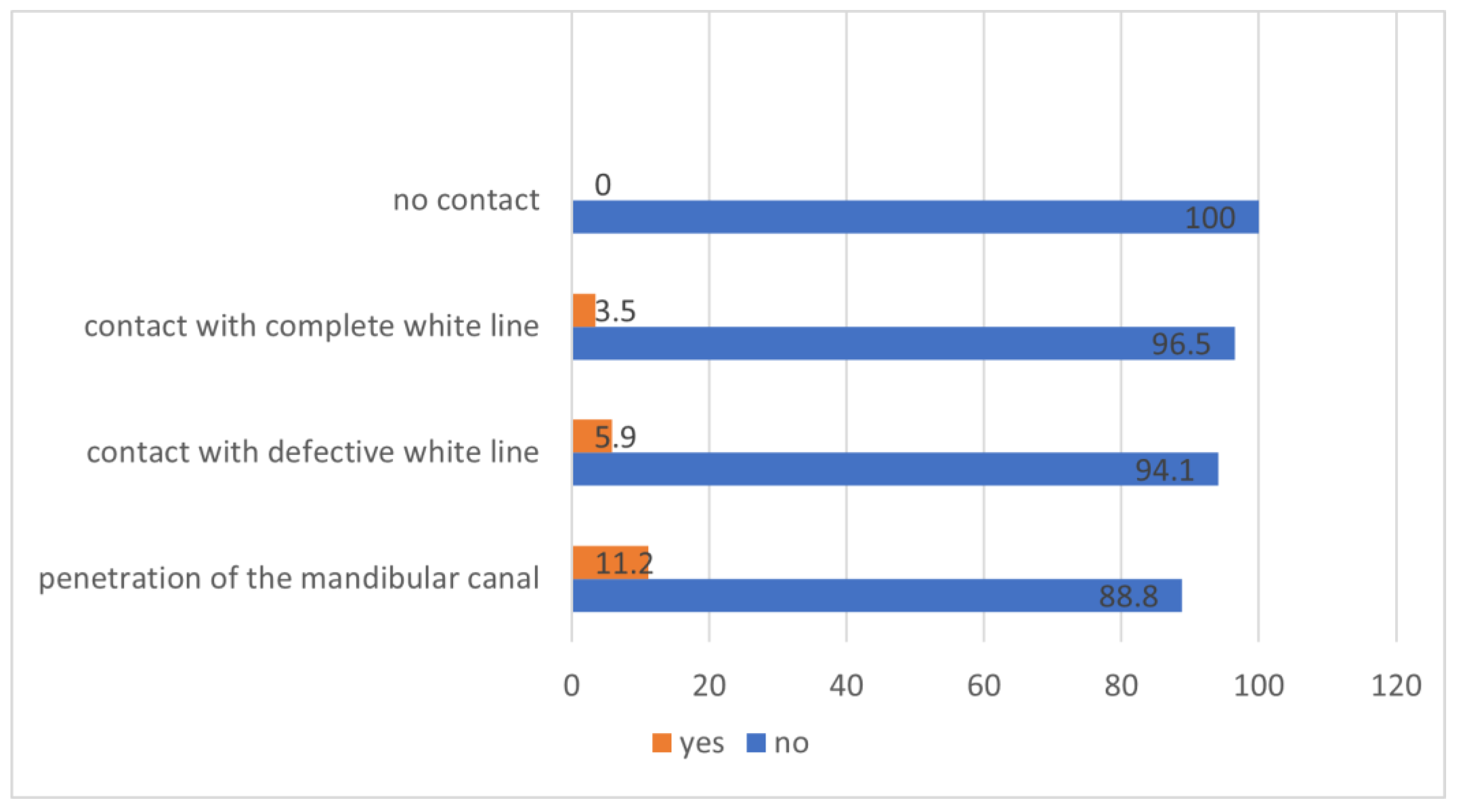
- The mandibular third molar has no contact with the mandibular canal.
- The mandibular third molar contacts with the mandibular canal with a complete white line.
- The mandibular third molar contacts with the mandibular canal with a defective white line.
- The mandibular third molar penetrates the mandibular canal.
3. Results
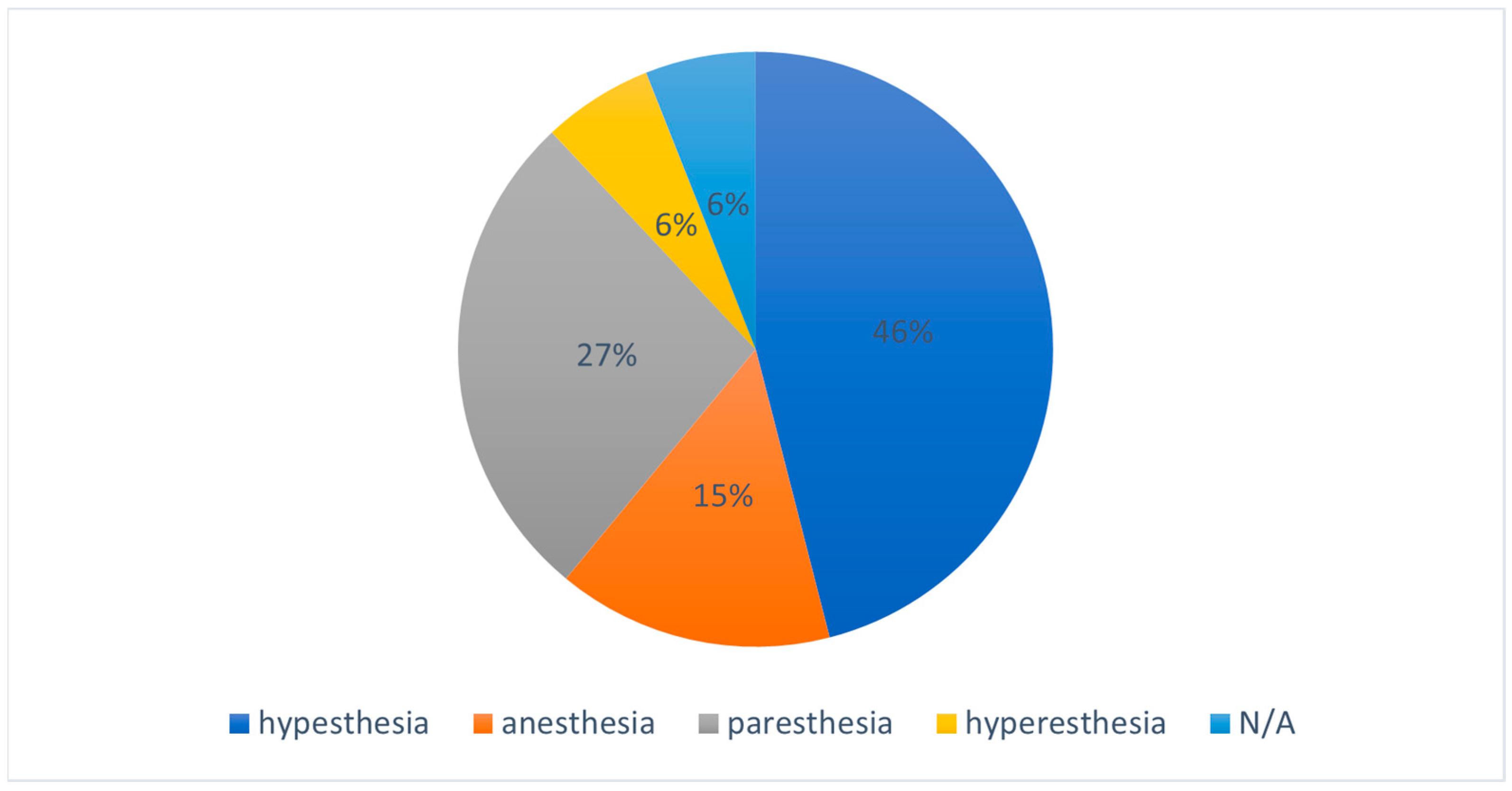
3.1. Incidence of Neurosensory Deficits
3.2. Type of Impaction
3.3. Position of the Mandibular Canal Relative to the Apex
3.4. Sex and Age
3.5. Experience of Operators
3.6. Recovery Patterns
Therapy Regime
- The combination of cortisone (prednisolone 5mg), vitamin B-complex, and low-level laser therapy was used in 26.3% (n = 5) of the cases and had a complete recovery in 60% (3/5).
- The combination of vitamin B-complex and low-level laser therapy in 21.1% (n = 4) with a recovery rate of 50% (2/4).
- Only low-level laser therapy in 15.8% (n = 3) with a recovery rate of 100% (3/3).
- The combination of cortisone and vitamin B-complex in 5.3% (n = 1) with a recovery rate of 0% (0/1).
- Only vitamin-b-complex in 21.1% (n = 4) with a recovery rate of 75% (3/4).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, A.H. Pattern of third molar impaction in a Saudi population. Clin. Cosmet. Investig. Dent. 2010, 2, 109–113. [Google Scholar] [CrossRef]
- Alfadil, L.; Almajed, E. Prevalence of impacted third molars and the reason for extraction in Saudi Arabia. Saudi Dent. J. 2020, 32, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Dachi, S.F.; Howell, F.V. A survey of 3874 routine full-mouth radiographs. I. A study of retained roots and teeth. Oral Surg. Oral Med. Oral Pathol. 1961, 14, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.S.; Lorton, L. The incidence of unerupted permanent teeth and related clinical cases. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.C.; Li, T.K.; Lui, V.K.; Newsome, P.R.; Chow, R.L.; Cheung, L.K. Prevalence of impacted teeth and associated pathologies—A radiographic study of the Hong Kong Chinese population. Hong Kong Med. J. 2003, 9, 158–163. [Google Scholar] [PubMed]
- Behbehani, F.; Artun, J.; Thalib, L. Prediction of mandibular third-molar impaction in adolescent orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 47–55. [Google Scholar] [CrossRef]
- Barone, S.; Antonelli, A.; Averta, F.; Diodati, F.; Muraca, D.; Bennardo, F.; Giudice, A. Does Mandibular Gonial Angle Influence the Eruption Pattern of the Lower Third Molar? A Three-Dimensional Study. J. Clin. Med. 2021, 10, 4057. [Google Scholar] [CrossRef]
- Gümrükçü, Z.; Balaban, E.; Karabağ, M. Is there a relationship between third-molar impaction types and the dimensional/angular measurement values of posterior mandible according to Pell & Gregory/Winter Classification? Oral Radiol. 2021, 37, 29–35. [Google Scholar] [CrossRef]
- Blondeau, F.; Daniel, N.G. Extraction of impacted mandibular third molars: Postoperative complications and their risk factors. J. Can. Dent. Assoc. 2007, 73, 325. [Google Scholar]
- Akashi, M.; Hiraoka, Y.; Hasegawa, T.; Komori, T. Temporal Evaluation of Neurosensory Complications after Mandibular Third Molar Extraction: Current Problems for Diagnosis and Treatment. Open Dent. J. 2016, 10, 728–732. [Google Scholar] [CrossRef][Green Version]
- Cheung, L.K.; Leung, Y.Y.; Chow, L.K.; Wong, M.C.; Chan, E.K.; Fok, Y.H. Incidence of neurosensory deficits and recovery after lower third molar surgery: A prospective clinical study of 4338 cases. Int. J. Oral Maxillofac. Surg. 2010, 39, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sigron, G.R.; Pourmand, P.P.; Mache, B.; Stadlinger, B.; Locher, M.C. The most common complications after wisdom-tooth removal: Part 1: A retrospective study of 1199 cases in the mandible. Swiss. Dent. J. 2014, 124, 1042–1046, 1052–1056. [Google Scholar] [PubMed]
- Bouloux, G.F.; Steed, M.B.; Perciaccante, V.J. Complications of third molar surgery. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, M.; Becker, J.; Boehme, P.; Engel, P.; Göz, G.; Haessler, D.; Heidemann, D.; Hellwig, E.; Kopp, I.; Kreusser, B.; et al. Surgical extraction of wisdom teeth. Mund. Kiefer Gesichtschir. 2006, 10, 205–211. [Google Scholar] [CrossRef]
- Antonelli, A.; Barone, S.; Bennardo, F.; Giudice, A. Three-dimensional facial swelling evaluation of pre-operative single-dose of prednisone in third molar surgery: A split-mouth randomized controlled trial. BMC Oral Health 2023, 23, 614. [Google Scholar] [CrossRef]
- Kiencało, A.; Jamka-Kasprzyk, M.; Panaś, M.; Wyszyńska-Pawelec, G. Analysis of complications after the removal of 339 third molars. Dent. Med. Probl. 2021, 58, 75–80. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Buenechea-Imaz, R.; Gay-Escoda, C. Lingual nerve protection during surgical removal of lower third molars: A prospective randomised study. Int. J. Oral Maxillofac. Surg. 2000, 29, 268–271. [Google Scholar] [CrossRef]
- Krafft, T.C.; Hickel, R. Clinical investigation into the incidence of direct damage to the lingual nerve caused by local anaesthesia. J. Cranio-Maxillofac. Surg. 1994, 22, 294–296. [Google Scholar] [CrossRef]
- Leung, Y.Y. Management and prevention of third molar surgery-related trigeminal nerve injury: Time for a rethink. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 233–240. [Google Scholar] [CrossRef]
- Kqiku, L.; Weiglein, A.H.; Pertl, C.; Biblekaj, R.; Städtler, P. Histology and intramandibular course of the inferior alveolar nerve. Clin. Oral Investig. 2011, 15, 1013–1016. [Google Scholar] [CrossRef]
- Bataineh, A.B. Sensory nerve impairment following mandibular third molar surgery. J. Oral Maxillofac. Surg. 2001, 59, 1012–1017; discussion 1017. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdóttir, I.S.; Wenzel, A.; Petersen, J.K.; Hintze, H. Mandibular third molar removal: Risk indicators for extended operation time, postoperative pain, and complications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.I.; Bøe, O.E. Predictor evaluation of postoperative morbidity after surgical removal of mandibular third molars. Acta Odontol. Scand. 1994, 52, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Brann, C.R.; Brickley, M.R.; Shepherd, J.P. Factors influencing nerve damage during lower third molar surgery. Br. Dent. J. 1999, 186, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.A.; Frederickson, G.C.; Small, G.S. Age of patients and morbidity associated with mandibular third molar surgery. J. Am. Dent. Assoc. 1980, 101, 240–245. [Google Scholar] [CrossRef]
- Hochwald, D.A.; Davis, W.H.; Martinoff, J. Modified distolingual splitting technique for removal of impacted mandibular third molars: Incidence of postoperative sequelae. Oral Surg. Oral Med. Oral Pathol. 1983, 56, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kipp, D.P.; Goldstein, B.H.; Weiss, W.W., Jr. Dysesthesia after mandibular third molar surgery: A retrospective study and analysis of 1,377 surgical procedures. J. Am. Dent. Assoc. 1980, 100, 185–192. [Google Scholar] [CrossRef]
- Lopes, V.; Mumenya, R.; Feinmann, C.; Harris, M. Third molar surgery: An audit of the indications for surgery, post-operative complaints and patient satisfaction. Br. J. Oral Maxillofac. Surg. 1995, 33, 33–35. [Google Scholar] [CrossRef]
- Miura, K.; Kino, K.; Shibuya, T.; Hirata, Y.; Shibuya, T.; Sasaki, E.; Komiyama, T.; Yoshimasu, H.; Amagasa, T. Nerve paralysis after third molar extraction. Kokubyo Gakkai Zasshi 1998, 65, 1–5. [Google Scholar] [CrossRef][Green Version]
- Queral-Godoy, E.; Valmaseda-Castellón, E.; Berini-Aytés, L.; Gay-Escoda, C. Incidence and evolution of inferior alveolar nerve lesions following lower third molar extraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 259–264. [Google Scholar] [CrossRef]
- Rood, J.P. Permanent damage to inferior alveolar and lingual nerves during the removal of impacted mandibular third molars. Comparison of two methods of bone removal. Br. Dent. J. 1992, 172, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Rud, J. The split-bone technic for removal of impacted mandibular third molars. J. Oral Surg. 1970, 28, 416–421. [Google Scholar] [PubMed]
- Schultze-Mosgau, S.; Reich, R.H. Assessment of inferior alveolar and lingual nerve disturbances after dentoalveolar surgery, and of recovery of sensitivity. Int. J. Oral Maxillofac. Surg. 1993, 22, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Sisk, A.L.; Hammer, W.B.; Shelton, D.W.; Joy, E.D., Jr. Complications following removal of impacted third molars: The role of the experience of the surgeon. J. Oral Maxillofac. Surg. 1986, 44, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Valmaseda-Castellón, E.; Berini-Aytés, L.; Gay-Escoda, C. Lingual nerve damage after third lower molar surgical extraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 90, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhu, C.; Chen, K.; Liu, X.; Tang, Z. Anatomic study of the position of the mandibular canal and corresponding mandibular third molar on cone-beam computed tomography images. Surg. Radiol. Anat. 2018, 40, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Rugani, P.; Kirnbauer, B.; Arnetzl, G.V.; Jakse, N. Cone beam computerized tomography: Basics for digital planning in oral surgery and implantology. Int. J. Comput. Dent. 2009, 12, 131–145. [Google Scholar] [PubMed]
- Baumann, P.; Widek, T.; Merkens, H.; Boldt, J.; Petrovic, A.; Urschler, M.; Kirnbauer, B.; Jakse, N.; Scheurer, E. Dental age estimation of living persons: Comparison of MRI with OPG. Forensic Sci. Int. 2015, 253, 76–80. [Google Scholar] [CrossRef]
- Kirnbauer, B.; Jakse, N.; Rugani, P.; Schwaiger, M.; Magyar, M. Assessment of impacted and partially impacted lower third molars with panoramic radiography compared to MRI-a proof of principle study. Dentomaxillofac. Radiol. 2018, 47, 20170371. [Google Scholar] [CrossRef]
- Ghaeminia, H.; Meijer, G.J.; Soehardi, A.; Borstlap, W.A.; Mulder, J.; Bergé, S.J. Position of the impacted third molar in relation to the mandibular canal. Diagnostic accuracy of cone beam computed tomography compared with panoramic radiography. Int. J. Oral Maxillofac. Surg. 2009, 38, 964–971. [Google Scholar] [CrossRef]
- Wang, D.; Lin, T.; Wang, Y.; Sun, C.; Yang, L.; Jiang, H.; Cheng, J. Radiographic features of anatomic relationship between impacted third molar and inferior alveolar canal on coronal CBCT images: Risk factors for nerve injury after tooth extraction. Arch. Med. Sci. 2018, 14, 532–540. [Google Scholar] [CrossRef]
- Sayed, N.; Bakathir, A.; Pasha, M.; Al-Sudairy, S. Complications of Third Molar Extraction: A retrospective study from a tertiary healthcare centre in Oman. Sultan Qaboos Univ. Med. J. 2019, 19, e230–e235. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Ball, R.; Patel, J.; Obisesan, O.; Shah, A.; Manoharan, A. Retrospective evaluation of sensory neuropathies after extraction of mandibular third molars with confirmed "high-risk" features on cone beam computed topography scans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Imai, T.; Nakazawa, M.; Uzawa, N. Risk stratification against inferior alveolar nerve injury after lower third molar extraction by scoring on cone-beam computed tomography image. Odontology 2020, 108, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Nakamori, K.; Shiratori, K.; Igarashi, T.; Sasaki, T.; Anbo, N.; Kaneko, T.; Suzuki, N.; Dehari, H.; Sonoda, T.; et al. Clinical significance of computed tomographic assessment and anatomic features of the inferior alveolar canal as risk factors for injury of the inferior alveolar nerve at third molar surgery. J. Oral Maxillofac. Surg. 2012, 70, 514–520. [Google Scholar] [CrossRef]
- Sklavos, A.; Delpachitra, S.; Jaunay, T.; Kumar, R.; Chandu, A. Degree of Compression of the Inferior Alveolar Canal on Cone-Beam Computed Tomography and Outcomes of Postoperative Nerve Injury in Mandibular Third Molar Surgery. J. Oral Maxillofac. Surg. 2021, 79, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Choudhury, S. Role of Panoramic Imaging and Cone Beam CT for Assessment of Inferior Alveolar Nerve Exposure and Subsequent Paresthesia Following Removal of Impacted Mandibular Third Molar. J. Maxillofac. Oral Surg. 2018, 17, 242–247. [Google Scholar] [CrossRef]
- Sarikov, R.; Juodzbalys, G. Inferior alveolar nerve injury after mandibular third molar extraction: A literature review. J. Oral Maxillofac. Res. 2014, 5, e1. [Google Scholar] [CrossRef]
- Xu, G.Z.; Yang, C.; Fan, X.D.; Yu, C.Q.; Cai, X.Y.; Wang, Y.; He, D. Anatomic relationship between impacted third mandibular molar and the mandibular canal as the risk factor of inferior alveolar nerve injury. Br. J. Oral Maxillofac. Surg. 2013, 51, e215–e219. [Google Scholar] [CrossRef]
- Fielding, A.F.; Rachiele, D.P.; Frazier, G. Lingual nerve paresthesia following third molar surgery: A retrospective clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1997, 84, 345–348. [Google Scholar] [CrossRef]
- Middlehurst, R.J.; Barker, G.R.; Rood, J.P. Postoperative morbidity with mandibular third molar surgery: A comparison of two techniques. J. Oral Maxillofac. Surg. 1988, 46, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Valmaseda-Castellón, E.; Berini-Aytés, L.; Gay-Escoda, C. Inferior alveolar nerve damage after lower third molar surgical extraction: A prospective study of 1117 surgical extractions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, K.; Nakamori, K.; Ueda, M.; Sonoda, T.; Dehari, H. Assessment of the shape of the inferior alveolar canal as a marker for increased risk of injury to the inferior alveolar nerve at third molar surgery: A prospective study. J. Oral Maxillofac. Surg. 2013, 71, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Feng, B.; Park, J.S.; Foo, M.; Kruger, E. Incidence of lingual nerve damage following surgical extraction of mandibular third molars with lingual flap retraction: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0282185. [Google Scholar] [CrossRef] [PubMed]
- Alling, C.C., 3rd. Dysesthesia of the lingual and inferior alveolar nerves following third molar surgery. J. Oral Maxillofac. Surg. 1986, 44, 454–457. [Google Scholar] [CrossRef]
- Blackburn, C.W.; Bramley, P.A. Lingual nerve damage associated with the removal of lower third molars. Br. Dent. J. 1989, 167, 103–107. [Google Scholar] [CrossRef]
- Jerjes, W.; Swinson, B.; Moles, D.R.; El-Maaytah, M.; Banu, B.; Upile, T.; Kumar, M.; Al Khawalde, M.; Vourvachis, M.; Hadi, H.; et al. Permanent sensory nerve impairment following third molar surgery: A prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, e1–e7. [Google Scholar] [CrossRef]
- Wofford, D.T.; Miller, R.I. Prospective study of dysesthesia following odontectomy of impacted mandibular third molars. J. Oral Maxillofac. Surg. 1987, 45, 15–19. [Google Scholar] [CrossRef]
| Angulation | n | Neurosensory Deficits |
|---|---|---|
| vertical | 80 (14.4%) | 5 (6.3%) |
| mesioangular | 217 (39.1%) | 14 (6.5%) |
| distoangular | 101 (18.2%) | 8 (7.9%) |
| horizontal | 148 (26.7%) | 6 (4.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rieder, M.; Remschmidt, B.; Schrempf, V.; Schwaiger, M.; Jakse, N.; Kirnbauer, B. Neurosensory Deficits of the Mandibular Nerve Following Extraction of Impacted Lower Third Molars—A Retrospective Study. J. Clin. Med. 2023, 12, 7661. https://doi.org/10.3390/jcm12247661
Rieder M, Remschmidt B, Schrempf V, Schwaiger M, Jakse N, Kirnbauer B. Neurosensory Deficits of the Mandibular Nerve Following Extraction of Impacted Lower Third Molars—A Retrospective Study. Journal of Clinical Medicine. 2023; 12(24):7661. https://doi.org/10.3390/jcm12247661
Chicago/Turabian StyleRieder, Marcus, Bernhard Remschmidt, Vera Schrempf, Matthäus Schwaiger, Norbert Jakse, and Barbara Kirnbauer. 2023. "Neurosensory Deficits of the Mandibular Nerve Following Extraction of Impacted Lower Third Molars—A Retrospective Study" Journal of Clinical Medicine 12, no. 24: 7661. https://doi.org/10.3390/jcm12247661
APA StyleRieder, M., Remschmidt, B., Schrempf, V., Schwaiger, M., Jakse, N., & Kirnbauer, B. (2023). Neurosensory Deficits of the Mandibular Nerve Following Extraction of Impacted Lower Third Molars—A Retrospective Study. Journal of Clinical Medicine, 12(24), 7661. https://doi.org/10.3390/jcm12247661






