Should Tinnitus Patients with Subclinical Hearing Impairment Be Offered Hearing Aids? A Comparison of Tinnitus Mitigation Following 3 Months Hearing Aid Use in Individuals with and without Clinical Hearing Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Hearing Assessment
2.3. Hearing Aid Fitting
2.4. Evaluation of Tinnitus Distress
2.5. Procedure
2.6. Data Analysis
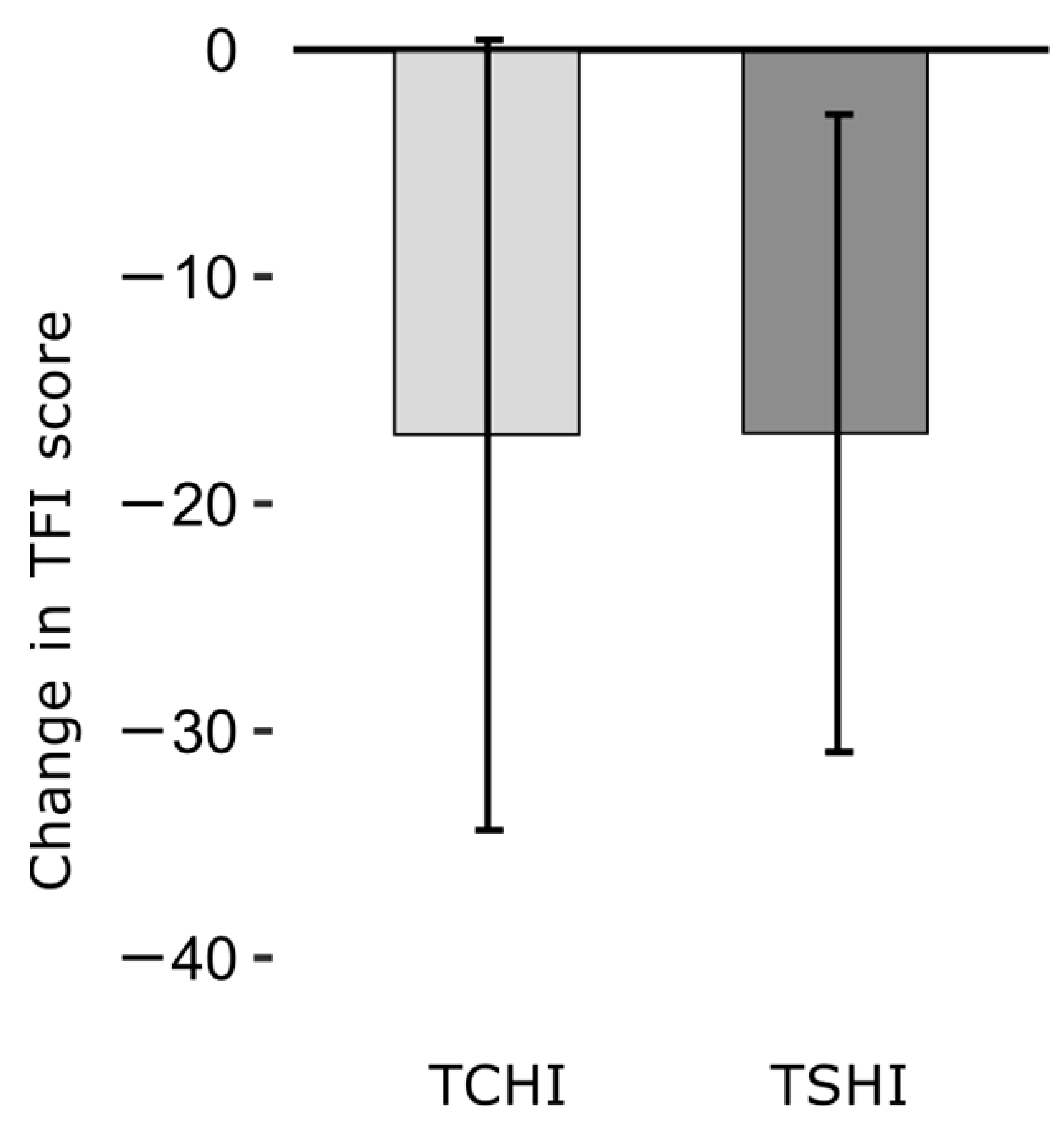
3. Results
4. Discussion
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| TCHI (n = 13) | TSHI (n = 14) | |
|---|---|---|
| TFI-I (mean, SD) | −14.4, 18.5 | −22.1, 17.1 |
| TFI-SoC (mean, SD) | −12.6, 32.2 | −16.2, 22.2 |
| TFI-C (mean, SD) | −13.8, 22.6 | −16.0, 14.7 |
| TFI-S (mean, SD) | −7.7, 22.7 | −15.0, 27.8 |
| TFI-A (mean, SD) | −26.7, 36.9 | −7.1, 25.5 |
| TFI-R (mean, SD) | −26.4, 25.4 | −31.7, 26.4 |
| TFI-QoL (mean, SD) | −22.5, 27.1 | −12.9, 16.6 |
| TFI-E (mean, SD) | −9.7, 28.2 | −15.2, 18.7 |
References
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-Analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.K.; Nayak, S.; Ravan, J.R.; Sahu, M.C. Tinnitus and its current treatment–Still an enigma in medicine. J. Formos. Med. Assoc. 2015, 115, 139–144. [Google Scholar] [CrossRef]
- Jackson, R.; Vijendren, A.; Phillips, J. Objective Measures of Tinnitus: A Systematic Review. Otol. Neurotol. 2019, 40, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Myers, P.J.; Newman, C.W.; Sandridge, S.; Turk, D.C.; et al. The Tinnitus Functional Index: Development of a New Clinical Measure for Chronic, Intrusive Tinnitus. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef] [PubMed]
- McFerran, D.J.; Stockdale, D.; Holme, R.; Large, C.H.; Baguley, D.M. Why Is There No Cure for Tinnitus? Front. Neurosci. 2019, 13, 802. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.T.; Gander, P.E.; Jansen, J.N.; Shen, S. Expectations for Tinnitus Treatment and Outcomes: A Survey Study of Audiologists and Patients. J. Am. Acad. Audiol. 2018, 29, 313–336. [Google Scholar] [CrossRef]
- Saltzman, M.; Ersner, M.S. A hearing aid for the relief of tinnitus aurium. Laryngoscope 1947, 57, 358–366. [Google Scholar] [CrossRef]
- Trotter, M.I.; Donaldson, I. Hearing aids and tinnitus therapy: A 25-year experience. J. Laryngol. Otol. 2008, 122, 1052–1056. [Google Scholar] [CrossRef]
- Langguth, B.; Kleinjung, T.; Schlee, W.; Vanneste, S.; De Ridder, D. Tinnitus Guidelines and Their Evidence Base. J. Clin. Med. 2023, 12, 3087. [Google Scholar] [CrossRef]
- Jacquemin, L.; Gilles, A.; Shekhawat, G.S. Hearing more to hear less: A scoping review of hearing aids for tinnitus relief. Int. J. Audiol. 2022, 61, 887–895. [Google Scholar] [CrossRef]
- Waechter, S.; Jönsson, A. Hearing Aids Mitigate Tinnitus, But Does It Matter if the Patient Receives Amplification in Accordance with Their Hearing Impairment or Not? A Meta-Analysis. Am. J. Audiol. 2022, 31, 789–818. [Google Scholar] [CrossRef]
- Del Bo, L.; Ambrosetti, U. Hearing Aids for the Treatment of Tinnitus. Prog. Brain Res. 2007, 166, 341–345. [Google Scholar] [CrossRef]
- Park, D.C.; Bischof, G.N. The aging mind: Neuroplasticity in response to cognitive training. Dialogues Clin. Neurosci. 2013, 15, 109–119. [Google Scholar] [CrossRef]
- Marks, E.; Smith, P.; McKenna, L. Living with tinnitus and the health care journey: An interpretative phenomenological analysis. Br. J. Health Psychol. 2019, 24, 250–264. [Google Scholar] [CrossRef]
- Van der Wal, A.; Luyten, T.; Cardon, E.; Jacquemin, L.; Vanderveken, O.M.; Topsakal, V.; Van de Heyning, P.; De Hertogh, W.; Van Looveren, N.; Van Rompaey, V.; et al. Sex Differences in the Response to Different Tinnitus Treatment. Front. Neurosci. 2020, 14, 422. [Google Scholar] [CrossRef]
- Boecking, B.; Psatha, S.; Nyamaa, A.; Dettling-Papargyris, J.; Funk, C.; Oppel, K.; Brueggemann, P.; Rose, M.; Mazurek, B. Hearing Aid Use Time Is Causally Influenced by Psychological Parameters in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss. J. Clin. Med. 2022, 11, 5869. [Google Scholar] [CrossRef]
- Jafari, Z.; Baguley, D.; Kolb, B.E.; Mohajerani, M.H. A Systematic Review and Meta-Analysis of Extended High-Frequency Hearing Thresholds in Tinnitus with a Normal Audiogram. Ear Hear. 2022, 43, 1643–1652. [Google Scholar] [CrossRef]
- Martines, F.; Sireci, F.; Cannizzaro, E.; Costanzo, R.; Martines, E.; Mucia, M.; Plescia, F.; Salvago, P. Clinical observations and risk factors for tinnitus in a Sicilian cohort. Eur. Arch. Otorhinolaryngol. 2015, 272, 2719–2729. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Report of the Informal Working Group on Prevention of Deafness and Hearing Impairment Programme Planning. 1991. Available online: http://www.who.int/iris/handle/10665/58839 (accessed on 14 October 2023).
- Sereda, M.; Hoare, D.J.; Nicholson, R.; Smith, S.; Hall, D.A. Consensus on Hearing Aid Candidature and Fitting for Mild Hearing Loss, with and without Tinnitus: Delphi Review. Ear Hear. 2015, 36, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Shinden, S.; Oishi, N.; Ueno, M.; Suzuki, D.; Ogawa, K.; Ozawa, H. Effectiveness of hearing aids in treating patients with chronic tinnitus with average hearing levels of <30 dBHL and no inconvenience due to hearing loss. Acta Oto-Laryngol. 2021, 141, 773–779. [Google Scholar] [CrossRef]
- Marcrum, S.C.; Picou, E.M.; Steffens, T.; Hannemann, R.; Vielsmeier, V.; Schecklmann, M.; Langguth, B.; Schlee, W. Conventional Versus Notch Filter Amplification for the Treatment of Tinnitus in Adults with Mild-to-Moderate Hearing Loss. Prog. Brain Res. 2021, 260, 235–252. [Google Scholar] [CrossRef]
- Haab, L.; Lehser, C.; Corona-Strauss, F.I.; Bernarding, C.; Seidler, H.; Hannemann, R.; Strauss, D.J. Implementation and Long-Term Evaluation of a Hearing Aid Supported Tinnitus Treatment Using Notched Environmental Sounds. IEEE J. Transl. Eng. Health Med. 2019, 7, 1600109. [Google Scholar] [CrossRef]
- Scollie, S.; Seewald, R.; Cornelisse, L.; Moodie, S.; Bagatto, M.; Laurnagaray, D.; Beaulac, S.; Pumford, J. The Desired Sensation Level Multistage Input/Output Algorithm. Trends Amplif. 2005, 9, 159–197. [Google Scholar] [CrossRef] [PubMed]
- Keidser, G.; Dillon, H.; Flax, M.; Ching, T.; Brewer, S. The NAL-NL2 Prescription Procedure. Audiol. Res. 2011, 1, e24. [Google Scholar] [CrossRef] [PubMed]
- Almufarrij, I.; Dillon, H.; Munro, K.J. Does Probe-Tube Verification of Real-Ear Hearing Aid Amplification Characteristics Improve Outcomes in Adults? A Systematic Review and Meta-Analysis. Trends Hear. 2021, 25, 2331216521999563. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.; Oeding, K.; Brockmeyer, A.; Smith, S.; Kallogjeri, D. Differences in Word and Phoneme Recognition in Quiet, Sentence Recognition in Noise, and Subjective Outcomes between Manufacturer First-Fit and Hearing Aids Programmed to NAL-NL2 Using Real-Ear Measures. J. Am. Acad. Audiol. 2018, 29, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Abrams, H.B.; Chisolm, T.H.; McManus, M.; McArdle, R. Initial-Fit Approach Versus Verified Prescription: Comparing Self-Perceived Hearing Aid Benefit. J. Am. Acad. Audiol. 2012, 23, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Hind, S.E.; Haines-Bazrafshan, R.; Benton, C.L.; Brassington, W.; Towle, B.; Moore, D.R. Prevalence of clinical referrals having hearing thresholds within normal limits. Int. J. Audiol. 2011, 50, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Neumann, K.J.; Saunders, J.E. The global burden of disabling hearing impairment: A call to action. Bull. World Health Organ. 2014, 92, 367–373. [Google Scholar] [CrossRef]
- Roup, C.M.; Post, E.; Lewis, J. Mild-Gain Hearing Aids as a Treatment for Adults with Self-Reported Hearing Difficulties. J. Am. Acad. Audiol. 2018, 29, 477–494. [Google Scholar] [CrossRef]
- Tunkel, D.E.; Bauer, C.A.; Sun, G.H.; Rosenfeld, R.M.; Chandrasekhar, S.S.; Cunningham, E.R., Jr.; Archer, S.M.; Blakley, B.W.; Carter, J.M.; Granieri, E.C.; et al. Clinical Practice Guideline: Tinnitus. Otolaryngol. Head Neck Surg. 2014, 151 (Suppl. 2), S1–S40. [Google Scholar] [CrossRef]
- Mazurek, B.; Hesse, G.; Dobel, C.; Kratzsch, V.; Lahmann, C.; Sattel, H. Clinical practice guideline: Chronic tinnitus—Diagnosis and treatment. Dtsch. Arztebl. Int. 2022, 119, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Attarha, M.; Bigelow, J.; Merzenich, M.M. Unintended Consequences of White Noise Therapy for Tinnitus—Otolaryngology’s Cobra Effect: A Review. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, Y.; Hatanaka, A.; Kitamura, K. Clinical prognostic factors for tinnitus retraining therapy with a sound generator in tinnitus patients. J. Med. Dent. Sci. 2010, 57, 45–53. [Google Scholar] [PubMed]
- Jalilvand, H.; Pourbakht, A.; Haghani, H. Hearing Aid or Tinnitus Masker: Which One Is the Best Treatment for Blast-Induced Tinnitus? The Results of a Long-Term Study on 974 Patients. Audiol. Neurotol. 2015, 20, 195–201. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Sawilowski, S.S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- ISO 8253-1; Acoustics—Audiometric Test Methods. International Organization for Standardization: Geneva, Switzerland, 2010.
- DiGiovanni, J.J.; Pratt, R.M. Verification of In Situ Thresholds and Integrated Real-Ear Measurements. J. Am. Acad. Audiol. 2010, 21, 663–670. [Google Scholar] [CrossRef]
- Hoff, M.; Kähäri, K. A Swedish cross-cultural adaptation and validation of the Tinnitus Functional Index. Int. J. Audiol. 2017, 56, 277–285. [Google Scholar] [CrossRef]
- Hornsby, B.W.; Johnson, E.E.; Picou, E. Effects of Degree and Configuration of Hearing Loss on the Contribution of High- and Low-Frequency Speech Information to Bilateral Speech Understanding. Ear Hear. 2011, 32, 543–555. [Google Scholar] [CrossRef]
- Wang, J.; Puel, J.L. Presbycusis: An Update on Cochlear Mechanisms and Therapies. J. Clin. Med. 2020, 9, 218. [Google Scholar] [CrossRef]
- Sanchez, T.G.; Mak, M.P.; Pedalini, M.E.B.; Levy, C.P.D.; Bento, R.F. Tinnitus and Hearing Evolution in Normal Hearing Patients. Int. Arch. Otorhinolaryngol. 2005, 9, 220–227. [Google Scholar]
- Waechter, S. Association between hearing status and tinnitus distress. Acta Oto-Laryngol. 2021, 141, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, B.; Olze, H.; Haupt, H.; Szczepek, A.J. The More the Worse: The Grade of Noise-Induced Hearing Loss Associates with the Severity of Tinnitus. Int. J. Environ. Res. Public Health 2010, 7, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Duckert, L.G.; Rees, T.S. Placebo Effect in Tinnitus Management. Otolaryngol. Head Neck Surg. 1984, 92, 697–699. [Google Scholar] [CrossRef]
- Searchfield, G.D.; Kaur, M.; Martin, W.H. Hearing aids as an adjunct to counseling: Tinnitus patients who choose amplification do better than those that don’t. Int. J. Audiol. 2010, 49, 574–579. [Google Scholar] [CrossRef]
- Han, J.J.; Ridder, D.; Vanneste, S.; Chen, Y.C.; Koo, J.W.; Song, J.J. Pre-treatment Ongoing Cortical Oscillatory Activity Predicts Improvement of Tinnitus After Partial Peripheral Reafferentation with Hearing Aids. Front. Neurosci. 2020, 14, 410. [Google Scholar] [CrossRef]

| Total (n = 27) | |
|---|---|
| Sex | |
| Female | 10 (37.0%) |
| Male | 17 (63.0%) |
| Age (years) | |
| Mean (SD) | 51.7 (13.1) |
| Range | 32.2–78.6 |
| Time since tinnitus debut (years) | |
| Mean (SD) | 14.8 (10.8) |
| Range | 0.3–35.0 |
| Tinnitus distress at baseline (TFI score) | |
| Mean (SD) | 46.3 (14.7) |
| Range | 16.4–72.4 |
| Degree of hearing impairment (PTA) | |
| Mean (SD) | 25.2 (15.6) |
| Range | −2.5–63.8 |
| TCHI (n = 13) | TSHI (n = 14) | p-Value | |
|---|---|---|---|
| Sex | 0.802 1 | ||
| Female | 4 (30.8%) | 6 (42.9%) | |
| Male | 9 (69.2%) | 8 (57.1%) | |
| Age (years) | 0.111 2 | ||
| Mean (SD) | 55.9 (14.5) | 47.8 (10.7) | |
| Range | 35.6–78.6 | 32.2–61.5 | |
| Time since tinnitus debut (years) | 0.318 2 | ||
| Mean (SD) | 17.0 (12.0) | 12.8 (9.6) | |
| Range | 0.3–35.0 | 0.3–28.3 | |
| Tinnitus distress at baseline (TFI score) | 0.101 2 | ||
| Mean (SD) | 51.1 (15.4) | 41.8 (13.0) | |
| Range | 23.2–72.4 | 16.4–64.0 | |
| Treatment adherence (average daily hearing aid use) | 0.879 3 | ||
| Mean (SD) | 9.6 (4.3) | 9.4 (3.7) | |
| Range | 1–14 | 3–14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waechter, S.; Olovsson, M.; Pettersson, P. Should Tinnitus Patients with Subclinical Hearing Impairment Be Offered Hearing Aids? A Comparison of Tinnitus Mitigation Following 3 Months Hearing Aid Use in Individuals with and without Clinical Hearing Impairment. J. Clin. Med. 2023, 12, 7660. https://doi.org/10.3390/jcm12247660
Waechter S, Olovsson M, Pettersson P. Should Tinnitus Patients with Subclinical Hearing Impairment Be Offered Hearing Aids? A Comparison of Tinnitus Mitigation Following 3 Months Hearing Aid Use in Individuals with and without Clinical Hearing Impairment. Journal of Clinical Medicine. 2023; 12(24):7660. https://doi.org/10.3390/jcm12247660
Chicago/Turabian StyleWaechter, Sebastian, Maria Olovsson, and Petter Pettersson. 2023. "Should Tinnitus Patients with Subclinical Hearing Impairment Be Offered Hearing Aids? A Comparison of Tinnitus Mitigation Following 3 Months Hearing Aid Use in Individuals with and without Clinical Hearing Impairment" Journal of Clinical Medicine 12, no. 24: 7660. https://doi.org/10.3390/jcm12247660
APA StyleWaechter, S., Olovsson, M., & Pettersson, P. (2023). Should Tinnitus Patients with Subclinical Hearing Impairment Be Offered Hearing Aids? A Comparison of Tinnitus Mitigation Following 3 Months Hearing Aid Use in Individuals with and without Clinical Hearing Impairment. Journal of Clinical Medicine, 12(24), 7660. https://doi.org/10.3390/jcm12247660




