Abstract
Background: Hospitalization often leads to a decline in activities of daily living (ADL) in older patients with heart failure. Although cardiac rehabilitation (CR) improves ADL, it can be difficult to perform CR due to the deconditioning of these patients. This study aimed to examine the factors associated with ADL at discharge in older patients with heart failure who underwent CR. Methods: A total of 86 of 110 older heart failure patients aged ≥ 75 years (average age, 86.9 ± 5.7 years) transferred to our institution for CR were enrolled and classified into high ADL at discharge (n = 54) and low ADL at discharge (n = 32) groups. Physical characteristics, comorbidities, medications, blood test data, echocardiographic data, and nutritional status (Geriatric Nutritional Risk Index [GNRI]) were retrospectively examined from medical records. ADL were assessed using the Barthel Index (BI) at admission and discharge. Considering multicollinearity, the relationship between high ADL (BI ≥ 60) at discharge and these assessments at admission was analyzed using multiple logistic regression analysis. The receiver operating characteristic curve was analyzed to calculate the cutoff values for the parameters identified by the multiple logistic regression analysis. Results: The GNRI was the only independent factor predicting high ADL at discharge (p = 0.041; odds ratio [OR], 1.125; 95% confidence interval [CI], 1.005–1.260). The area under the receiver operating characteristic curve for the GNRI was 0.770 (95% CI, 0.664–0.876). The cutoff value for the GNRI was 83.4 (sensitivity, 85.2%; specificity, 62.5%). Conclusion: These findings suggest that the GNRI score at admission predicts high ADL at discharge in older patients with heart failure who underwent CR.
1. Introduction
The increase in the incidence of heart failure in the older population is a global problem [1], which is accompanied by an increased prevalence of multimorbidity [2,3]. Many older patients with heart failure require physical and social care in addition to disease management [2,3]. Therefore, activities of daily living (ADL) tend to decline before hospitalization, and proceed to decline after hospitalization due to bed rest [4]. Low ADL, particularly an impaired Barthel Index (BI < 60), is closely associated with a poor prognosis [3,4,5], but inpatient cardiac rehabilitation (CR) improves ADL in older patients with heart failure [3,6]. However, it can be difficult to perform ordinary inpatient CR due to the deconditioning of these patients; therefore, their exercise prescriptions involve very low-intensity and intermittent training [7]. As a result, it can be difficult to achieve high ADL scores at discharge in older patients with heart failure.
Local edema of the intestinal wall in patients with heart failure may contribute to malabsorption [8]. Additionally, sympathetic nerve activity and appetite loss in heart failure patients increase in proportion to the severity of heart failure [9,10]. Therefore, heart failure patients are prone to malnutrition. Recently, many older patients with heart failure have had a high risk of malnutrition. A recent study found that 46.5% of hospitalized older patients with heart failure exhibited signs of malnutrition [11]. Additionally, poor nutritional status is an independent poor prognostic factor. Low body weight due to malnutrition was associated with increased mortality in older patients with heart failure [12,13]. Therefore, the clinical importance of nutritional management in these patients has increased. Kinugasa et al. suggested that nutritional status is associated with ADL [14]. Studies have addressed the relationship between nutritional status and ADL in older patients with heart failure who underwent CR [15,16]; however, the association between nutritional status and a high ADL (BI ≥ 60) remains unreported.
This study aimed to examine the factors associated with high ADL scores at discharge in older patients with heart failure.
2. Materials and Methods
2.1. Participants
This retrospective cohort study recruited 110 older patients with heart failure aged ≥ 75 years who transferred to our institution for CR between 1 April 2015 and 31 July 2022. Our institution accepts patients with stable conditions who have been treated at acute care hospitals. Inclusion criteria were as follows: (1) patients with heart failure who transferred to our institution for CR, and (2) patients aged ≥ 75 years. Exclusion criteria were as follows: (1) in-hospital mortality, (2) missing data, (3) undergoing surgical treatment, and (4) difficulty consenting to participate. Among the 110 recruited patients, 24 were excluded owing to in-hospital mortality (n = 16) and missing data (n = 8), and 86 patients were eventually included in the study. Heart failure was diagnosed by a cardiologist based on the criteria of the Japanese Circulation Society guidelines (JCS 2017/JHFS 2017) for the Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure [17].
These patients were referred for inpatient late phase II CR by a cardiologist after examination at admission and contraindications to exercise therapy were confirmed. The primary physician determined the prescription and timing of CR. CR at our institution is recommended for all older patients with heart failure. CR was performed according to the guidelines of the Japanese Circulation Society for the rehabilitation of inpatients with cardiovascular disease (JCS 2012, JCS 2021) [7,18]. The participants were divided into two groups based on their ADL scores at discharge using the BI. Patients who had high ADL (BI ≥ 60) and low ADL (BI < 60) scores at discharge were classified into the high ADL (n = 54) and low ADL groups (n = 32). The evaluation parameters were retrospectively examined from medical records. A flowchart of the inclusion of participants is shown in Figure 1.
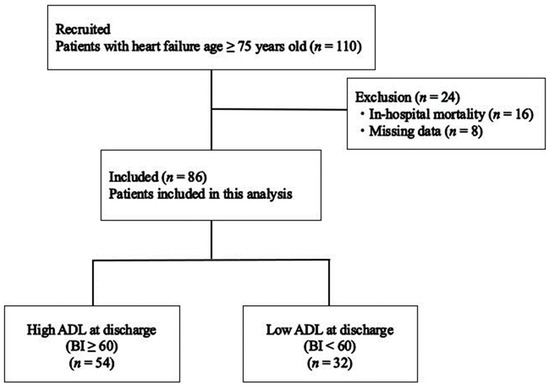
Figure 1.
Flowchart of study participants. BI—Barthel Index.
2.2. Evaluation Parameters
Physical characteristics including age, sex, prior heart failure hospitalization, body mass index (BMI), length of hospital stays, nursing-care insurance, New York Heart Association (NYHA) functional class, comorbidities, medication data, blood test data, and echocardiographic data at admission were retrospectively examined from medical records. Comorbidities were examined for coronary artery disease, hypertension, atrial fibrillation, diabetes, valvular disease, cardiomyopathy, respiratory disease, cerebrovascular disease, dyslipidemia, chronic kidney disease (CKD), and dementia. Medication data were examined for angiotensin-converting enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), diuretics, β-blockers, and angiotensin receptor neprilysin inhibitor (ARNI). Blood test data were examined for brain natriuretic peptide (BNP), hemoglobin (Hb), blood urea nitrogen (BUN), creatinine (Cre), estimated glomerular filtration rate (eGFR), albumin (Alb), total protein (TP), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), and C-reactive protein (CRP). Echocardiographic data were examined for left atrial dimension (LAD) and left ventricular ejection fraction (LVEF). ADL scores at admission and discharge were retrospectively assessed from medical records using the BI.
In this study, BNP was measured as N-terminal proBNP (NT-proBNP) is more affected by decline in renal function than BNP [19]. Additionally, the medical costs of NTproBNP are higher than those of BNP.
The BI is a 10-item scoring assessment scale used to measure a patient’s functional ability to perform basic activities of daily living [20]. The BI includes self-care independence (feeding, grooming, bathing, dressing, bowel and bladder care, and using the toilet) and mobility independence (ambulation, transferring, and going up and down stairs), with a score of 0 (completely dependent) to 100 points (completely independent). In this study, BI scores were assessed by a physical therapist.
The cutoff value of the BI used to divide the patients into groups was 60, based on a previous study demonstrating poor prognosis in older patients with heart failure who underwent CR [5].
Nutritional status was also retrospectively assessed based on medical records at admission using the Geriatric Nutritional Risk Index (GNRI). This nutritional scale precisely predicts mortality rates in older people [21]. The GNRI score was calculated using serum albumin and BMI at admission and assessed using the following formula:
GNRI = [14.89 × serum Alb value (g/L)] + [41.7 × (actual weight/reference weight)]
Reference weight (kg) = (height)2(m2) × 22
The reference (ideal) weight was set as the weight resulting in a BMI of 22 kg/m2. When the actual weight exceeded the reference weight, actual weight/reference weight was calculated as 1. The correlation coefficient between the GNRI calculated by the ideal BMI of 22, and that calculated by the Lorentz formula, is high [22,23]. The GNRI scores were reported as 4 grades of nutrition-related risk: major risk (GNRI < 82), moderate risk (GNRI: 82 to <92), low risk (GNRI: 92 to ≤98), and no risk (98 < GNRI) [21]. In this study, the score of GNRI < 92 was defined as low GNRI based on a previous study [21].
2.3. Statistical Analysis
To identify factors associated with high ADL (BI > 60) at discharge, continuous variables, including physical characteristics, blood test data, echocardiographic data, and GNRI at admission, were compared using an unpaired t-test for normally distributed variables and the Mann–Whitney U-test for non-normally distributed variables. Categorical variables were compared using chi-square and Fisher’s exact tests, as required. Multiple logistic regression analysis using the forced-entry method was used to assess the effect of factors associated with high ADL at discharge. A high ADL score at discharge was used as the dependent variable. Parameters with statistically significant differences between the two groups were set as explanatory variables.
A receiver operating characteristic (ROC) curve analysis was performed on the parameters identified via multiple logistic regression to calculate cutoff values, sensitivity, and specificity using the Youden index. Statistical analysis was performed using SPSS (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY, USA: IBM Corp.) and JMP (JMP®, Version 16. SAS Institute Inc., Cary, NC, USA, 1989–2023.), with significance set at p < 0.05.
3. Results
The baseline characteristics of the participants at admission are shown in Table 1. The cohort had a mean age of 86.9 ± 5.7 years, with 34.9% being men, 32.6% having prior heart failure hospitalization, a mean BMI of 20.3 (18.7, 23.1), 61.6% having NYHA functional class III/IV, and an admission BI score of 43.2 ± 25.8. Overall, 46.5% of the participants had coronary artery disease, and the most common comorbidity was dyslipidemia (72.5%), followed by hypertension (69.8%) and atrial fibrillation (51.2%). A total of 7% of the participants had cardiomyopathy. The cohort included hypertrophic cardiomyopathy (n = 2), ischemic cardiomyopathy (n = 2), and drug-induced cardiomyopathy (n = 2). ACEI/ARB, diuretics, and β-blockers and ARNI were prescribed to 59.3%, 79.1%, 68.6%, and 5.8% of the patients, respectively. The median LVEF was 60.8 (47.2, 69.2). The cohort comprised 12 patients with heart failure with reduced ejection fraction (HFrEF), 12 patients with heart failure with mid-range ejection fraction (HFmrEF), and 62 patients with heart failure with preserved ejection fraction (HFpEF) based on the guidelines, respectively [17].

Table 1.
Baseline characteristics of the high ADL and low ADL groups.
Significant differences were observed between the high and low ADL groups in terms of age, sex, BMI, NYHA functional class III/IV, admission BI scores, dyslipidemia, Cre levels, eGFR levels, Alb levels, TP levels, CRP levels, and admission GNRI scores (Table 1).
Pearson’s product–moment correlation coefficient was used to account for multicollinearity. The correlation coefficient for the GNRI score and Alb levels was r = 0.913 (p < 0.0001). The correlation coefficient for eGFR and Cre levels was r = −0.811 (p < 0.0001). The correlation ratios between high ADL at discharge and factors showing multicollinearity were η = 0.479 for the GNRI, η = 0.395 for Alb levels, η = 0.270 for eGFR, and η = 0.250 for Cre levels. Therefore, the GNRI and eGFR were selected as explanatory variables for the multiple logistic regression analysis.
Table 2 shows the results of the multiple logistic regression analysis used to examine the association between high ADL at discharge and the evaluation parameters associated with it. The multiple logistic regression analysis was performed using the GNRI, BI, age, sex, BMI, NYHA functional class III/IV, dyslipidemia, eGFR, CRP, and TP levels as explanatory variables. GNRI was identified as the only independent factor predicting high ADL at discharge (p = 0.041; odds ratio [OR], 1.125; 95% confidence interval [CI], 1.005–1.260). The model χ2 test result was significant at p < 0.01.

Table 2.
Multiple logistic regression analysis regarding factors associated with high ADL at discharge.
Figure 2 shows the ROC curve for the GNRI. The area under the curve was 0.770 (95% CI, 0.664–0.876, p < 0.001). The cutoff value for the GNRI was 83.4 (sensitivity, 85.2%; specificity, 62.5%).
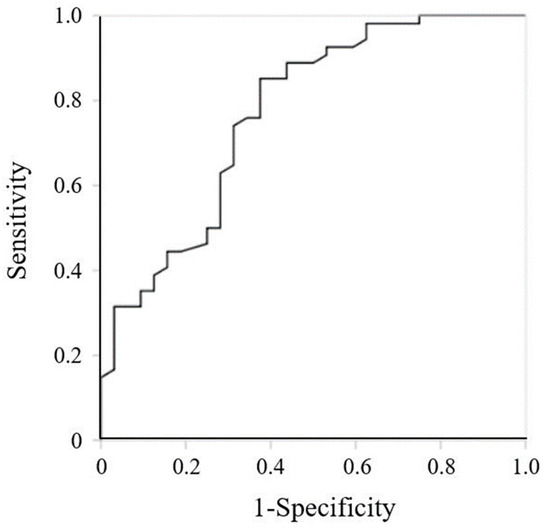
Figure 2.
Receiver operating characteristic curve for Geriatric Nutritional Risk Index at admission.
Table 3 shows the results of the main parameters according to LVEF classification. No differences were observed between groups.

Table 3.
Main parameters according to LVEF classification.
4. Discussion
This is the first study to demonstrate the association between nutritional status and ADL at discharge in older patients with heart failure aged ≥ 75 years who underwent CR. The GNRI specifically was identified as a factor associated with high ADL at discharge; thus, malnutrition at admission is an independent risk factor for low ADL at discharge. These findings provide valuable insights for achieving high ADL scores at discharge and promoting CR in older patients with heart failure.
Factors associated with the pathophysiology and treatment of heart failure, such as cardiopulmonary failure, bed rest, myopathy, and malnutrition, may cause and exacerbate muscle weakness, leading to decreased ADL [14,24]. The quadriceps muscle mass (the major muscle of knee extension) decreased by approximately 12.5% after only 7 days of bed rest [25]. In addition, knee extension strength, which is essential for independent ambulation and walking, positively correlates with ADL [26]. In the present study, a low GNRI score at admission was identified as a factor associated with low ADL (BI < 60) at discharge in older patients with heart failure who underwent cardiac rehabilitation. Thus, muscle weakness resulting from the pathophysiology, treatment background, and malnutrition may have led to a decline in the ADL of the patients.
Malnutrition in patients with heart failure tends to result from gut malabsorption, appetite loss, catabolic and anabolic imbalance, and physiological changes due to aging [7,27]. A study proposed that increased intestinal wall edema translocates bacterial endotoxins from the intestine, ultimately leading to the production of proinflammatory cytokines from monocytes in the bloodstream [28]. Consequently, catabolism is exacerbated by increased levels of these proinflammatory mediators. Reduced intestinal circulation may contribute to local edema and malabsorption in the intestinal wall [8]. The causes of appetite loss include proinflammatory cytokines, gastrointestinal congestion, and gastrointestinal dysfunction [27,29]. Thus, older patients with heart failure may develop malnutrition based on this pathophysiology.
Early initiation of feeding may maintain high ADL in older patients with heart failure [30]. However, the mean age of patients in our study was higher than that in the previously mentioned study. Exercise training and nutritional intervention improve muscle strength and energy intake in older people with various comorbidities (mean age 87 years) [31]. In our study, GNRI at admission was identified as a factor associated with high ADL (BI ≥ 60) at discharge in older patients with heart failure who underwent cardiac rehabilitation; thus, in addition to cardiac rehabilitation, nutritional intervention from the time of admission may be important to achieve high ADL at discharge.
Recently, the prevalence of HFpEF in older patients has gradually increased with age and is higher in women than in men of all ages [32]. The severity of HFpEF increases more rapidly with age than that of HFrEF [33]. Additionally, non-cardiac comorbidities are highly prevalent in patients with HFpEF and can induce a systemic inflammatory state [34,35]. Therefore, the elevated levels of circulating inflammatory biomarkers were more pronounced in patients with HFpEF than in those with HFrEF. The control of proinflammatory pathways has been associated with reduced severity and improved outcomes in patients with HFpEF [33]. Studies suggest that exercise training and nutritional support improve the levels of inflammatory markers in patients with heart failure [36,37]; therefore, they are necessary interventions.
The cutoff value of GNRI < 92 is commonly used to assess the risk of morbidity and mortality in hospitalized older patients [21]. In this study, the cutoff value was 83.4 for high ADL (BI ≥ 60) at discharge, giving a sensitivity of 85.2% and specificity of 62.5%. A relationship between nutritional status and continuous walking distance has been reported [38]. While heart failure patients with a mean GNRI ≥ 92 could walk at least 50 m, those who have a mean GNRI of 87 ± 10 could only walk within 50 m. Ambulatory independence is defined as being able to walk on level ground for more than 45 m [20]. Additionally, Shah et al. reported that a BI < 60 indicates a severe decline in function [39]. Thus, most patients in the present study may not have been capable of walking independently, considering the relationship between the low GNRI score and decline in ADL.
Previous studies reported that the mean age of hospitalized older patients with heart failure was 78.0 ± 12.5 years, and most older patients with heart failure in Japan were older than 75 [40]. The distribution of older patients hospitalized with heart failure peaked in the 80–89-year-old age group for both men and women [3]. Additionally, the median ADL score based on the BI at admission was as low as 25 (0–80), and the proportion of patients capable of walking and using the toilet independently was 37.6% and 29.3%, respectively [3]. In this study, the mean age and mean ADL at admission of the overall cohort were 86.9 ± 5.7 years and 43.2 ± 25.8, respectively, and there were more women (65.1%) than men. Thus, this study reflects the real-world clinical data of older patients with heart failure.
This study has several limitations. First, this was a single-center retrospective observational study with a small sample size. Second, the leg muscle strength and frailty were not assessed. Third, this study was insufficient for assessment at discharge and did not assess changes in GNRI scores. Therefore, further multicenter and prospective studies with a large sample size are required.
In conclusion, the present study showed that malnutrition at admission reduced ADL at discharge (BI < 60) in older patients with heart failure who underwent cardiac rehabilitation. Appropriate nutritional intervention is important for high ADL at discharge in these patients and may lead to an improved prognosis.
Author Contributions
Conceptualization: Y.M. and S.E.; methodology: S.E.; data acquisition: T.M., M.M., T.O., and K.O.; data analysis: T.M., M.M., T.O., T.K. and K.O.; writing—original draft preparation: Y.M. and S.E.; writing—review and editing: S.E. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Funding for Longevity Sciences from the National Center for Geriatrics and Gerontology (22-1); JSPS KAKENHI (19H03984, 19K22821, and 22K19760), Japan Agency for Medical Research and Development (23zf0127001h0003) and JST Strategic International Collaborative Research Program (SICORP) (JPMJSC), Japan.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Tohoku University Graduate School of Medicine (approval number 2022-1-716, 24 November 2022). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all the participants through the opt-out consent process before any study procedure began.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We would like to thank all of the patients who participated in this study and all of the physicians, therapists, and physiotherapists.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shimokawa, H.; Miura, M.; Nochioka, K.; Sakata, Y. Heart failure as a general pandemic in Asia. Eur. J. Heart Fail. 2015, 17, 884–892. [Google Scholar] [CrossRef]
- Takabayashi, K.; Ikuta, A.; Okazaki, Y.; Ogami, M.; Iwatsu, K.; Matsumura, K.; Ikeda, T.; Ichinohe, T.; Morikami, Y.; Yamamoto, T.; et al. Clinical Characteristics and Social Frailty of Super-Elderly Patients With Heart Failure—The Kitakawachi Clinical Background and Outcome of Heart Failure Registry. Circ. J. 2016, 81, 69–76. [Google Scholar] [CrossRef]
- Obata, H.; Izumi, T.; Yamashita, M.; Mitsuma, W.; Suzuki, K.; Noto, S.; Morimoto, T.; Isobe, M. Characteristics of Elderly Patients with Heart Failure and Impact on Activities of Daily Living: A Registry Report from Super-Aged Society. J. Card. Fail. 2021, 27, 1203–1213. [Google Scholar] [CrossRef]
- Saitoh, M.; Takahashi, Y.; Okamura, D.; Akiho, M.; Suzuki, H.; Noguchi, N.; Yamaguchi, Y.; Hori, K.; Adachi, Y.; Takahashi, T. Prognostic impact of hospital-acquired disability in elderly patients with heart failure. ESC Heart Fail. 2021, 8, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Motoki, H.; Nishimura, M.; Kanai, M.; Kimura, K.; Minamisawa, M.; Yamamoto, S.; Saigusa, T.; Ebisawa, S.; Okada, A.; Kuwahara, K. Impact of inpatient cardiac rehabilitation on Barthel Index score and prognosis in patients with acute decompensated heart failure. Int. J. Cardiol. 2019, 293, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, S.; Ono, T.; Ebihara, S. Geriatric nutritional risk index and 100-m walk achievement predict discharge to home in elderly patients with heart failure. Geriatr. Gerontol. Int. 2020, 20, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Makita, S.; Yasu, T.; Akashi, Y.J.; Adachi, H.; Izawa, H.; Ishihara, S.; Iso, Y.; Ohuchi, H.; Omiya, K.; Ohya, Y.; et al. Japanese Circulation Society/the Japanese Association of Cardiac Rehabilitation Joint Working Group. JCS/JACR 2021 Guideline on Rehabilitation in Patients With Cardiovascular Disease. Circ. J. 2022, 87, 155–235. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Ando, H.; Mitsuoka, W.; Egashira, S.; Masaki, H.; Ashihara, T.; Fukuyama, T. Iodine-123 metaiodobenzylguanidine images reflect intense myocardial adrenergic nervous activity in congestive heart failure independent of underlying cause. J. Am. Coll. Cardiol. 1995, 26, 1594–1599. [Google Scholar] [CrossRef]
- Xia, T.; Chai, X.; Shen, J. Pancreatic exocrine insufficiency in patients with chronic heart failure and its possible association with appetite loss. PLoS ONE 2017, 12, e0187804. [Google Scholar] [CrossRef]
- Hirose, S.; Matsue, Y.; Kamiya, K.; Kagiyama, N.; Hiki, M.; Dotare, T.; Sunayama, T.; Konishi, M.; Saito, H.; Saito, K.; et al. Prevalence and prognostic implications of malnutrition as defined by GLIM criteria in elderly patients with heart failure. Clin. Nutr. 2021, 40, 4334–4340. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Pocock, S.J.; Wang, D.; Finn, P.V.; Zornoff, L.A.; Skali, H.; Pfeffer, M.A.; Yusuf, S.; Swedberg, K.; Michelson, E.L.; et al. Body mass index and prognosis in patients with chronic heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007, 116, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, S.; Tsuchihashi-Makaya, M.; Kinugawa, S.; Goto, D.; Yokota, T.; Goto, K.; Yamada, S.; Yokoshiki, H.; Takeshita, A.; Tsutsui, H. Body mass index is an independent predictor of long-term outcomes in patients hospitalized with heart failure in Japan. Circ. J. 2010, 74, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, Y.; Kato, M.; Sugihara, S.; Hirai, M.; Yamada, K.; Yanagihara, K.; Yamamoto, K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ. J. 2013, 77, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Izawa, K.P.; Yaekura, M.; Mimura, Y.; Nagashima, H.; Oka, K. Differences in nutritional status and activities of daily living and mobility in elderly hospitalized patients with heart failure. ESC Heart Fail. 2019, 6, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kojima, I.; Tanaka, S.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kimura, Y.; Ishiyama, D.; Maetani, Y.; Kusumi, H.; Terao, Y.; et al. What is the optimal nutritional assessment tool for predicting decline in the activity of daily living among older patients with heart failure? Heart Vessel. 2022, 37, 1356–1362. [Google Scholar] [CrossRef]
- Tsutsui, H.; Isobe, M.; Ito, H.; Ito, H.; Okumura, K.; Ono, M.; Kitakaze, M.; Kinugawa, K.; Kihara, Y.; Goto, Y.; et al. Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure- Digest Version. Circ. J. 2019, 83, 2084–2184. [Google Scholar] [CrossRef] [PubMed]
- JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef]
- Horii, M.; Matsumoto, T.; Uemura, S.; Sugawara, Y.; Takitsume, A.; Ueda, T.; Nakagawa, H.; Nishida, T.; Soeda, T.; Okayama, S.; et al. Prognostic value of B-type natriuretic peptide and its amino-terminal proBNP fragment for cardiovascular events with stratification by renal function. J. Cardiol. 2013, 61, 410–416. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Sucher, K.; Hollenbeck, C.B. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr. Clin. Pract. 2006, 21, 312–319. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Takemoto, K.; Koyasu, M.; Ishikawa, S.; Murohara, T.; Watarai, M. Prognostic Impact of the Preservation of Activities of Daily Living on Post-Discharge Outcomes in Patients With Acute Heart Failure. Circ. J. 2018, 82, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Hart, N. Skeletal muscle mass and mortality—But what about functional outcome? Crit. Care. 2014, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Kim, H.; Saito, K.; Yoshida, H.; Yoshida, Y.; Hirano, H.; Obuchi, S.; Shimada, H.; Suzuki, T. Association of knee-extension strength with instrumental activities of daily living in community-dwelling older adults. Geriatr. Gerontol. Int. 2014, 14, 674–680. [Google Scholar] [CrossRef]
- Saitoh, M.; Rodrigues, D.S.M.; von Haehling, S. Muscle wasting in heart failure: The role of nutrition. Wien. Klin. Wochenschr. 2016, 128, 455–465. [Google Scholar] [CrossRef]
- Anker, S.D.; von Haehling, S. Inflammatory mediators in chronic heart failure: An overview. Heart 2004, 90, 464–470. [Google Scholar] [CrossRef]
- Von, H.S.; Doehner, W.; Anker, S.D. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc. Res. 2007, 73, 298–309. [Google Scholar]
- Kaneko, H.; Itoh, H.; Morita, K.; Sugimoto, T.; Konishi, M.; Kamiya, K.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Michihata, N.; et al. Early Initiation of Feeding and In-Hospital Outcomes in Patients Hospitalized for Acute Heart Failure. Am. J. Cardiol. 2021, 145, 85–90. [Google Scholar] [CrossRef]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Ceia, F.; Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; de Sousa, A.; Oliveira, A. Prevalence of chronic heart failure in Southwestern Europe: The EPICA study. Eur. J. Heart Fail. 2002, 4, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, T.; Lin, Y.N.; Ibrahim, A. Chronic low-grade inflammation in heart failure with preserved ejection fraction. Aging Cell 2021, 20, e13453. [Google Scholar] [CrossRef] [PubMed]
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.; Deswal, A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Adams, V.; Möbius-Winkler, S.; Linke, A.; Erbs, S.; Yu, J.; Kempf, W.; Schubert, A.; Schuler, G.; Hambrecht, R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 861–868. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, H. Relationship between enteral nutrition and serum levels of inflammatory factors and cardiac function in elderly patients with heart failure. Clin. Interv. Aging 2018, 13, 397–401. [Google Scholar] [CrossRef]
- Yasumura, K.; Abe, H.; Iida, Y.; Kato, T.; Nakamura, M.; Toriyama, C.; Nishida, H.; Idemoto, A.; Shinouchi, K.; Mishima, T.; et al. Prognostic impact of nutritional status and physical capacity in elderly patients with acute decompensated heart failure. ESC Heart Fail. 2020, 7, 1801–1808. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Ide, T.; Kaku, H.; Matsushima, S.; Tohyama, T.; Enzan, N.; Funakoshi, K.; Sumita, Y.; Nakai, M.; Nishimura, K.; Miyamoto, Y.; et al. Clinical Characteristics and Outcomes of Hospitalized Patients With Heart Failure From the Large-Scale Japanese Registry Of Acute Decompensated Heart Failure (JROADHF). Circ. J. 2021, 85, 1438–1450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).