Effect of HPV Vaccination on Virus Disappearance in Cervical Samples of a Cohort of HPV-Positive Polish Patients
Abstract
:1. Introduction
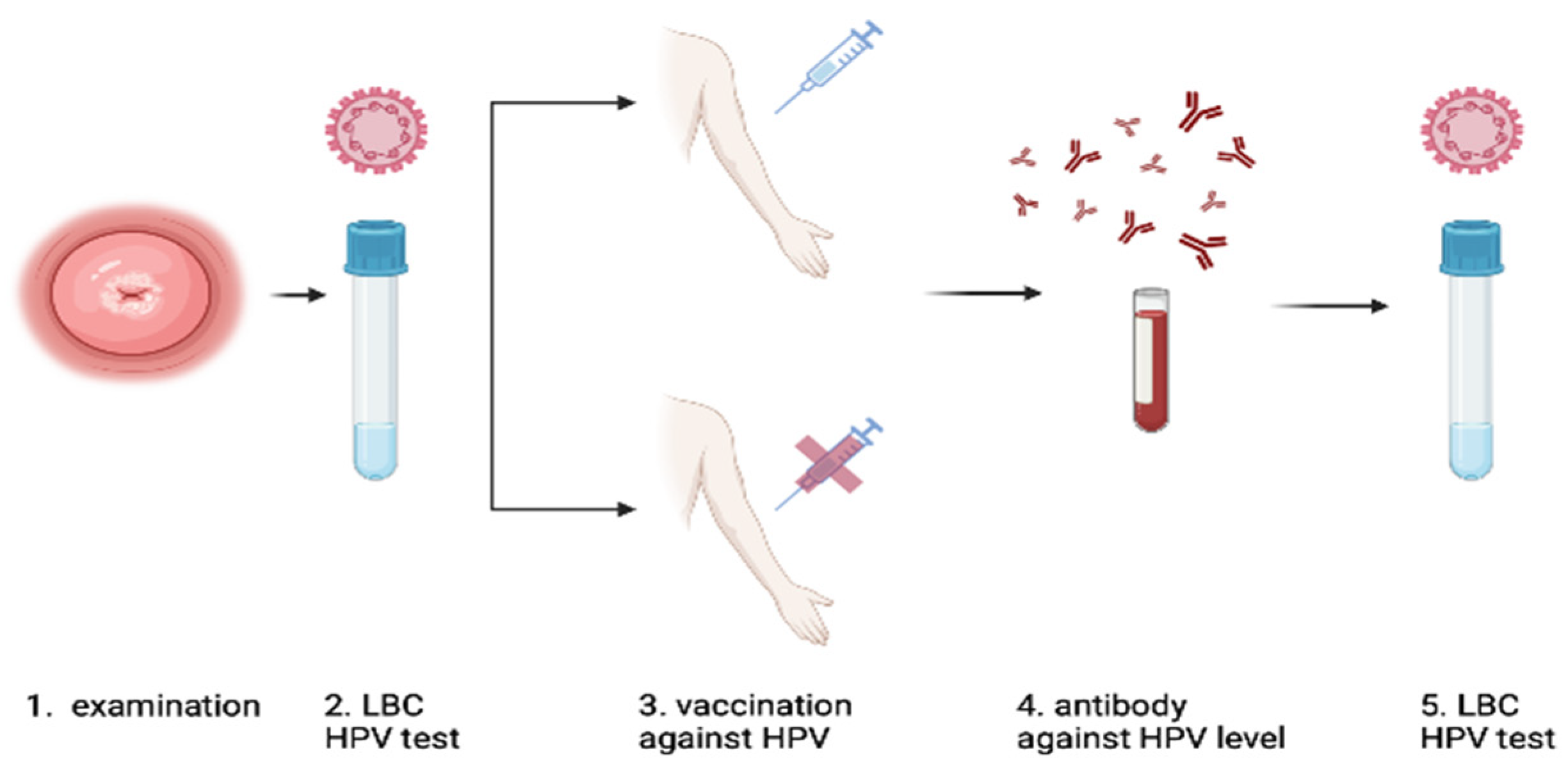
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria for the Study
- Adult females only.
- Non-pregnant or postpartum individuals.
- Subjects not undergoing immunosuppressive drug treatment.
- No prior vaccination with other HPV vaccines.
- Expressing informed written consent to participate.
- Consenting to proposed surgical diagnostics and potential surgical excision treatment if indicated.
- Having received three doses of the nine-valent HPV vaccination following the 0-2-6 month schedule.
- Providing blood samples at least six months after the last vaccination dose.
- Refusal of potential treatment for squamous intraepithelial lesions.
- Failure to complete the full vaccination regimen.
2.3. HPV Genotyping Test and LBC
2.4. Colposcopy and Punch Biopsy
2.5. Specimen Collection and Handling
2.6. HPV Serological Measurements
2.7. Statistical Analysis
3. Results
3.1. Vaccinated Group—HPV Overall Positive/Negative
3.2. Non-Vaccinated Group—HPV Positive/Negative Considering Gardasil®9 HPV Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGC | atypical glandular cells |
| ASC-H | atypical squamous cells of undetermined significance cannot exclude HSIL |
| ASC-US | atypical cells of undetermined significance |
| CC | cervical cancer |
| CI | confidence interval |
| CIN | cervical intraepithelial neoplasia |
| CIN 2+ | CIN 2+ CIN 3 + early-stage cervical cancer. |
| CIN 3+ | CIN 3 + cervical cancer |
| EMA | European Medical Agency |
| HPV | human papillomavirus |
| HSIL | high-grade squamous intraepithelial neoplasia |
| LBC | liquid-based cytology |
| LEEP | Loop Electrosurgical Excision Procedure |
| LLETZ | Large Loop Excision of the Transformation Zone |
| LSIL | low-grade squamous intraepithelial neoplasia |
| M | mean |
| MD | mean difference |
| OD | odds ratio |
| p | p-value |
| SD | standard deviation |
| VLP | virus-like particles |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Didkowska, J. Nowotwory w Polsce w 2019. NOWOTWORY J. Oncol. 2019, 63, 197–216. [Google Scholar] [CrossRef]
- Przybylski, M.; Pruski, D.; Wszołek, K.; de Mezer, M.; Żurawski, J.; Jach, R.; Millert-Kalińska, S. Prevalence of HPV and Assessing Type-Specific HPV Testing in Cervical High-Grade Squamous Intraepithelial Lesions in Poland. Pathogens 2023, 12, 350. [Google Scholar] [CrossRef]
- Woodman, C.; Collins, S.; Young, L. The natural history of cervical HPV infection: Unresolved issues. Nat. Rev. Cancer 2007, 7, 11–22 . [Google Scholar] [CrossRef]
- Xu, Y.F.; Zhang, Y.Q.; Xu, X.M.; Song, G.X. Papillomavirus virus-like particles as vehicles for the delivery of epitopes or genes. Arch. Virol. 2006, 151, 2133–2148. [Google Scholar] [CrossRef]
- Pelkmans, L.; Helenius, A. Insider information: What viruses tell us about endocytosis. Curr. Opin. Cell Biol. 2003, 15, 414–422. [Google Scholar] [CrossRef]
- Marsh, M.; Helenius, A. Virus entry: Open sesame. Cell 2006, 124, 729–740. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- GLOBOCAN. Cervical Cancer Fact Sheet: Cervical Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012; International Agency for Research on Cancer: Lyon, France, 2012; Available online: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp (accessed on 2 August 2023).
- Schiller, J.T.; Lowy, D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012, 10, 681–692. [Google Scholar] [CrossRef]
- Hall, M.T.; Smith, M.A.; Lew, J.-B.; O’Hallahan, J.; Fentiman, G.; Neal, H.; Sage, M.; Canfell, K. The combined impact of implementing HPV immunisation and primary HPV screening in New Zealand: Transitional and long-term benefits, costs and resource utilisation implications. Gynecol. Oncol. 2019, 152, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Hall, M.; Lew, J.-B.; Canfell, K. Potential for HPV vaccination and primary HPV screening to reduce cervical cancer disparities: Example from New Zealand. Vaccine 2018, 36, 6314–6324. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ährlund-Richter, A.; Näsman, A.; Dalianis, T. Human papilloma virus (HPV) prevalence upon HPV vaccination in Swedish youth: A review based on our findings 2008–2018, and perspectives on cancer prevention. Arch. Gynecol. Obstet. 2021, 303, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Brotherton, J.M.; Pillsbury, A.; Jayasinghe, S.; Donovan, B.; Macartney, K.; Marshall, H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: What additional disease burden will a nonavalent vaccine prevent? Eurosurveillance 2018, 23, 1700737. [Google Scholar] [CrossRef]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Hagensee, M.E.; Yaegashi, N.; Galloway, D.A. Self-assembly of Human Papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 1993, 67, 315–322. [Google Scholar] [CrossRef]
- Harper, D.M.; De Mars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitchener, H.C. Cervarix—A Bivalent L1 Virus-Like Particle Vaccine for Prevention of Human Papillomavirus Type 16- and 18-Associated Cervical Cancer. Expert. Opin. Biol. Ther. 2007, 7, 391–396. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Zhang, H.; Shen, J.; Chen, J.; Hong, J.; Xu, Y.; Qian, C. Prophylactic and Therapeutic HPV Vaccines: Current Scenario and Perspectives. Front. Cell Infect. Microbiol. 2022, 12, 909223. [Google Scholar] [CrossRef]
- Rosenberg, T.; Philipsen, B.B.; Mehlum, C.S.; Dyrvig, A.-K.; Wehberg, S.; Chirilǎ, M.; Godballe, C. Therapeutic use of the human papillomavirus vaccine on recurrent respiratory papillomatosis: A systematic review and meta-analysis. J. Infect. Dis. 2019, 219, 1016–1025. [Google Scholar] [CrossRef]
- Choi, H. Can quadrivalent human papillomavirus prophylactic vaccine be an effective alternative for the therapeutic management of genital warts? An exploratory study. Int. Braz. J. Urol. 2019, 45, 361–368. [Google Scholar] [CrossRef]
- Pieralli, A.; Bianchi, C.; Auzzi, N.; Fallani, M.G.; Bussani, C.; Fambrini, M.; Cariti, G.; Scarselli, G.; Petraglia, F.; Ghelardi, A. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch. Gynecol. Obstet. 2018, 298, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Bartels, H.C.; Postle, J.; Rogers, A.C.; Brennan, D. Prophylactic human papillomavirus vaccination to prevent recurrence of cervical intraepithelial neoplasia: A meta-analysis. Int. J. Gynecol. Cancer 2020, 30, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Lichter, K.; Krause, D.; Xu, J.; Tsai, S.H.L.; Hage, C.; Weston, E.; Eke, A.; Levinson, K. Adjuvant Human Papillomavirus Vaccine to Reduce Recurrent Cervical Dysplasia in Unvaccinated Women: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2020, 135, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, M.; Kampers, J.; Becker, J.; Sibbertsen, P.; Hillemanns, P. Prophylactic HPV vaccination after conization: A systematic review and meta-analysis. Vaccine 2020, 38, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Chhieng, D.C.; Crothers, B.; Darragh, T.M.; Davey, D.D.; Eisenhut, C.; Goulart, R.; Huang, E.C.; Tabbara, S.O. Moving forward—The 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: Implications and suggestions for laboratories. J. Am. Soc. Cytopathol. 2020, 9, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Stumbar, S.E.; Stevens, M.; Feld, Z. Cervical cancer and its precursors: A preventative approach to screening, diagnosis, and management prim. Care Clin. Off. Pract. 2019, 46, 117–134. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Arbyn, M.; Redman, C.W.E.; Verdoodt, F.; Kyrgiou, M.; Tzafetas, M.; Ghaem-Maghami, S.; Petry, K.U.; Leeson, S.; Bergeron, C.; Nieminen, P.; et al. Incomplete excision of cervical precancer as a predictor of treatment failure: A systematic review and meta-analysis. Lancet Oncol. 2017, 18, 1665–1679. [Google Scholar] [CrossRef]
- Tjalma, W.A.A.; van Heerden, J.; Van den Wyngaert, T. If prophylactic HPV vaccination is considered in a woman with CIN2+, what is the value and should it be given before or after the surgical treatment? Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 269, 98–101. [Google Scholar] [CrossRef]
- Sand, F.L.; Kjaer, S.K.; Frederiksen, K.; Dehlendorff, C. Risk of cervical intraepithelial neoplasia grade 2 or worse after conization in relation to HPV vaccination status. Int. J. Cancer 2020, 147, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Casajuana-Pérez, A.; Ramírez-Mena, M.; Ruipérez-Pacheco, E.; Gil-Prados, I.; García-Santos, J.; Amo, M.B.-D.; Hernández-Aguado, J.J.; de la Fuente-Valero, J.; Zapardiel, I.; Coronado-Martín, P.J. Effectiveness of Prophylactic Human Papillomavirus Vaccine in the Prevention of Recurrence in Women Conized for HSIL/CIN 2–3: The VENUS Study. Vaccines 2022, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Kocken, M.; Helmerhorst, T.J.; Berkhof, J.; Louwers, J.A.; Nobbenhuis, M.A.; Bais, A.G.; Hogewoning, C.J.; Zaal, A.; Verheijen, R.H.; Snijders, P.J.; et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: A long-term multi-cohort study. Lancet Oncol. 2011, 12, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Pruski, D.; Fraszczak, J.; Iwaniec, K.; Przybylski, M.; Kedzia, W.; Gretkiewicz-Tomczyk, A.; Karowicz-Bilińska, A.; Spaczyński, M. Assessment of frequency of regression and progression of mild cervical neoplasia-LGSIL in women with positive high-risk HPV DNA test result. Ginekol. Pol. 2012, 83, 572–575. [Google Scholar] [PubMed]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Panici, P.B.; et al. Adjuvant hpv vaccination to prevent recurrent cervical dysplasia after surgical treatment: A meta-analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Fogleman, C.; Leaman, L. Prophylactic vaccination against human papillomavirus to prevent cervical cancer and its precursors. Am. Fam. Phys. 2019, 99, 548–549. [Google Scholar] [CrossRef]
- Valasoulis, G.; Pouliakis, A.; Michail, G.; Kottaridi, C.; Spathis, A.; Kyrgiou, M.; Paraskevaidis, E.; Daponte, A. Alterations of HPV-Related Biomarkers after Prophylactic HPV Vaccination. A Prospective Pilot Observational Study in Greek Women. Cancers 2020, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Mariz, F.C.; Gray, P.; Bender, N.; Eriksson, T.; Kann, H.; Apter, D.; Paavonen, J.; Pajunen, E.; Prager, K.M.; Sehr, P.; et al. Sustainability of neutralising antibodies induced by bivalent or quadrivalent HPV vaccines and correlation with efficacy: A combined follow-up analysis of data from two randomised, double-blind, multicentre, phase 3 trials. Lancet Infect. Dis. 2021, 21, 1458–1468. [Google Scholar] [CrossRef]
- Coskuner, E.R.; Ozkan, T.A.; Karakose, A.; Dillioglugil, O.; Cevik, I. Impact of the quadrivalent HPV vaccine on disease recurrence in men exposed to HPV infection: A randomized study. J. Sex. Med. 2014, 11, 2785–2791. [Google Scholar] [CrossRef]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.K.; Haupt, R.M.; et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ 2012, 344, e1401. [Google Scholar] [CrossRef]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Sehnal, B.; Dusek, L.; Cibula, D.; Zima, T.; Halaska, M.; Driak, D.; Slama, J. The relationship between the cervical and anal HPV infection in women with cervical intraepithelial neoplasia. J. Clin. Virol. 2014, 59, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, A.; Marrai, R.; Bogani, G.; Sopracordevole, F.; Bay, P.; Tonetti, A.; Lombardi, S.; Bertacca, G.; Joura, E.A. Surgical treatment of vulvar HSIL: Adjuvant HPV vaccine reduces recurrent disease. Vaccines 2021, 9, 83. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Palefsky, J.M.; Goldstone, S.; Moreira, E.D., Jr.; Penny, M.E.; Aranda, C.; Vardas, E.; Moi, H.; Jessen, H.; Hillman, R.; et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011, 364, 401–411, Erratum in N. Engl. J. Med. 2011, 364, 1481. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef]
- Kim, S.; Kang, W.; Choi, H. Efficacy of the human papillomavirus vaccination in women aged 20–45 years with high-grade cervical intraepithelial neoplasia treated by loop electrosurgical excision procedure. Gynecol. Oncol. 2013, 130, e15–e16. [Google Scholar] [CrossRef]
- Ciccarese, G.; Herzum, A.; Rebora, A.; Drago, F. Prevalence of genital, oral, and anal HPV infection among STI patients in Italy. J. Med. Virol. 2017, 89, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Paavonen, J.; Jaisamrarn, U.; Naud, P.; Salmerón, J.; Chow, S.N.; Apter, D.; Castellsagué, X.; Teixeira, J.C.; Skinner, S.R.; et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial. Int. J. Cancer 2016, 139, 2812–2826. [Google Scholar] [CrossRef] [PubMed]
- Bissett, S.L.; Godi, A.; Jit, M.; Beddows, S. Seropositivity to non-Vaccine Incorporated Genotypes Induced by the Bivalent and Quadrivalent HPV Vaccines: A Systematic Review and Meta-Analysis. Vaccine 2017, 35, 3922–3929. [Google Scholar] [CrossRef]
- Leung, T.F.; Liu, A.P.-Y.; Lim, F.S.; Thollot, F.; Oh, H.M.L.; Lee, B.W.; Rombo, L.; Tan, N.C.; Rouzier, R.; De Simoni, S.; et al. Comparative Immunogenicity and Safety of Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Vaccine and 4vhpv Vaccine Administered According to Two-or Three-Dose Schedules in Girls Aged 9–14 Years: Results to Month 36 From a Randomized Trial. Vaccine 2018, 36, 98–106. [Google Scholar] [CrossRef]
- Hildesheim, A.; Herrero, R.; Wacholder, S.; Rodriguez, A.C.; Solomon, D.; Bratti, M.C.; Schiller, J.T.; Gonzalez, P.; Dubin, G.; Porras, C.; et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: A randomized trial. JAMA 2007, 298, 743–753. [Google Scholar] [CrossRef]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. Available online: www.OpenEpi.com (accessed on 4 December 2023).
| Characteristic | Vaccinated Group | Unvaccinated Group | MD (95% CI) | p |
|---|---|---|---|---|
| N | 51 | 9 | - | - |
| Age, years, M ± SD | 34.71 ± 6.91 | 33.33 ± 7.73 | 1.38 (−3.71; 6.46) | 0.591 1 |
| Any of 32 HPV genotypes (before vac.), positive, n (%) | 51 (100.0) | 9 (100.0) | - | - |
| HPV Gardasil®9 type (6, 11, 16, 18, 31, 33, 45, 52, 58) before vac., n (%) | ||||
| positive, n (%) | 45 (88.2) | 8 (88.9) | - | |
| HPV Gardasil®9 type (6, 11, 16, 18, 31, 33, 45, 52, 58) after vac., n (%) | ||||
| Positive | 6 (11.8) | 6 (66.7) | - | 0.001 |
| Negative | 45 (88.2) | 3 (33.3) | - | |
| Antibody level, Me (Q1; Q3) | 1.78 (1.24; 2.42) | 0.17 (0.08; 0.66) | 1.61 (0.67; 1.96) | <0.001 2 |
| Reactive, n (%) | 51 (100.0) | 3 (33.3) | - | <0.001 |
| LEEP, n (%) | ||||
| HSIL (CIN 2 + CIN 3) + early-stage CC | 37 (72.5) | 5 (55.6) | - | 0.431 |
| No indication | 14 (27.5) | 4 (44.4) | - |
| Case | Group | HPV Result Before | HPV Result after Gardasil®9 or Observation | Antibody Level | Age | LBC Result | Biopsy Result | Leep Conization Result |
|---|---|---|---|---|---|---|---|---|
| 1 | Gardasil | 6 | negative | 2.938 | 29 | LSIL | HSIL | HSIL |
| 2 | Gardasil | 16 | negative | 0.307 | 35 | LSIL | HSIL | HSIL |
| 3 | Control | 31, 53, 56, 58, 59 | 54, 56 | 0.063 | 21 | LSIL | HSIL | HSIL |
| 4 | Gardasil | 16 | negative | 1.681 | 34 | LSIL | HSIL | HSIL |
| 5 | Control | 16, 33, 56, 66, 68 | 66, 68b | 1.761 | 26 | ASC-US | HSIL | No indication |
| 6 | Gardasil | 16, 18 | 51 | 2.655 | 36 | NILM | NILM | No indication |
| 7 | Gardasil | 16 | negative | 1.308 | 46 | LSIL | HSIL | HSIL |
| 8 | Gardasil | 18 | 6, 18, 56 | 3.348 | 45 | ASC-US | LSIL | LSIL |
| 9 | Gardasil | 52 | 44, 51 | 0.667 | 48 | LSIL | NILM | No indication |
| 10 | Control | 66 | negative | 0.061 | 39 | ASC-US | NILM | No indication |
| 11 | Gardasil | 16 | negative | 1.783 | 40 | LSIL | HSIL | No pathology |
| 12 | Gardasil | 16 | negative | 1.766 | 35 | LSIL | HSIL | HSIL |
| 13 | Control | 16 | 16 | 0,663 | 31 | HSIL | HSIL | HSIL |
| 14 | Gardasil | 16, 18 | negative | 1.654 | 40 | ASC-US | LSIL | No pathology |
| 15 | Gardasil | 31, 51 | negative | 0.675 | 30 | LSIL | LSIL | No indication |
| 16 | Gardasil | 16, 68, 83 | negative | 2.781 | 37 | ASC-US | HSIL | HSIL |
| 17 | Gardasil | 52 | 52 | 2.472 | 37 | LSIL | LSIL | No indication |
| 18 | Gardasil | 6, 16, 56, 89 | 16 | 1.454 | 25 | ASC-US | LSIL | No pathology |
| 19 | Control | 6, 39, 67 | 39, 51, 52 | 1.306 | 27 | LSIL | NILM | No indication |
| 20 | Gardasil | 53 | negative | 2.579 | 32 | AGC | NILM | No indication |
| 21 | Gardasil | 16 | negative | 2.027 | 32 | LSIL | HSIL | HSIL |
| 22 | Gardasil | 31, 56 | 31, 56 | 2.353 | 21 | ASC-H | LSIL | No indication |
| 23 | Gardasil | 16, 18, 67 | 56 | 2.952 | 45 | NILM | NILM | No pathology |
| 24 | Gardasil | 16 | negative | 2.221 | 36 | LSIL | HSIL | HSIL |
| 25 | Gardasil | 73 | negative | 0.586 | 29 | ASC-US | NILM | No pathology |
| 26 | Gardasil | 16, 53, 68, 73 | 56 | 2.888 | 27 | ASC-H | HSIL | HSIL |
| 27 | Control | 52, 62 | 52, 62 | 0.171 | 36 | HSIL | HSIL | No pathology |
| 28 | Control | 16 | 18 | 0.077 | 37 | ASC-US | HSIL | CANCER |
| 29 | Gardasil | 6, 11, 45, 51, 52, 53, 58, 61, 66, 73 | 45, 51, 73 | 2.716 | 24 | NILM | HSIL | LSIL |
| 30 | Control | 51, 52, 62, 89 | 51, 52, 62, 89 | 0.217 | 46 | LSIL | LSIL | No indication |
| 31 | Gardasil | 16, 53 | negative | 2.099 | 35 | HSIL | HSIL | HSIL |
| 32 | Gardasil | 16 | negative | 0.497 | 40 | LSIL | LSIL | LSIL |
| 33 | Gardasil | 16 | negative | 0.596 | 45 | LSIL | HSIL | CANCER |
| 34 | Control | 16 | 16 | 0.08 | 37 | ASC-H | HSIL | HSIL |
| 35 | Gardasil | 16, 18 | 16, 52 | 3.043 | 30 | ASC-H | NILM | No indication |
| 36 | Gardasil | 16 | negative | 0.972 | 31 | HSIL | HSIL | HSIL |
| 37 | Gardasil | 31, 39, 62, 70, 81, 82, CP6108 | negative | 0.901 | 45 | LSIL | HSIL | No pathology |
| 38 | Gardasil | 18, 31, 59 | negative | 1.346 | 24 | LSIL | HSIL | HSIL |
| 39 | Gardasil | 16 | negative | 1.176 | 28 | HSIL | LSIL | HSIL |
| 40 | Gardasil | 31 | negative | 1.377 | 41 | LSIL | HSIL | HSIL |
| 41 | Gardasil | 31, 53, 59 | negative | 2.692 | 29 | NILM | HSIL | No pathology |
| 42 | Gardasil | 16 | negative | 1.751 | 44 | HSIL | HSIL | HSIL |
| 43 | Gardasil | 6, 16, 62, CP6108 | negative | 1.081 | 29 | ASC-US | NILM | No indication |
| 44 | Gardasil | 16, 84 | 53 | 01.cze | 40 | AGC | CANCER | CANCER |
| 45 | Gardasil | 56 | negative | 2.378 | 35 | ASC-H | LSIL | HSIL |
| 46 | Gardasil | 16, 31, 43, 68 | negative | lut.62 | 35 | ASC-US | NILM | No indication |
| 47 | Gardasil | 16 | negative | 0.704 | 32 | HSIL | HSIL | HSIL |
| 48 | Gardasil | 18 | negative | 2.129 | 38 | ASC-US | LSIL | HSIL |
| 49 | Gardasil | 73 | negative | 0.733 | 33 | NILM | NILM | No indication |
| 50 | Gardasil | 73, 84 | negative | lut.34 | 36 | ASC-H | LSIL | No pathology |
| 51 | Gardasil | 16 | negative | 1.945 | 34 | ASC-H | HSIL | HSIL |
| 52 | Gardasil | 6, 53, CP6108 | negative | 1.439 | 32 | LSIL | LSIL | No pathology |
| 53 | Gardasil | 52, 54, 56 | negative | 1.934 | 27 | ASC-H | HSIL | HSIL |
| 54 | Gardasil | 31 | 84 | 2.063 | 45 | LSIL | LSIL | No indication |
| 55 | Gardasil | 16 | negative | 0.626 | 33 | ASC-H | HSIL | HSIL |
| 56 | Gardasil | 16 | negative | 1.709 | 49 | LSIL | LSIL | No pathology |
| 57 | Gardasil | 16, 18 | negative | 1.802 | 31 | ASC-H | HSIL | HSIL |
| 58 | Gardasil | 51, 54, 67, 82 | negative | 3.132 | 25 | HSIL | HSIL | HSIL |
| 59 | Gardasil | 6, 16 | negative | 1.503 | 26 | NILM | HSIL | LSIL |
| 60 | Gardasil | 16 | negative | 2.203 | 35 | CANCER | HSIL | HSIL |
| Characteristic | HPV—Positive | HPV—Negative | MD (95% CI) | p |
|---|---|---|---|---|
| Any of the 32 HPV genotypes | n = 12 | n = 39 | ||
| Age, years, M ± SD | 35.25 ± 9.55 | 34.54 ± 6.02 | 0.71 (−5.56; 6.99) | 0.811 2 |
| Antibody level, M ± SD | 2.35 ± 0.78 | 1.64 ± 0.75 | 0.71 (0.21; 1.21) | 0.007 1 |
| LEEP-conization result, n (%) | ||||
| HSIL (CIN 2 + CIN 3) + early-stage CC | 5 (41.7) | 32 (82.1) | - | 0.011 |
| No indication | 7 (58.3) | 7 (17.9) | - | |
| HPV Gardasil®9 type (6, 11, 16, 18, 31, 33, 45, 52, 58) | n = 6 | n = 45 | ||
| Age, years, M ± SD | 30.33 ± 9.11 | 35.29 ± 6.47 | −4.96 (−10.88; 0.97) | 0.099 1 |
| Antibody level, M ± SD | 2.56 ± 0.66 | 1.71 ± 0.78 | 0.85 (0.19; 1.53) | 0.013 1 |
| LEEP-conization result, n (%) | ||||
| HSIL (CIN 2 + CIN 3) + early-stage CC | 3 (50.0) | 34 (75.6) | - | 0.327 |
| No indication | 3 (50.0) | 11 (24.4) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruski, D.; Millert-Kalińska, S.; Łagiedo, M.; Sikora, J.; Jach, R.; Przybylski, M. Effect of HPV Vaccination on Virus Disappearance in Cervical Samples of a Cohort of HPV-Positive Polish Patients. J. Clin. Med. 2023, 12, 7592. https://doi.org/10.3390/jcm12247592
Pruski D, Millert-Kalińska S, Łagiedo M, Sikora J, Jach R, Przybylski M. Effect of HPV Vaccination on Virus Disappearance in Cervical Samples of a Cohort of HPV-Positive Polish Patients. Journal of Clinical Medicine. 2023; 12(24):7592. https://doi.org/10.3390/jcm12247592
Chicago/Turabian StylePruski, Dominik, Sonja Millert-Kalińska, Małgorzata Łagiedo, Jan Sikora, Robert Jach, and Marcin Przybylski. 2023. "Effect of HPV Vaccination on Virus Disappearance in Cervical Samples of a Cohort of HPV-Positive Polish Patients" Journal of Clinical Medicine 12, no. 24: 7592. https://doi.org/10.3390/jcm12247592
APA StylePruski, D., Millert-Kalińska, S., Łagiedo, M., Sikora, J., Jach, R., & Przybylski, M. (2023). Effect of HPV Vaccination on Virus Disappearance in Cervical Samples of a Cohort of HPV-Positive Polish Patients. Journal of Clinical Medicine, 12(24), 7592. https://doi.org/10.3390/jcm12247592









