Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience
Abstract
1. Introduction
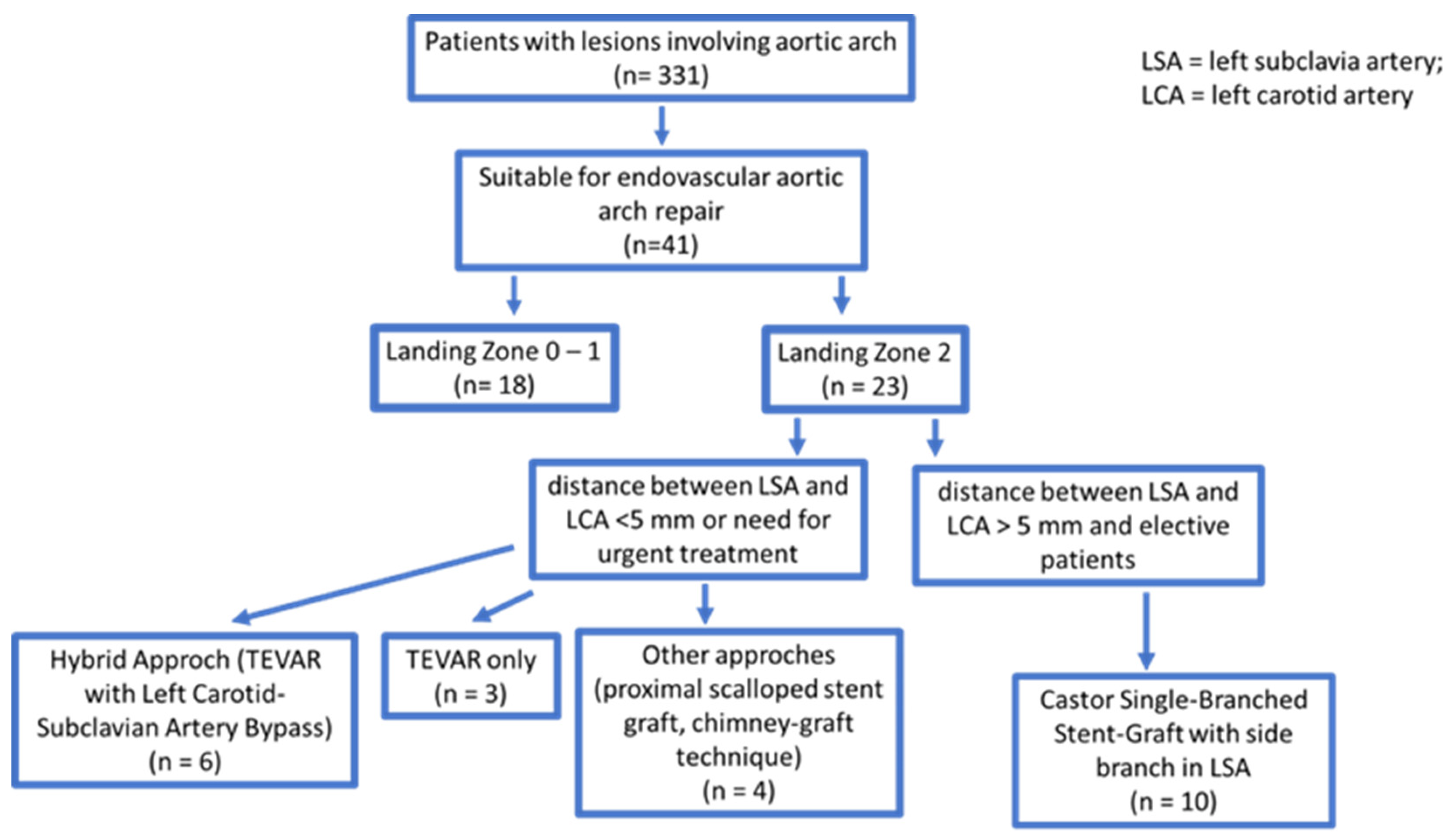
2. Materials and Methods
2.1. Patient Population
- Adults older than 18 years of age, regardless of gender, excluding pregnant women;
- Individuals diagnosed with distal aortic arch diseases, such as aortic dissection (AD), penetrating aortic ulcer (PAU), thoracic aortic aneurysm (TAA) exceeding 5.5 cm in diameter, or cases in which the aorta demonstrated rapid growth (greater than 1 cm per year);
- The distance between the proximal end of the aortic lesion and the ostium of the LSA had to be less than 15 mm;
- Assurance that the aortic disease did not affect the left common carotid artery (LCCA), with a minimum distance from the proximal aortic lesion to the LCCA ostium of more than 15 mm;
- The distance between the origin of the left vertebral artery (LVA) and the foramen of the LSA had to be greater than 25 mm;
- A minimum separation of 5 mm between the ostium of the LCCA and the ostium of the LSA was necessary; and
- A maximum aortic arch diameter of 40 mm.
- Patients with connective tissue diseases, such as Marfan syndrome and Ehlers–Danlos syndrome;
- Individuals with severe organ diseases which would hinder surgery or anesthesia;
- Cases of Stanford type A aortic dissection;
- Patients with an aberrant right subclavian artery;
- Those in whom the diameter of the external iliac artery or common femoral artery (FA) was less than 7 mm; and
- Individuals with allergies to nitinol or iodine contrast agents.
2.2. Stent Graft
2.3. Stent Graft Deployment
2.4. Definition of the Clinical Parameters
2.5. Clinical Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Perioperative Results
4. Follow-Up
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suenaga, E.; Sato, M.; Fumoto, H. Ascending aortic replacement for acute type A aortic dissection in octogenarians. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 138–143. [Google Scholar] [CrossRef]
- Rylski, B.; Hoffmann, I.; Beyersdorf, F.; Suedkamp, M.; Siepe, M.; Nitsch, B.; Blettner, M.; Borger, M.A.; Weigang, E.; Multicenter Prospective Observational Study. Acute aortic dissection type A: Age-related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann. Surg. 2014, 259, 598–604. [Google Scholar] [CrossRef]
- Walsh, S.R.; Tang, T.Y.; Sadat, U.; Naik, J.; Gaunt, M.E.; Boyle, J.R.; Hayes, P.D.; Varty, K. Endovascular stenting versus open surgery for thoracic aortic disease: Systematic review and meta-analysis of perioperative results. J. Vasc. Surg. 2008, 47, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Abraha, I.; Romagnoli, C.; Montedori, A.; Cirocchi, R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database Syst. Rev. 2016, 2016, CD006796. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liu, L.; Chang, G.; Chen, X.; Feng, H.; Zhang, X.; Fu, W.; Dong, Z.; Jing, Z. Mid-term outcomes from a multicenter study: Is TEVAR safe for ascending aortic dissection? Int. J. Cardiol. 2018, 265, 218–222. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Sakalihasan, N.; Clough, R.E.; Aboukoura, M.; Mancuso, E.; Yeh, J.S.; Defraigne, J.O.; Cheshire, N.; Rosendahl, U.P.; Quarto, C.; et al. Thoracic endovascular aortic repair (TEVAR) in proximal (type A) aortic dissection: Ready for a broader application? J. Thorac. Cardiovasc. Surg. 2017, 153, S3–S11. [Google Scholar] [CrossRef]
- Katada, Y.; Kondo, S.; Tsuboi, E.; Rokkaku, K.; Irie, Y.; Yokoyama, H. Endovascular Total Arch Repair Using In Situ Fenestration for Arch Aneurysm and Chronic Type A Dissection. Ann. Thorac. Surg. 2016, 101, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Schmidli, J.; Adler, S.; van den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: An expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur. J. Cardiothorac. Surg. 2019, 55, 133–162. [Google Scholar]
- Wada, T.; Yamamoto, H.; Takagi, D.; Kadohama, T.; Yamaura, G.; Kiryu, K.; Igarashi, I. Aortic Remodeling, Reintervention, and Survival after Zone 0 Arch Repair with Frozen Elephant Trunks for Acute Type A Aortic Dissection: Midterm Results. JTCVS Tech. 2022, 14, 29–38. [Google Scholar] [CrossRef]
- Makaroun, M.S.; Dillavou, E.D.; Kee, S.T.; Sicard, G.; Chaikof, E.; Bavaria, J.; Williams, D.; Cambria, R.P.; Mitchell, R.S. Endovascular treatment of thoracic aortic aneurysms: Results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J. Vasc. Surg. 2005, 41, 1–9. [Google Scholar] [CrossRef]
- Voskresensky, I.; Scali, S.T.; Feezor, R.J.; Fatima, J.; Giles, K.A.; Tricarico, R.; Berceli, S.A.; Beck, A.W. Outcomes of thoracic endovascular aortic repair using aortic arch chimney stents in high-risk patients. J. Vasc. Surg. 2017, 66, 9–20.e3. [Google Scholar] [CrossRef]
- De Rango, P.; Ferrer, C.; Coscarella, C.; Musumeci, F.; Verzini, F.; Pogany, G.; Montalto, A.; Cao, P. Contemporary comparison of aortic arch repair by endovascular and open surgical reconstructions. J. Vasc. Surg. 2015, 61, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Waterford, S.D.; Chou, D.; Bombien, R.; Uzun, I.; Shah, A.; Khoynezhad, A. Left Subclavian Arterial Coverage and Stroke During Thoracic Aortic Endografting: A Systematic Review. Ann. Thorac. Surg. 2016, 101, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Zamor, K.C.; Eskandari, M.K.; Rodriguez, H.E.; Ho, K.J.; Morasch, M.D.; Hoel, A.W. Outcomes of Thoracic Endovascular Aortic Repair and Subclavian Revascularization Techniques. J. Am. Coll. Surg. 2015, 221, 93–100. [Google Scholar] [CrossRef]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G. Editor’s Choice—Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- D’Oria, M.; Mani, K.; DeMartino, R.; Czerny, M.; Donas, K.P.; Wanhainen, A.; Lepidi, S. Narrative Review on Endovascular Techniques for Left Subclavian Artery Revascularization during Thoracic Endovascular Aortic Repair and Risk Factors for Postoperative Stroke. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Criado, F.J. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): Retrograde catheterization and stenting. J. Endovasc. Ther. 2007, 14, 54–58. [Google Scholar] [CrossRef]
- Lindblad, B.; Bin Jabr, A.; Holst, J.; Malina, M. Chimney Grafts in Aortic Stent Grafting: Hazardous or Useful Technique? Systematic Review of Current Data. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 722–731. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, L.; Zheng, J.; Huang, X.; Guo, X.; Li, T.; Huang, L. The chimney technique for preserving the left subclavian artery in thoracic endovascular aortic repair. Eur. J. Cardiothorac. Surg. 2015, 47, 623–629. [Google Scholar] [CrossRef]
- Mangialardi, N.; Ronchey, S.; Malaj, A.; Fazzini, S.; Alberti, V.; Ardita, V.; Orrico, M.; Lachat, M. Value and limitations of chimney grafts to treat arch lesions. J. Cardiovasc. Surg. 2015, 56, 503–511. [Google Scholar]
- Moulakakis, K.G.; Mylonas, S.N.; Avgerinos, E.; Papapetrou, A.; Kakisis, J.D.; Brountzos, E.N.; Liapis, C.D. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J. Vasc. Surg. 2012, 55, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.H.; Dimaio, J.M.; Dean, W.; Jessen, M.E.; Arko, F.R. Endovascular repair of acute traumatic thoracic aortic transection with laser-assisted in-situ fenestration of a stent-graft covering the left subclavian artery. J. Endovasc. Ther. 2009, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.G.; Murphy, M.; Hartley, D.; Lawrence-Brown, M.M.D.; Harris, P.L. In situ stent-graft fenestration to preserve the left subclavian artery. J. Endovasc. Ther. 2004, 11, 170–174. [Google Scholar] [CrossRef]
- Crawford, S.A.; Sanford, R.M.; Forbes, T.L.; Amon, C.H.; Doyle, M.G. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J. Vasc. Surg. 2016, 64, 244–250. [Google Scholar] [CrossRef]
- Inoue, K.; Hosokawa, H.; Iwase, T.; Sato, M.; Yoshida, Y.; Ueno, K.; Tsubokawa, A.; Tanaka, T.; Tamaki, S.; Suzuki, T. Aortic arch reconstruction by transluminally placed endovascular branched stent graft. Circulation 1999, 100, II316–II321. [Google Scholar] [CrossRef] [PubMed]


| Baseline Clinical Characteristics | |
|---|---|
| Age (median IQR) | 76 61–79 |
| BMI (median IQR) | 29.7 20.6–34.5 |
| Male (n; %) | 9; 90% |
| Smoker (n; %) | 4; 40% |
| Arterial hypertension (n; %) | 9; 90% |
| Diabetes mellitus (n; %) | 1; 10% |
| Dislipidemia (n; %) | 8; 80% |
| Hb (g/dL) pre (median IQR) | 13.4 11.9–15.6 |
| Previous Myocardial Infarction (n; %) | 2; 20% |
| Previous stroke (n; %) | 2; 20% |
| Carotid artery disease (n; %) | 2; 20% |
| Lower limb arterial disease (n; %) | 1; 10% |
| Ejection fraction (%) (median IQR) | 58 41–65 |
| Indications | |
| Type B Aortic dissection (n; %) | 4; 40% |
| Intramural haematoma (n; %) | 1; 10% |
| Penetrating aortic ulceration (n; %) | 3; 30% |
| Aortic aneurysm (n; %) | 4; 40% |
| LSA pathology (re-entry, aneurysm) (n; %) | 0; 0% |
| Preoperative CTA measurements | ||
|---|---|---|
| Proximal landing mm (Median IQR) | 31.4 27–35 | |
| Distal landing mm (Median IQR) | 32.1 26.0–40 | |
| Proximal oversizing mm (Median IQR) | 5 3–7 | |
| Distal LSA mm (Median IQR) | 10.7 9.5–12.9 | |
| LSA oversizing mm (Median IQR) | 1.3 0–2.5 | |
| Arch type (n; %) | I | 1; 10% |
| II | 3; 30% | |
| III | 6; 60% | |
| Procedural data | ||
| Fluoroscopy time (Median IQR) | 26′ 1″ 17′ 9″–34′ 1″ | |
| Contrast medium (mL) (Median IQR) | 286.4 120–580 | |
| General Anesthesia (n; %) | 7; 70% | |
| Surgical access (n; %) | 1; 10% | |
| Hospitalization (Median IQR) | 4.3 2–10 | |
| Percutaneous access (n; %) | 9; 90% | |
| Technical succes (n; %) | 10; 100% | |
| Short and Mid-term complications | ||
| Bird Beak (n; %) | 0; 0% | |
| Endoleak Tvpe 1 (n; %) | 0; 0% | |
| Endoleak Type 2 (n; %) | 0; 0% | |
| Endoleak Type 3 (n; %) | 0; 0% | |
| Intraoperative complications (n; %) | 0; 0% | |
| Intraoperative cardiac arrest (n; %) | 0; 0% | |
| Intraoperative death (n; %) | 0; 0% | |
| Preoperative CTA measurements | ||
| Proximal landing mm (Median IQR) | 31.4 27–35 | |
| Distal landing mm (Median IQR) | 32.1 26.0–40 | |
| Proximal oversizing mm (Median IQR) | 5 3–7 | |
| Distal LSA mm (Median IQR) | 10.7 9.5–12.9 | |
| LSA oversizing mm (Median IQR) | 1.3 0–2.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizza, A.; Trimarchi, G.; Di Sibio, S.; Bastiani, L.; Murzi, M.; Palmieri, C.; Foffa, I.; Berti, S. Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience. J. Clin. Med. 2023, 12, 7593. https://doi.org/10.3390/jcm12247593
Rizza A, Trimarchi G, Di Sibio S, Bastiani L, Murzi M, Palmieri C, Foffa I, Berti S. Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience. Journal of Clinical Medicine. 2023; 12(24):7593. https://doi.org/10.3390/jcm12247593
Chicago/Turabian StyleRizza, Antonio, Giancarlo Trimarchi, Silvia Di Sibio, Luca Bastiani, Michele Murzi, Cataldo Palmieri, Ilenia Foffa, and Sergio Berti. 2023. "Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience" Journal of Clinical Medicine 12, no. 24: 7593. https://doi.org/10.3390/jcm12247593
APA StyleRizza, A., Trimarchi, G., Di Sibio, S., Bastiani, L., Murzi, M., Palmieri, C., Foffa, I., & Berti, S. (2023). Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience. Journal of Clinical Medicine, 12(24), 7593. https://doi.org/10.3390/jcm12247593








