The Novel Ceramide- and Phosphatidylcholine-Based Risk Score for the Prediction of New-Onset of Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of Prevalent and New-Onset Hypertension
2.3. Blood Pressure Measurements
2.4. Laboratory Measurements
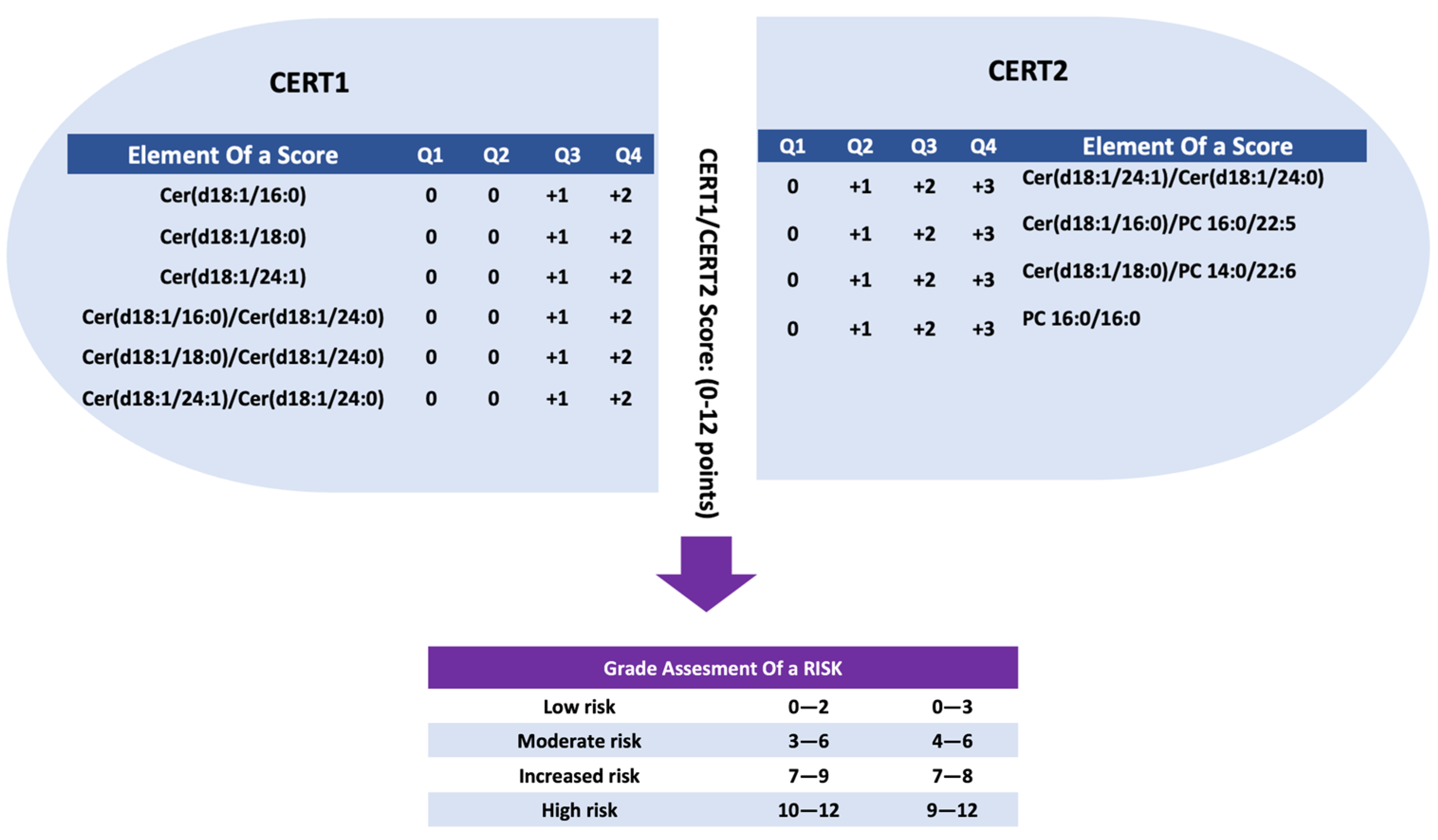
2.5. Coronary Event Test Score 1 (CERT1)
2.6. Coronary Event Test Score 2 (CERT2)
2.7. Statistical Analyses
2.8. Statistical Software and Packages
3. Results
3.1. Baseline Characteristics
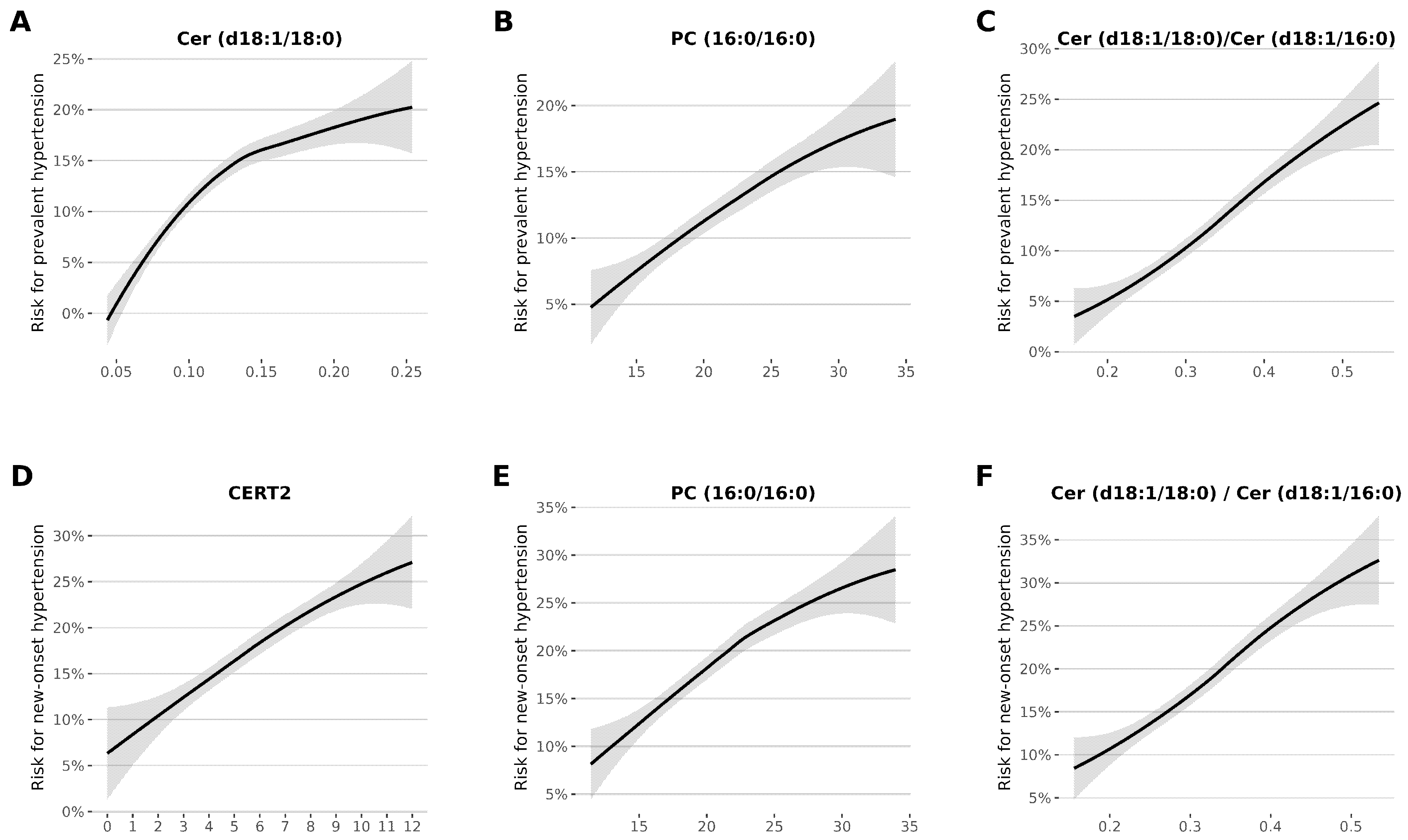
3.2. Association of CERT1 and CERT2 Scores with Prevalent and New-Onset Hypertension
3.3. Association between Lipid Species and Prevalent and New-Onset Hypertension
3.4. Factors Linked to Prevalent and New-Onset Hypertension
3.5. A Closer Look at the Effect of Sex on Baseline Characteristics and Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, W.; Li, F.; Tan, X.; Wang, H.; Jiang, W.; Wang, X.; Li, S.; Zhang, Y.; Han, Q.; Wang, Y.; et al. Plasma Ceramides and Cardiovascular Events in Hypertensive Patients at High Cardiovascular Risk. Am. J. Hypertens. 2021, 34, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.S.; Stalikas, N.; Moysidis, D.V.; Otountzidis, N.; Kartas, A.; Karagiannidis, E.; Giannakoulas, G.; Sianos, G. CERT2 ceramide-and phospholipid-based risk score and major adverse cardiovascular events: A systematic review and meta-analysis. J. Clin. Lipidol. 2022, 16, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Morrow, D.A.; Braunwald, E.; Goodrich, E.L.; Hilvo, M.; Kauhanen, D.; Sabatine, M.S.; Laaksonen, R.; O’Donoghue, M.L. Plasma ceramide and phospholipid-based risk score and the risk of cardiovascular death in patients after acute coronary syndrome. Eur. J. Prev. Cardiol. 2022, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating ceramides predict cardiovascular outcomes in the population based FINRISK 2002 Cohort. Arterioscler Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Borodulin, K.; Vartiainen, E.; Peltonen, M.; Jousilahti, P.; Juolevi, A.; Laatikainen, T.; Männistö, S.; Salomaa, V.; Sundvall, J.; Puska, P. Forty-year trends in cardiovascular risk factors in Finland. Eur. J. Public Health 2015, 25, 539–546. [Google Scholar] [CrossRef]
- Luepker, R.V. WHO MONICA project: What have we learned and where to go from here? Public Health Rev. 2011, 33, 373–396. [Google Scholar] [CrossRef]
- WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): A major international collaboration. J. Clin. Epidemiol. 1988, 41, 105–114. [Google Scholar] [CrossRef]
- Hilvo, M.; Jylhä, A.; Lääperi, M.; Jousilahti, P.; Laaksonen, R. Absolute and relative risk prediction in cardiovascular primary prevention with a modified SCORE chart incorporating ceramide-phospholipid risk score and diabetes mellitus. Eur. Heart J. Open 2021, 1, oeab010. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front. Endocrinol. 2020, 11, 570628. [Google Scholar] [CrossRef] [PubMed]
- Mundra, P.A.; Barlow, C.K.; Nestel, P.J.; Barnes, E.H.; Kirby, A.; Thompson, P.; Sullivan, D.R.; Alshehry, Z.H.; Mellett, N.A.; Huynh, K.; et al. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018, 3, 121326. [Google Scholar] [CrossRef]
- Bai, X.; Dee, R.; Mangum, K.D.; Mack, C.P.; Taylor, J.M. RhoA signaling and blood pressure: The consequence of failing to “Tone it Down”. World J. Hypertens. 2016, 6, 18–35. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, Q.; Cheng, Y.; Wang, J.; Li, Y. Circulating ceramide as a potential biomarker for isolated nocturnal hypertension. J. Hypertens. 2021, 39, e106. [Google Scholar] [CrossRef]
- Liu, J.; de Vries, P.S.; Del Greco, M.F.; Johansson, Å.; Schraut, K.E.; Hayward, C.; van Dijk, K.W.; Franco, O.H.; Hicks, A.A.; Vitart, V.; et al. A multi-omics study of circulating phospholipid markers of blood pressure. Sci. Rep. 2022, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.; Mehak, F.; Khan, Z.M.; Ahmad, W.; Khan, M.R.; Zia, S.; Rahaman, A.; Aadil, R.M. Interplay between ceramides and phytonutrients: New insights in metabolic syndrome. Trends Food Sci. Technol. 2021, 111, 483–494. [Google Scholar] [CrossRef]
- Tu, C.; Xie, L.; Wang, Z.; Zhang, L.; Wu, H.; Ni, W.; Li, C.; Li, L.; Zeng, Y. Association between ceramides and coronary artery stenosis in patients with coronary artery disease. Lipids Health Dis. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Birk, M.; Baum, E.; Zadeh, J.K.; Manicam, C.; Pfeiffer, N.; Patzak, A.; Helmstädter, J.; Steven, S.; Kuntic, M.; Daiber, A.; et al. Angiotensin II induces oxidative stress and endothelial dysfunction in mouse ophthalmic arteries via involvement of AT1 receptors and NOX2. Antioxidants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Cogolludo, A.; Villamor, E.; Perez-Vizcaino, F.; Moreno, L. Ceramide and Regulation of Vascular Tone. Int. J. Mol. Sci. 2019, 20, 411. [Google Scholar] [CrossRef]
- Fenger, M.; Linneberg, A.; Jeppesen, J. Network-based analysis of the sphingolipid metabolism in hypertension. Front. Genet. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Fenger, M.; Linneberg, A.; Jørgensen, T.; Madsbad, S.; Søbye, K.; Eugen-Olsen, J.; Jeppesen, J. Genetics of the ceramide/sphingosine-1- 499 phosphate rheostat in blood pressure regulation and hypertension. BMC Genet. 2011, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells 502 by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Hla, T.; Dannenberg, A.J. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012, 16, 420–434. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Domingues, A.; Jolibois, J.; Marquet de Rougé, P.; Nivet-Antoine, V. The Emerging Role of TXNIP in Ischemic and Cardiovascular Diseases, A Novel Marker and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 4. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, Y.; Wu, J.; Ji, Y. Mechanisms of pulmonary microvascular endothelial cells barrier dysfunction induced by LPS: The roles of ceramides and the Txnip/NLRP3 inflammasome. Microvasc. Res. 2023, 147, 104491. [Google Scholar] [CrossRef]
- Alvim, R.O.; Santos, P.C.J.L.; Ferreira, N.E.; Mill, J.G.; Krieger, J.E.; Pereira, A.C. Thioredoxin interacting protein (TXNIP) rs7212 polymorphism is associated with arterial stiffness in the Brazilian general population. J. Hum. Hypertens. 2012, 26, 340–342. [Google Scholar] [CrossRef]
- Wang, X.B.; Han, Y.D.; Zhang, S.; Cui, N.H.; Liu, Z.J.; Huang, Z.L.; Li, C.; Zheng, F. Associations of polymorphisms in TXNIP and gene-environment interactions with the risk of coronary artery disease in a Chinese Han population. J. Cell. Mol. Med. 2016, 20, 2362–2373. [Google Scholar] [CrossRef]
- Field, B.C.; Gordillo, R.; Scherer, P.E. The Role of Ceramides in Diabetes and Cardiovascular Disease Regulation of Ceramides by Adipokines. Front. Endocrinol. 2020, 11, 569250. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Salonurmi, T.; Havulinna, A.S.; Kauhanen, D.; Pedersen, E.R.; Tell, G.S.; Meyer, K.; Teeriniemi, A.M.; Laatikainen, T.; Jousilahti, P.; et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia 2018, 61, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Serine metabolism in health and disease and as a conditionally essential amino acid. Nutrients 2022, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- McGurk, K.A.; Keavney, B.D.; Nicolaou, A. Circulating ceramides as biomarkers of cardiovascular disease: Evidence from phenotypic and genomic studies. Atherosclerosis 2021, 327, 18–30. [Google Scholar] [CrossRef]
| Study on Prevalent Hypertension | |||
| Variable | People without Hypertension at Baseline (n = 6847) | People with Hypertension at Baseline (n = 875) | p-Value |
| Age (years) | 46.7 (12.9) | 59.2 (8.8) | <0.001 |
| Men n (%) | 3157 (46.1%) | 462 (52.8%) | <0.001 |
| BMI (Kg/m2) | 26.5 (4.5) | 30.1 (4.9) | <0.001 |
| Waist circumference (cm) | 88.2 (13.1) | 99.0 (13.1) | <0.001 |
| Serum triglycerides (mmol/L) | 1.4 (0.9) | 1.8 (1.2) | <0.001 |
| Serum total cholesterol (mmol/L) | 5.6 (1.1) | 5.7 (1.0) | 0.002 |
| Serum HDL (mmol/L) | 1.5 (0.4) | 1.4 (0.4) | <0.001 |
| Serum CRP (mg/dL) | 2.4 (4.9) | 3.8 (7.9) | <0.001 |
| Current smoking | 1840 (26.9%) | 132 (15.1%) | <0.001 |
| Systolic BP (mmHg) | 133 (19.5) | 162 (19.7) | <0.001 |
| Diastolic BP (mmHg) | 78 (11.1) | 84 (10.4) | <0.001 |
| Diabetes mellitus | 290 (4.2%) | 159 (18.2%) | <0.001 |
| History of lipid-lowering drug treatment | 355 (5.2%) | 220 (25.1%) | <0.001 |
| History of BP-lowering drug treatment | 330 (4.8%) | 793 (90.6%) | <0.001 |
| CERT1 | 4.4 (3.2) | 5.5 (3.2) | <0.001 |
| CERT2 | 5.9 (2.4) | 6.7 (2.3) | <0.001 |
| Death during the follow-up | 631 (9.2%) | 217 (24.8%) | <0.001 |
| Study on New-onset Hypertension | |||
| Variable | People without Hypertension at the Baseline (n = 5621) | People with Hyper-tension at the Baseline (n = 1225) | p-Value |
| Age (years) | 45.0 (12.7) | 54.3 (10.9) | <0.001 |
| Men n (%) | 2550 (45.4%) | 606 (49.5%) | 0.010 |
| BMI (Kg/m2) | 26.0 (4.2) | 28.8 (4.9) | <0.001 |
| Waist circumference (cm) | 86.8 (12.5) | 94.8 (14.0) | <0.001 |
| Serum triglycerides (mmol/L) | 1.3 (0.9) | 1.7 (1.1) | <0.001 |
| Serum total cholesterol (mmol/L) | 5.5 (1.1) | 5.8 (1.1) | <0.001 |
| Serum HDL (mmol/L) | 1.5 (0.4) | 1.5 (0.4) | <0.001 |
| Serum CRP (mg/dL) | 2.2 (4.7) | 3.3 (5.6) | <0.001 |
| Current smoking | 1545 (27.5%) | 294 (24.0%) | 0.014 |
| Systolic BP (mean) | 129 (16.9) | 150(21.1) | <0.001 |
| Diastolic BP (mean) | 77 (10.4) | 86 (11.3) | <0.001 |
| Diabetes mellitus | 177 (3.1%) | 112 (9.1%) | <0.001 |
| Lipid-lowering drug treatment | 225 (4.0%) | 130 (10.6%) | <0.001 |
| BP-lowering drug treatment | 121 (2.2%) | 209 (17.1%) | <0.001 |
| CERT1 | 4.2 (3.2) | 5.3 (3.3) | <0.001 |
| CERT2 | 5.8 (2.4) | 6.5 (2.3) | <0.001 |
| Death during the follow-up | 451 (8.0%) | 179 (14.6%) | <0.001 |
| Variables | Study on Prevalent Hypertension | Study on New-Onset Hypertension | |||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | HR (95% CI) | p Value | ||
| CERT1 | (Unadjusted) | 1.42 (1.32–1.52) | <0.001 | 1.16 (1.10–1.23) | <0.001 |
| CERT2 | (Unadjusted) | 1.36 (1.26–1.46) | <0.001 | 1.16 (1.09–1.23) | <0.001 |
| CERT1 | (Adjusted for M1) | 1.12 (1.04–1.21) | 0.002 | - | - |
| CERT2 | (Adjusted for M1) | 1.06 (0.98–1.15) | 0.140 | - | - |
| CERT1 | (Adjusted for M2) | 1.11(1.02–1.20) | 0.013 | 1.12 (1.06–1.19) | <0.001 |
| CERT2 | (Adjusted for M2) | 1.05(0.96–1.13) | 0.280 | 1.13 (1.06–1.20) | <0.001 |
| CERT1 | (Adjusted for M3) | 1.03(0.94–1.12) | 0.550 | 1.05 (0.98–1.11) | 0.160 |
| CERT2 | (Adjusted for M3) | 1.04(0.96–1.14) | 0.350 | 1.09 (1.02–1.16) | 0.009 |
| Study on Prevalent Hypertension | ||||||||
| Unadjusted | Adjustment for Model 1 | Adjustment for Model 2 | Adjustment for Model 3 | |||||
| Components | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Cer (d18:1/16:0) | 1.22 (1.14–1.31) | <0.001 | 0.98 (0.90–1.06) | 0.58 | 0.98 (0.89–1.09) | 0.75 | 1.00 (0.90–1.11) | 0.95 |
| Cer (d18:1/18:0) | 1.61 (1.50–1.73) | <0.001 | 1.30 (1.20–1.41) | <0.001 | 1.37 (1.25–1.51) | <0.001 | 1.14 (1.03–1.26) | 0.012 |
| Cer (d18:1/24:0) | 1.22 (1.13–1.31) | <0.001 | 1.03 (0.95–1.12) | 0.43 | 1.09 (0.98–1.21) | 0.10 | 1.06 (0.95–1.18) | 0.32 |
| Cer (d18:1/24:1) | 1.49 (1.39–1.60) | <0.001 | 1.16 (1.07–1.26) | 0.0027 | 1.23 (1.11–1.37) | 0.0091 | 1.18 (1.06–1.31) | 0.0030 |
| PC (14:0/22:6) | 1.33 (1.24–1.44) | <0.001 | 1.09 (1.00–1.18) | 0.039 | 1.16 (1.06–1.27) | 0.0019 | 1.1 (1.00–1.24) | 0.049 |
| PC (16:0/16:0) | 1.36 (1.27–1.46) | <0.001 | 1.04 (0.97–1.13) | 0.29 | 1.21 (1.09–1.33) | 0.0030 | 1.2 (1.08–1.34) | 0.0064 |
| PC (16:0/22:5) | 1.14 (1.06–1.22) | 0.0033 | 1.05 (0.97–1.13) | 0.22 | 1.15 (1.05–1.27) | 0.002 | 1.05 (0.95–1.16) | 0.34 |
| Cer (d18:1/16:0)/Cer (d18:1/24:0) ratio | 0.98 (0.91–1.05) | 0.51 | 0.94 (0.87–1.01) | 0.10 | 0.93 (0.86–1.00) | 0.057 | 0.96 (0.89–1.04) | 0.36 |
| Cer (d18:1/18:0)/Cer (d18:1/24:0) ratio | 1.49 (1.38–1.60) | <0.001 | 1.32 (1.22–1.43) | <0.001 | 1.26 (1.16–1.36) | <0.001 | 1.09 (1.00–1.19) | 0.060 |
| Cer (d18:1/24:1)/Cer (d18:1/24:0) ratio | 1.35 (1.25–1.45) | <0.001 | 1.16 (1.07–1.25) | 0.0001 | 1.10 (1.02–1.19) | 0.012 | 1.09 (1.01–1.19) | 0.031 |
| Cer (d18:1/16:0)/PC (16:0/22:5) ratio | 1.07 (1.00–1.15) | 0.045 | 0.94 (0.87–1.01) | 0.094 | 0.9 (0.83–0.97) | 0.0071 | 0.97 (0.89–1.05) | 0.47 |
| Cer (d18:1/18:0)/PC (14:0/22:6) ratio | 1.08 (1.00–1.15) | 0.039 | 1.1 (1.02–1.19) | 0.012 | 1.06 (0.98–1.15) | 0.17 | 1.00 (0.91–1.09) | 0.93 |
| Cer (d18:1/18:0)/Cer (d18:1/16:0) ratio | 1.63 (1.52–1.76) | <0.001 | 1.46 (1.35–1.58) | <0.001 | 1.40 (1.29–1.52) | <0.001 | 1.14 (1.05–1.25) | 0.0027 |
| Study on New-onset Hypertension | ||||||||
| Unadjusted | Adjustment for Model 1 | Adjustment for Model 2 | Adjustment for Model 3 | |||||
| Components | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Cer (d18:1/16:0) | 1.11 (1.05–1.18) | 0.0005 | ― | ― | 1.08 (1.00–1.16) | 0.055 | 1.07 (1.00–1.16) | 0.059 |
| Cer (d18:1/18:0) | 1.29 (1.22–1.37) | <0.001 | ― | ― | 1.29 (1.20–1.38) | <0.001 | 1.13 (1.05–1.21) | 0.0016 |
| Cer (d18:1/24:0) | 1.12 (1.06–1.19) | 0.0014 | ― | ― | 1.10 (1.02–1.19) | 0.011 | 1.09 (1.01–1.18) | 0.027 |
| Cer (d18:1/24:1) | 1.19 (1.11–1.26) | <0.001 | ― | ― | 1.16 (1.07–1.25) | 0.0019 | 1.1 (1.02–1.19) | 0.013 |
| PC (14:0/22:6) | 1.01 (0.95–1.07) | 0.87 | ― | ― | 0.99 (0.92–1.05) | 0.69 | 0.97 (0.90–1.04) | 0.36 |
| PC (16:0/16:0) | 1.10 (1.04–1.17) | 0.0016 | ― | ― | 1.15 (1.07–1.24) | 0.0003 | 1.12 (1.04–1.21) | 0.0031 |
| PC (16:0/22:5) | 1.06 (1.00–1.12) | 0.046 | ― | ― | 1.07 (1.00–1.15) | 0.062 | 1.04 (0.97–1.11) | 0.29 |
| Cer (d18:1/16:0)/Cer (d18:1/24:0) ratio | 0.97 (0.92–1.03) | 0.31 | ― | ― | 0.97 (0.92–1.03) | 0.37 | 0.98 (0.93–1.04) | 0.57 |
| Cer (d18:1/18:0)/Cer (d18:1/24:0) ratio | 1.22 (1.15–1.29) | <0.001 | ― | ― | 1.18 (1.11–1.25) | <0.001 | 1.05 (0.99–1.12) | 0.099 |
| Cer (d18:1/24:1)/Cer (d18:1/24:0) ratio | 1.06 (1.00–1.12) | 0.055 | ― | ― | 1.04 (0.98–1.10) | 0.19 | 1.01 (0.95–1.07) | 0.77 |
| Cer (d18:1/16:0)/PC (16:0/22:5) ratio | 1.04 (0.98–1.10) | 0.18 | ― | ― | 1 (0.94–1.06) | 0.95 | 1.02 (0.96–1.08) | 0.54 |
| Cer (d18:1/18:0)/PC (14:0/22:6) ratio | 1.17 (1.11–1.24) | <0.001 | ― | ― | 1.15 (1.08–1.22) | 0.00007 | 1.09 (1.02–1.16) | 0.0098 |
| Cer (d18:1/18:0)/Cer (d18:1/16:0) ratio | 1.29 (1.22–1.37) | <0.001 | ― | ― | 1.24 (1.17–1.32) | <0.001 | 1.08 (1.02–1.15) | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoghli, M.; Lokki, A.I.; Lääperi, M.; Sinisalo, J.; Lokki, M.-L.; Hilvo, M.; Jylhä, A.; Tuomilehto, J.; Laaksonen, R. The Novel Ceramide- and Phosphatidylcholine-Based Risk Score for the Prediction of New-Onset of Hypertension. J. Clin. Med. 2023, 12, 7524. https://doi.org/10.3390/jcm12247524
Shoghli M, Lokki AI, Lääperi M, Sinisalo J, Lokki M-L, Hilvo M, Jylhä A, Tuomilehto J, Laaksonen R. The Novel Ceramide- and Phosphatidylcholine-Based Risk Score for the Prediction of New-Onset of Hypertension. Journal of Clinical Medicine. 2023; 12(24):7524. https://doi.org/10.3390/jcm12247524
Chicago/Turabian StyleShoghli, Mohammadreza, A. Inkeri Lokki, Mitja Lääperi, Juha Sinisalo, Marja-Liisa Lokki, Mika Hilvo, Antti Jylhä, Jaakko Tuomilehto, and Reijo Laaksonen. 2023. "The Novel Ceramide- and Phosphatidylcholine-Based Risk Score for the Prediction of New-Onset of Hypertension" Journal of Clinical Medicine 12, no. 24: 7524. https://doi.org/10.3390/jcm12247524
APA StyleShoghli, M., Lokki, A. I., Lääperi, M., Sinisalo, J., Lokki, M.-L., Hilvo, M., Jylhä, A., Tuomilehto, J., & Laaksonen, R. (2023). The Novel Ceramide- and Phosphatidylcholine-Based Risk Score for the Prediction of New-Onset of Hypertension. Journal of Clinical Medicine, 12(24), 7524. https://doi.org/10.3390/jcm12247524







