To Test or Not to Test: Routine Thrombophilia Diagnostic Screening of Women with Reproductive Failures
Abstract
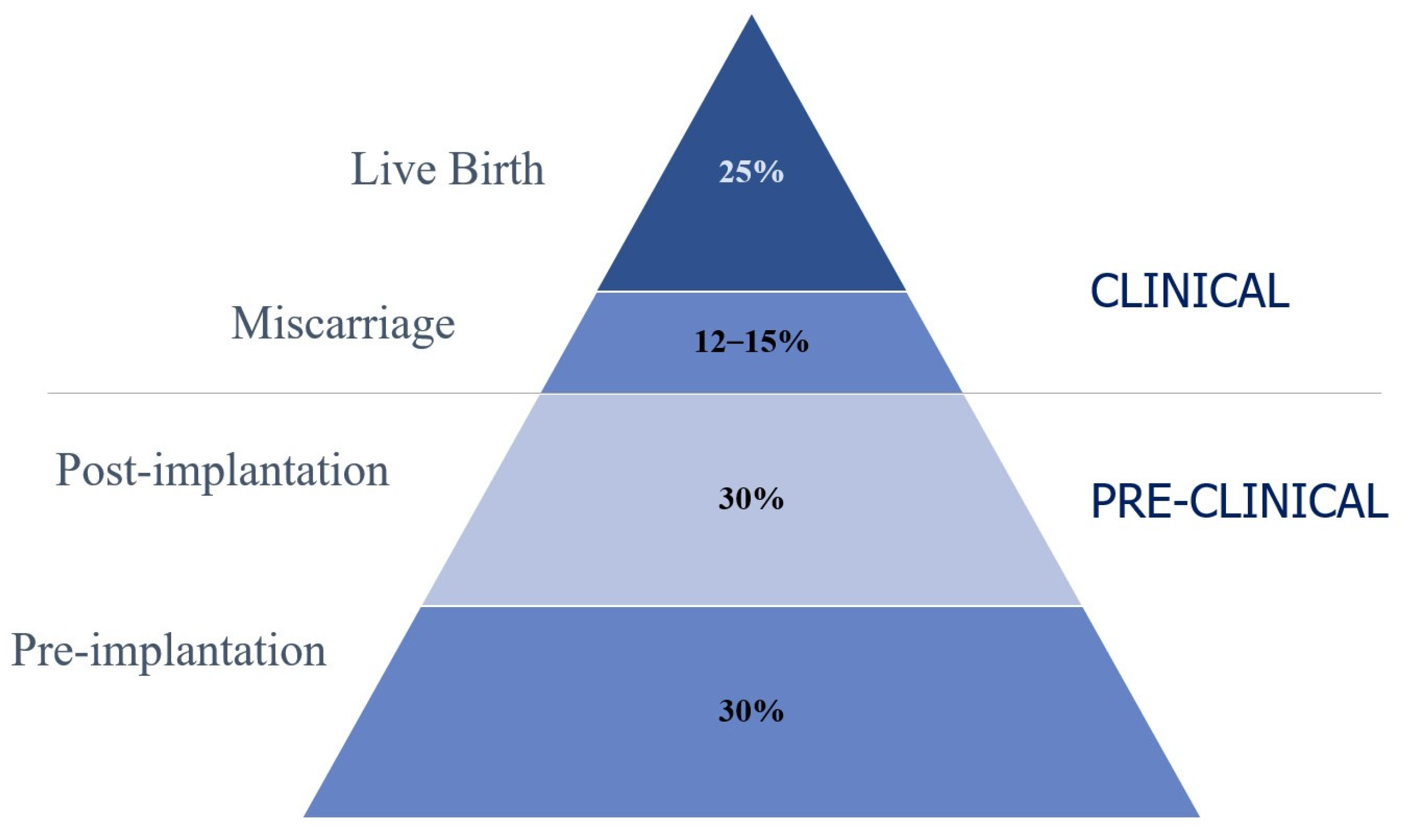
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Examination and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Homer, H.A. Modern management of recurrent miscarriage. Aust. New Zeal. J. Obstet. Gynaecol. 2019, 59, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.J.; Schust, D.J. Genetic considerations in recurrent pregnancy loss. Cold Spring Harb. Perspect Med. 2015, 5, a023119. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.C.; Christiansen, O.B.; Kolte, A.M.; Macklon, N. New insights into mechanisms behind miscarriage. BMC Med. 2013, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Chard, T. Factors of importance for implantation. Baillidre’s Clin. Obs. Gynaecol. Baillière Tindall 1991, 5, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Macklon, N.S.; Geraedts, J.P.M.; Fauser, B.C.J.M. Conception to ongoing pregnancy: The “black box” of early pregnancy loss. Hum. Reprod. 2002, 8, 333–343. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO. Infertility. 2021. Available online: https://www.who.int/health-topics/infertility#tab=tab_1 (accessed on 4 November 2021).
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 26, 65–76. [Google Scholar] [CrossRef]
- Maddirevula, S.; Awartani, K.; Coskun, S.; AlNaim, L.F.; Ibrahim, N.; Abdulwahab, F.; Hashem, M.; Alhassan, S.; Alkuraya, F.S. A genomics approach to females with infertility and recurrent pregnancy loss. Hum. Genet. 2020, 139, 605–613. [Google Scholar] [CrossRef]
- Fatini, C.; Conti, L.; Turillazzi, V.; Sticchi, E.; Romagnuolo, I.; Milanini, M.N.; Cozzi, C.; Abbate, R.; Noci, I. Unexplained infertility: Association with inherited thrombophilia. Thromb. Res. 2012, 129, e185-8. [Google Scholar] [CrossRef]
- Favaloro, E.J. Genetic testing for thrombophilia-related genes: Observations of testing patterns for factor V Leiden (G1691A) and prothrombin gene mutation (G20210A). Semin. Thromb. Hemost. 2019, 45, 730–742. [Google Scholar] [CrossRef]
- Romualdi, D.; Ata, B.; Bhattacharya, S.; Bosch, E.; Costello, M.; Somers, S.; Sunkara, S.K.; Verhoeve, H.R.; Le Clef, N. Evidence-based guideline: Unexplained infertility. Hum Reprod. 2023, 38, 1881–1890. [Google Scholar]
- Undas, A.; Windyga, J.; Podolak-Dawidziak, M.; Klukowska, A.; Zdziarska, J.; Chojnowski, K.; Łętowska, M.; Łaguna, P.; Treliński, J.; Musiał, J.; et al. Congenital/inherited thrombophilia in adults—Characteristics, laboratory testing and management. Recommendations of the Hemostasis Group of the Polish Society of Hematology and Transfusiology 2022. J. Transfus. Med. 2022, 15, 171–182. [Google Scholar] [CrossRef]
- Fabregues, F.; Antonio García-Velasco, J.; Llácer, J.; Requena, A.; Ángel Checa, M.; Bellver, J.; José Espinós, J. The role of thrombophilias in reproduction: A swot analysis. Eur. J. Obstet. Gynecol. Reprod Biol. 2023, 280, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Vermeulen, N.; Lashley, E.E.L.O.; Goddijn, M.; van der Hoorn, M.L.P. Comparison and appraisal of (inter)national recurrent pregnancy loss guidelines. Reprod Biomed. Online 2019, 39, 497–503. [Google Scholar] [CrossRef]
- Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil Steril. 2020, 113, 533–535. [CrossRef] [PubMed]
- Ford, H.B.; Schust, D.J. Recurrent pregnancy loss: Etiology, diagnosis, and therapy. Rev. Obstet. Gynecol. 2009, 2, 76–83. [Google Scholar] [PubMed]
- Malinowski, A.K. The Pathophysiology of Hypercoagulability and Infertility. Semin. Reprod Med. 2021, 39, 34–61. [Google Scholar] [CrossRef]
- Jeve, Y.B.; Davies, W. Evidence-based management of recurrent miscarriages. J. Hum. Reprod. Sci. 2014, 7, 159–169. [Google Scholar] [CrossRef]
- Ata, B.; Urman, B. Thrombophilia and assisted reproduction technology—Any detrimental impact or unnecessary overuse? J. Assist. Reprod. Genet. 2016, 33, 1305–1310. [Google Scholar] [CrossRef]
- Lindqvist, P.G.; Dahlbäck, B. Carriership of Factor V Leiden and Evolutionary Selection Advantage. Curr. Med. Chem. 2008, 15, 1541–1544. [Google Scholar] [CrossRef]
- Wawrusiewicz-Kurylonek, N.; Krȩtowski, A.J.; Posmyk, R. Frequency of thrombophilia associated genes variants: Population-based study. BMC Med. Genet. 2020, 21, 198. [Google Scholar] [CrossRef]
- Ślęzak, R.; Karpiński, P.; Łaczmański, Ł.; Reszczyńska-Ślęzak, D. The role of 1691G>A (Leiden) mutation in Factor V gene, 20210G>A in prothrombin gene and 677C>T in MTHFR gene in etiology of early pregnancy loss. Ginekol. Pol. 2011, 82, 447–450. [Google Scholar]
- Zangari, M.; Elice, F.; Tricot, G.; Fink, L. Thrombophilia. Drug Target Insights 2008, 3, 87–97. [Google Scholar] [CrossRef]
- Lim, M.Y.; Moll, S. Thrombophilia. Vasc. Med. 2015, 20, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.A.; Walker, M.C.; Smith, G.N.; Wells, P.S.; Ramsay, T.; Langlois, N.J.; Carson, N.; Carrier, M.; Rennicks White, R.; Shachkina, S.; et al. Is thrombophilia associated with placenta-mediated pregnancy complications? A prospective cohort study. J. Thromb. Haemost. 2014, 12, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Ormesher, L.; Simcox, L.E.; Tower, C.; Greer, I.A. ‘To test or not to test’, the arguments for and against thrombophilia testing in obstetrics. Obstet. Med. 2017, 10, 61–66. [Google Scholar] [CrossRef]
- Gerhardt, A.; Scharf, R.E.; Beckmann, M.W.; Struve, S.; Bender, H.G.; Pillny, M.; Sandmann, W.Z.R. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N. Engl. J. Med. 2000, 342, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.; Wu, O.; Langhorne, P.; Twaddle, S.; Clark, P.; Lowe, G.D.O.; Walker, I.D.; Greaves, M.; Brenkel, I.; Regan, L.; et al. Thrombophilia in pregnancy: A systematic review. Br. J. Haematol. 2006, 132, 171–196. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, A.; Scharf, R.E.; Greer, I.A.; Zotz, R.B. Hereditary risk factors for thrombophilia and probability of venous thromboembolism during pregnancy and the puerperium. Blood 2016, 128, 2343–2349. [Google Scholar] [CrossRef]
- Skrzypczak, J.; Rajewski, M.; Wirstlein, P.; Goździewicz, T.; Bręborowicz, G.; Leszczyńska-Gorzelak, B.; Ludwikowski, G.; Preis, K.; Wołczyński, S.; Zimmer, M. Incidence of hereditary thrombophilia in women with pregnancy loss in multi-center studies in Poland. Ginekol. Pol. 2012, 83, 330–336. [Google Scholar]
- Pasińska, M.; Soszyńska, K.; Runge, A.; Dąbrowska, A.; Juraszek, A.; Janiszewska, T.; Haus, O. Molecular diagnostic tests for thrombophilia in patients referred to genetic counseling clinic because due to recurrent pregnancy failure. One center’s experience. Ginekol. Pol. 2012, 83, 178–182. [Google Scholar]
- Wolski, H.; Barlik, M.; Drews, K.; Klejewski, A.; Kurzawińska, G.; Ozarowski, M.; Łowicki, Z.; Seremak-Mrozikiewicz, A. Contribution of inherited thrombophilia to recurrent miscarriage in the Polish population. Ginekol. Pol. 2017, 88, 385–392. [Google Scholar] [CrossRef]
- Barlik, M.; Seremak-Mrozikiewicz, A.; Kraśnik, W.; Drews, K. The 20210G>A and 19911A>G polymorphisms of prothrombin gene and recurrent miscarriages. Ginekol. Pol. 2013, 84, 830–834. [Google Scholar] [CrossRef]
- Bleker, S.M.; Coppens, M.; Middeldorp, S. Sex, thrombosis and inherited thrombophilia. Blood Rev. 2014, 28, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Spiezia, L.; Adamo, A.; Simioni, P. Thrombophilia, risk factors and prevention. Expert Rev. Hematol. 2019, 12, 147–158. [Google Scholar] [CrossRef]
- Campello, E.; Spiezia, L.; Simioni, P. Diagnosis and management of factor V Leiden. Expert Rev. Hematol. 2016, 9, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.F.; Dahm, A.; Bergrem, A.; Jacobsen, E.M.; Sandset, P.M. Risk of venous thrombosis in pregnancy among carriers of the factor V Leiden and the prothrombin gene G20210A polymorphisms. J. Thromb. Haemost. 2010, 8, 2443–2449. [Google Scholar] [CrossRef]
- Bałajewicz-Nowak, M.; Pityński, K.; Milewicz, T. The 1691 G>A (Factor V Leiden) and 1328 T>C V Coagulation Factor polymorphisms and recurrent miscarriages. Ginekol. Pol. 2015, 86, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Adler, G.; Clark, J.S.C.; Łoniewska, B.; Czerska, E.; Salkic, N.N.; Ciechanowicz, A. Prevalence of 1691G>A FV mutation in Poland compared with that in other Central, Eastern and South-Eastern European countries. Bosn. J. Basic Med. Sci. 2012, 12, 82–87. [Google Scholar] [CrossRef][Green Version]
- Walker, P.; Gregg, A.R. Screening, Testing, or Personalized Medicine: Where do Inherited Thrombophilias Fit Best? Obstet. Gynecol. Clin. N. Am. 2010, 37, 87–107. [Google Scholar] [CrossRef]
- Rodger, M.A.; Betancourt, M.T.; Clark, P.; Lindqvist, P.G.; Dizon-Townson, D.; Said, J.; Seligsohn, U.; Carrier, M.; Salomon, O.; Greer, I.A. The association of factor V leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: A systematic review and meta-analysis of prospective cohort studies. PLoS Med. 2010, 7, 1000292. [Google Scholar] [CrossRef]
- Ekwutosi, M.; Okoroh, M.D.; Ijeoma, C.; Azonobi, M.D.; Scott, D.; Grosse, D.; Althea, M.; Grant, D.; Hani, K.; Atrash, M.D.; et al. Prevention of Venous Thromboembolism in Pregnancy: A Review of Guidelines, 2000–2011. J. Womens Heal. 2012, 21, 611–615. [Google Scholar] [CrossRef]
- Colucci, G.; Tsakiris, D.A. Thrombophilia screening revisited: An issue of personalized medicine. J. Thromb. Thrombolysis 2020, 49, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Atik, R.B.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; Vermeulen, N.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open. 2018, 10, hoy004. [Google Scholar] [CrossRef]
- Bradley, L.A.; Palomaki, G.E.; Bienstock, J.; Varga, E.; Scott, J.A. Can Factor v Leiden and prothrombin G20210A testing in women with recurrent pregnancy loss result in improved pregnancy outcomes?: Results from a targeted evidence-based review. Genet. Med. 2012, 14, 39–50. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Jeve, Y.; Polanski, L.; Sharpe, A.; Yasmin, E.; Bhandari, H.M. Management of recurrent implantation failure: British Fertility Society policy and practice guideline. Hum. Fertil. 2021, 25, 813–837. [Google Scholar] [CrossRef] [PubMed]
- Lissalde-Lavigne, G.; Fabbro-Peray, P.; Marès, P.; Gris, J.C. Factor V Leiden and prothrombin G20210A polymorphisms as risk factors for miscarriage during a first intended pregnancy: The matched case-control “NOHA First” study. J. Thromb. Haemost. 2006, 4, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Preston, F.E.; Rosendaal, F.R.; Walker, I.D.; Briët, E.; Berntorp, E.; Conard, J.; Fontcuberta, J.; Makris, M.; Mariani, G.; Noteboom, W.; et al. Increased fetal loss in women with heritable thrombophilia. Lancet 1996, 348, 913–916. [Google Scholar] [CrossRef]
- Dizon-Townson, D.; Miller, C.; Sibai, B.; Spong, C.Y.; Thom, E.; Wendel, G., Jr.; Wenstrom, K.; Samuels, P.; Cotroneo, M.A.; Moawad, A.; et al. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obs. Gynecol. 2005, 106, 517–524. [Google Scholar] [CrossRef]
- Silver, R.M.; Zhao, Y.; Spong, C.Y.; Sibai, B.; Wendel, G.; Wenstrom, K.; Samuels, P.; Caritis, S.N.; Sorokin, Y.; Miodovnik, M.; et al. Prothrombin gene G20210A mutation and obstetric complications. Obstet. Gynecol. 2010, 115, 14–20. [Google Scholar] [CrossRef]
- De Jong, P.G.; Goddijn, M.; Middeldorp, S. Testing for inherited thrombophilia in recurrent miscarriage. Semin. Reprod. Med. 2011, 29, 540–547. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
| rs Number Genotype/Allele | Study Group | Patients with RPL | Patients with Infertility |
|---|---|---|---|
| N = 1186 | N = 545 | N = 641 | |
| F2 (rs1799963) | |||
| GG | 1169 (98.6%) | 541 (99.3%) | 628 (98%) |
| GA | 17 (1.4%) | 4 (0.7%) | 13 (2%) |
| AA | - | - | - |
| G | 2355 (99.3%) | 1086 (99.6%) | 1269 (99%) |
| A | 17(0.7%) | 4 (0.4%) | 13 (1%) |
| F5 (rs6025) | |||
| GG | 1119 (94.4%) | 517 (94.9%) | 602 (93.9%) |
| GA | 67 (5.6%) | 28 (5.1%) | 39 (6.1%) |
| AA | - | - | - |
| G | 2305 (97.2%) | 1062 (97.4%) | 1243 (97%) |
| A | 67 (2.8%) | 28 (2.6%) | 39 (3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocka, U.; Sałacińska, K.; Pinkier, I.; Kępczyński, Ł.; Ałaszewski, W.; Dudarewicz, L.; Gach, A. To Test or Not to Test: Routine Thrombophilia Diagnostic Screening of Women with Reproductive Failures. J. Clin. Med. 2023, 12, 7527. https://doi.org/10.3390/jcm12247527
Wysocka U, Sałacińska K, Pinkier I, Kępczyński Ł, Ałaszewski W, Dudarewicz L, Gach A. To Test or Not to Test: Routine Thrombophilia Diagnostic Screening of Women with Reproductive Failures. Journal of Clinical Medicine. 2023; 12(24):7527. https://doi.org/10.3390/jcm12247527
Chicago/Turabian StyleWysocka, Urszula, Kinga Sałacińska, Iwona Pinkier, Łukasz Kępczyński, Wojciech Ałaszewski, Lech Dudarewicz, and Agnieszka Gach. 2023. "To Test or Not to Test: Routine Thrombophilia Diagnostic Screening of Women with Reproductive Failures" Journal of Clinical Medicine 12, no. 24: 7527. https://doi.org/10.3390/jcm12247527
APA StyleWysocka, U., Sałacińska, K., Pinkier, I., Kępczyński, Ł., Ałaszewski, W., Dudarewicz, L., & Gach, A. (2023). To Test or Not to Test: Routine Thrombophilia Diagnostic Screening of Women with Reproductive Failures. Journal of Clinical Medicine, 12(24), 7527. https://doi.org/10.3390/jcm12247527