Correlations between Negative Symptoms and Cognitive Deficits in Individuals at First Psychotic Episode or at High Risk of Psychosis: A Systematic Review
Abstract
1. Introduction
- Summarize the available evidence on the correlations between negative symptoms and dysfunctions in neurocognition and social cognition in subjects with first-episode psychosis or who are at-risk.
- Identify possible methodological limitations especially relevant to the non-standardized and heterogeneous conceptualization of negative symptoms and the use of different scales for their evaluation.
2. Methods
3. Results
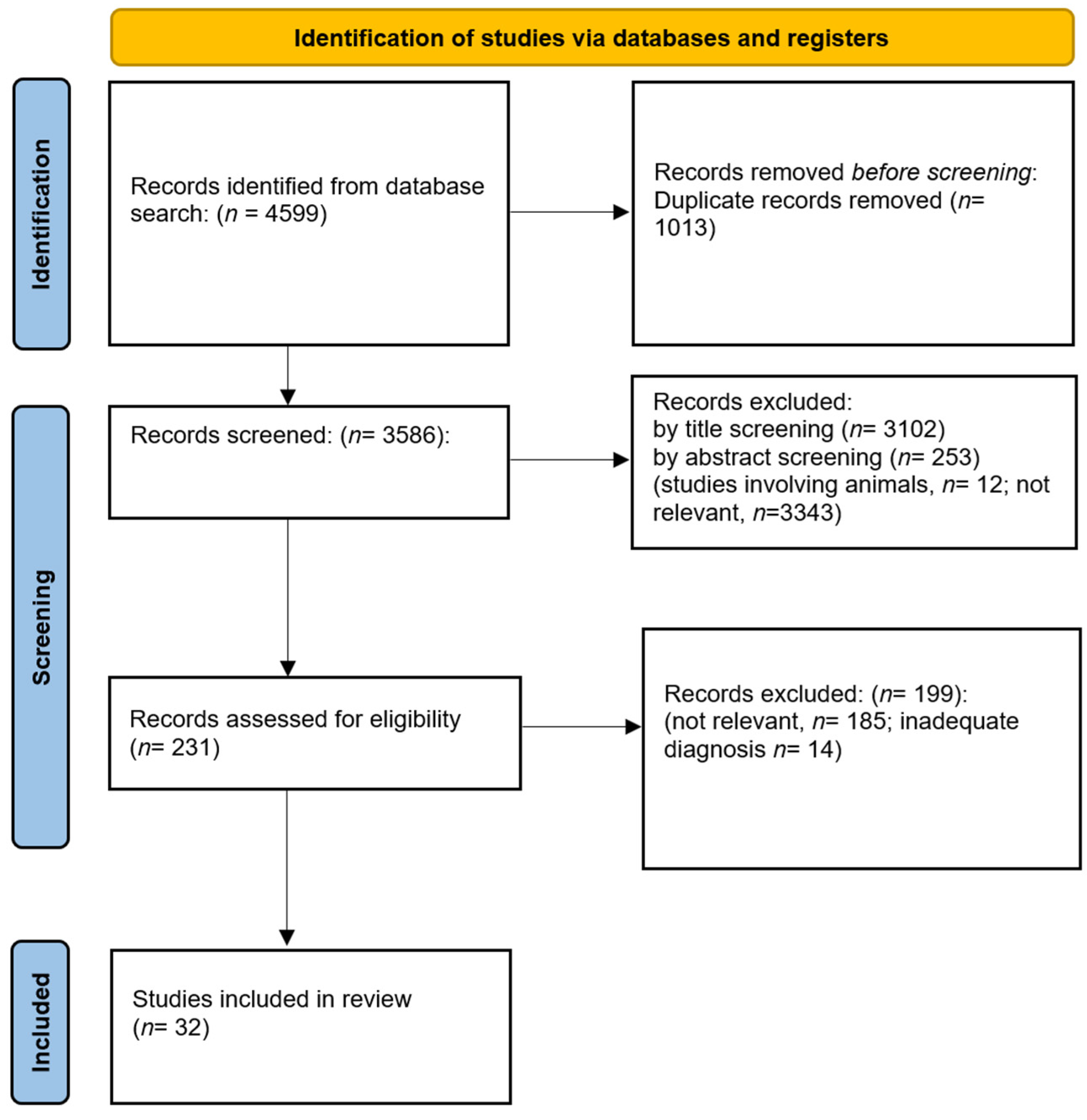
3.1. Search Results
3.2. Risk of Bias and the Methodological Quality of the Retrieved Studies
3.3. Sample Inclusion/Exclusion Criteria and Features of FEP Subjects
3.4. Sample Inclusion/Exclusion Criteria and Features of HR Subjects
| (a). Demographic and Clinical Features of FEP Subjects | FEP = 3086 |
| Diagnosis, n. of subjects | SCZ = 1508 SCZ-A = 75 Schizophreniform = 290 DD = 227 BPD = 175 SSD-NOS = 451 PD-NOS = 360 |
| Psychopharmacological treatment, n. of subjects | SGA = 912 FGA = 142 Anticholinergics = 50 Antidepressants = 13 Antidepressants + benzodiazepines = 1 Antipsychotic-naïve = 179 * |
| Mean age of onset range across studies (min.–max.) | 21.9–36.6 ** years |
| Mean duration of untreated psychosis/illness across studies (min.–max.) | 1–108 *** months |
| Mean age across studies (min.–max.) | 21.4–38.3 years |
| Gender, n. of male subjects (% of male subjects) | 1656 (53.7%) |
| Mean education across studies (min.–max.) | 10.2–12.6 years |
| (b). Demographic and clinical features of HR subjects | HR = 1732 |
| Gender, n. of male subjects (% of male subjects) | 1051 (60.6%) |
| Diagnosis, n. of subjects | AD = 48; Anxiety disorder = 50; SI-PD = 9; Somatoform disorder = 3; ED = 2; Adjustment disorder = 2; Personality disorder = 15 |
| Mean age across studies (min.–max.) | 15.5–26.9 years |
| Mean education across studies (min.−max.) | 10.3–14.3 years # |
| Psychopharmacological treatment, n. of subjects | Antipsychotic, NOS = 44 SGA = 34 Antipsychotic-naïve = 107 Antidepressants = 52 Mood stabilizers = 11 Benzodiazepines = 11 Psychostimulants = 3 Not currently under antipsychotic medication = 594 ## |
3.5. NS Assessment
3.5.1. Assessment of Negative Symptoms in FEP Subjects
3.5.2. Assessment of Negative Symptoms in HR Subjects
3.6. Assessment of Cognitive Impairment in FEP and HR Subjects
3.7. Correlations between NS and Cognitive Functions in FEP Subjects
3.8. Correlations between NS and Cognitive Functions in Subjects at High Risk of Psychosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Carpenter, W.T. Primary psychosis: More to know, much more to do. World Psychiatry 2021, 20, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sass, L. Subjectivity, psychosis and the science of psychiatry. World Psychiatry 2022, 21, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; van Os, J.; De Hert, M.; Gaebel, W.; Galderisi, S.; Green, M.F.; Guloksuz, S.; Harvey, P.D.; Jones, P.B.; Malaspina, D.; et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021, 20, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Dollfus, S.; Nordentoft, M.; Falkai, P.; Kaiser, S.; Giordano, G.M.; Vandevelde, A.; Nielsen, M.O.; Glenthoj, L.B.; et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e23. [Google Scholar] [CrossRef]
- Marder, S.R.; Galderisi, S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 2017, 16, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bleuer, E. Dementia Praecox or the Group of Schizophrenias; NY International Universities Press: New York, NY, USA, 1950. [Google Scholar]
- Kraepelin, E. Dementia praecox and paraphrenia. In Textbook of Psychiatry, 8th ed.; Barclay, E.S., Ed.; Livingston: Edinburgh, UK, 1919. [Google Scholar]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Giordano, G.M.; Caporusso, E.; Pezzella, P.; Galderisi, S. Updated perspectives on the clinical significance of negative symptoms in patients with schizophrenia. Expert Rev. Neurother. 2022, 22, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Solmi, M.; Croatto, G.; Schneider, L.K.; Rohani-Montez, S.C.; Fairley, L.; Smith, N.; Bitter, I.; Gorwood, P.; Taipale, H.; et al. Mortality in people with schizophrenia: A systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry 2022, 21, 248–271. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fenton, W.S.; Carpenter, W.T., Jr.; Marder, S.R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006, 32, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Amodio, A.; Quarantelli, M.; Mucci, A.; Prinster, A.; Soricelli, A.; Vignapiano, A.; Giordano, G.M.; Merlotti, E.; Nicita, A.; Galderisi, S. Avolition-Apathy and White Matter Connectivity in Schizophrenia: Reduced Fractional Anisotropy Between Amygdala and Insular Cortex. Clin. EEG Neurosci. 2018, 49, 55–65. [Google Scholar] [CrossRef]
- Peralta, V.; Gil-Berrozpe, G.J.; Sánchez-Torres, A.; Cuesta, M.J. Clinical relevance of general and specific dimensions in bifactor models of psychotic disorders. World Psychiatry 2021, 20, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.J.; Cohen, A.S. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr. Bull. 2006, 32, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.S.; Rekhi, G.; Lee, J. Validation of the Brief Negative Symptom Scale and its association with functioning. Schizophr. Res. 2019, 208, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.P.; Esfahlani, F.Z.; Galderisi, S.; Mucci, A.; Rossi, A.; Bucci, P.; Rocca, P.; Maj, M.; Kirkpatrick, B.; Ruiz, I.; et al. Network Analysis Reveals the Latent Structure of Negative Symptoms in Schizophrenia. Schizophr. Bull. 2019, 45, 1033–1041. [Google Scholar] [CrossRef]
- Ahmed, A.O.; Kirkpatrick, B.; Galderisi, S.; Mucci, A.; Rossi, A.; Bertolino, A.; Rocca, P.; Maj, M.; Kaiser, S.; Bischof, M.; et al. Cross-cultural Validation of the 5-Factor Structure of Negative Symptoms in Schizophrenia. Schizophr. Bull. 2019, 45, 305–314. [Google Scholar] [CrossRef]
- Mucci, A.; Vignapiano, A.; Bitter, I.; Austin, S.F.; Delouche, C.; Dollfus, S.; Erfurth, A.; Fleischhacker, W.W.; Giordano, G.M.; Gladyshev, I.; et al. A large European, multicenter, multinational validation study of the Brief Negative Symptom Scale. Eur. Neuropsychopharmacol. 2019, 29, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Piskulic, D.; Addington, J.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; Heinssen, R.; Perkins, D.O.; Seidman, L.J.; Tsuang, M.T.; Walker, E.F.; et al. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012, 196, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.R.; Yuen, H.P.; McGorry, P.D.; Phillips, L.J.; Kelly, D.; Dell’Olio, M.; Francey, S.M.; Cosgrave, E.M.; Killackey, E.; Stanford, C.; et al. Mapping the onset of psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust. N. Z. J. Psychiatry 2005, 39, 964–971. [Google Scholar] [CrossRef]
- Velthorst, E.; Nieman, D.H.; Becker, H.E.; van de Fliert, R.; Dingemans, P.M.; Klaassen, R.; de Haan, L.; van Amelsvoort, T.; Linszen, D.H. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr. Res. 2009, 109, 60–65. [Google Scholar] [CrossRef]
- Häfner, H.; Maurer, K.; Ruhrmann, S.; Bechdolf, A.; Klosterkötter, J.; Wagner, M.; Maier, W.; Bottlender, R.; Möller, H.J.; Gaebel, W.; et al. Early detection and secondary prevention of psychosis: Facts and visions. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 117–128. [Google Scholar] [CrossRef]
- Iyer, S.N.; Boekestyn, L.; Cassidy, C.M.; King, S.; Joober, R.; Malla, A.K. Signs and symptoms in the pre-psychotic phase: Description and implications for diagnostic trajectories. Psychol. Med. 2008, 38, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Lencz, T.; Smith, C.W.; Auther, A.; Correll, C.U.; Cornblatt, B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr. Res. 2004, 68, 37–48. [Google Scholar] [CrossRef]
- Demjaha, A.; Valmaggia, L.; Stahl, D.; Byrne, M.; McGuire, P. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophr. Bull. 2012, 38, 351–359. [Google Scholar] [CrossRef]
- Galderisi, S.; Kaiser, S.; Bitter, I.; Nordentoft, M.; Mucci, A.; Sabé, M.; Giordano, G.M.; Nielsen, M.; Glenthøj, L.B.; Pezzella, P.; et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e21. [Google Scholar] [CrossRef]
- Strauss, G.P.; Pelletier-Baldelli, A.; Visser, K.F.; Walker, E.F.; Mittal, V.A. A review of negative symptom assessment strategies in youth at clinical high-risk for psychosis. Schizophr. Res. 2020, 222, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Devoe, D.J.; Farris, M.S.; Townes, P.; Addington, J. Interventions and social functioning in youth at risk of psychosis: A systematic review and meta-analysis. Early Interv. Psychiatry 2019, 13, 169–180. [Google Scholar] [CrossRef]
- Carrión, R.E.; Demmin, D.; Auther, A.M.; McLaughlin, D.; Olsen, R.; Lencz, T.; Correll, C.U.; Cornblatt, B.A. Duration of attenuated positive and negative symptoms in individuals at clinical high risk: Associations with risk of conversion to psychosis and functional outcome. J. Psychiatr. Res. 2016, 81, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Glenthøj, L.B.; Hjorthøj, C.; Kristensen, T.D.; Davidson, C.A.; Nordentoft, M. The effect of cognitive remediation in individuals at ultra-high risk for psychosis: A systematic review. NPJ Schizophr. 2017, 3, 20. [Google Scholar] [CrossRef]
- Üçok, A.; Direk, N.; Kaya, H.; Çağlar, N.; Çıkrıkçılı, U.; Noyan, H.; Yokuşoğlu, Ç.; Devrim-Üçok, M. Relationship of negative symptom severity with cognitive symptoms and functioning in subjects at ultra-high risk for psychosis. Early Interv. Psychiatry 2021, 15, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, D.A.; Campellone, T.R.; Biagianti, B.; Delucchi, K.L.; Gard, D.E.; Fulford, D.; Stuart, B.K.; Fisher, M.; Loewy, R.L.; Vinogradov, S. Modeling the role of negative symptoms in determining social functioning in individuals at clinical high risk of psychosis. Schizophr. Res. 2015, 169, 204–208. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, K.R.; Lee, S.Y.; An, S.K. Impaired Social and Role Function in Ultra-High Risk for Psychosis and First-Episode Schizophrenia: Its Relations with Negative Symptoms. Psychiatry Investig. 2017, 14, 539–545. [Google Scholar] [CrossRef]
- Mason, O.; Startup, M.; Halpin, S.; Schall, U.; Conrad, A.; Carr, V. Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states’. Schizophr. Res. 2004, 71, 227–237. [Google Scholar] [CrossRef]
- Santesteban-Echarri, O.; Paino, M.; Rice, S.; González-Blanch, C.; McGorry, P.; Gleeson, J.; Alvarez-Jimenez, M. Predictors of functional recovery in first-episode psychosis: A systematic review and meta-analysis of longitudinal studies. Clin. Psychol. Rev. 2017, 58, 59–75. [Google Scholar] [CrossRef]
- Best, M.W.; Grossman, M.; Oyewumi, L.K.; Bowie, C.R. Examination of the Positive and Negative Syndrome Scale factor structure and longitudinal relationships with functioning in early psychosis. Early Interv. Psychiatry 2016, 10, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Milev, P.; Ho, B.C.; Arndt, S.; Andreasen, N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 2005, 162, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Noto, C.; Bressan, R.A.; Brietzke, E. Determinants of adherence to treatment in first-episode psychosis: A comprehensive review. Braz. J. Psychiatry 2015, 37, 168–176. [Google Scholar] [CrossRef]
- Watson, P.; Zhang, J.P.; Rizvi, A.; Tamaiev, J.; Birnbaum, M.L.; Kane, J. A meta-analysis of factors associated with quality of life in first episode psychosis. Schizophr. Res. 2018, 202, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Challis, S.; Nielssen, O.; Harris, A.; Large, M. Systematic meta-analysis of the risk factors for deliberate self-harm before and after treatment for first-episode psychosis. Acta Psychiatr. Scand. 2013, 127, 442–454. [Google Scholar] [CrossRef]
- Bucci, P.; Mucci, A.; van Rossum, I.W.; Aiello, C.; Arango, C.; Baandrup, L.; Buchanan, R.W.; Dazzan, P.; Demjaha, A.; Díaz-Caneja, C.M.; et al. Persistent negative symptoms in recent-onset psychosis: Relationship to treatment response and psychosocial functioning. Eur. Neuropsychopharmacol. 2020, 34, 76–86. [Google Scholar] [CrossRef]
- Perrottelli, A.; Giordano, G.M.; Brando, F.; Giuliani, L.; Mucci, A. EEG-Based Measures in At-Risk Mental State and Early Stages of Schizophrenia: A Systematic Review. Front. Psychiatry 2021, 12, 653642. [Google Scholar] [CrossRef]
- Hill, S.K.; Reilly, J.L.; Keefe, R.S.; Gold, J.M.; Bishop, J.R.; Gershon, E.S.; Tamminga, C.A.; Pearlson, G.D.; Keshavan, M.S.; Sweeney, J.A. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am. J. Psychiatry 2013, 170, 1275–1284. [Google Scholar] [CrossRef]
- Toulopoulou, T.; Goldberg, T.E.; Mesa, I.R.; Picchioni, M.; Rijsdijk, F.; Stahl, D.; Cherny, S.S.; Sham, P.; Faraone, S.V.; Tsuang, M.; et al. Impaired intellect and memory: A missing link between genetic risk and schizophrenia? Arch. Gen. Psychiatry 2010, 67, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.; Gaebel, W.; Mucci, A.; Sachs, G.; Barlati, S.; Giordano, G.M.; Nibbio, G.; Nordentoft, M.; Wykes, T.; Galderisi, S. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur. Psychiatry 2022, 65, e57. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Deste, G.; Smieskova, R.; Barlati, S.; Yung, A.R.; Howes, O.; Stieglitz, R.D.; Vita, A.; McGuire, P.; Borgwardt, S. Cognitive functioning in prodromal psychosis: A meta-analysis. Arch. Gen. Psychiatry 2012, 69, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Lin, A.; Wood, S.J.; Yung, A.R.; McGorry, P.D.; Pantelis, C. Cognitive deficits in youth with familial and clinical high risk to psychosis: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2014, 130, 1–15. [Google Scholar] [CrossRef]
- Green, M.F.; Nuechterlein, K.H.; Gold, J.M.; Barch, D.M.; Cohen, J.; Essock, S.; Fenton, W.S.; Frese, F.; Goldberg, T.E.; Heaton, R.K.; et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry 2004, 56, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.; Gaebel, W.; Mucci, A.; Sachs, G.; Erfurth, A.; Barlati, S.; Zanca, F.; Giordano, G.M.; Birkedal Glenthøj, L.; Nordentoft, M.; et al. European Psychiatric Association guidance on assessment of cognitive impairment in schizophrenia. Eur. Psychiatry 2022, 65, e58. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.; Poe, M.; Walker, T.M.; Harvey, P.D. The relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to functional capacity and real-world functional outcome. J. Clin. Exp. Neuropsychol. 2006, 28, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Mesholam-Gately, R.; Giuliano, A.; Kirsten, P.G.; Faraone, S.; Seidman, L. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology 2009, 23, 315–336. [Google Scholar] [CrossRef]
- González-Blanch, C.; Rodríguez-Sánchez, J.M.; Pérez-Iglesias, R.; Pardo-García, G.; Martínez-García, O.; Vázquez-Barquero, J.L.; Crespo-Facorro, B. First-episode schizophrenia patients neuropsychologically within the normal limits: Evidence of deterioration in speed of processing. Schizophr. Res. 2010, 119, 18–26. [Google Scholar] [CrossRef]
- Zabala, A.; Rapado, M.; Arango, C.; Robles, O.; de la Serna, E.; González, C.; Rodríguez-Sánchez, J.M.; Andrés, P.; Mayoral, M.; Bombín, I. Neuropsychological functioning in early-onset first-episode psychosis: Comparison of diagnostic subgroups. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 225–233. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Byrne, M.; Valmaggia, L.; Day, F.; Tabraham, P.; Johns, L.; McGuire, P. Social dysfunction predicts two years clinical outcome in people at ultra high risk for psychosis. J. Psychiatr. Res. 2010, 44, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Radua, J.; Jauhar, S. Lack of robust meta-analytic evidence to favour cognitive behavioural therapy for prevention of psychosis. World Psychiatry 2021, 20, 443–444. [Google Scholar] [CrossRef]
- Albert, N.; Weibell, M.A. The outcome of early intervention in first episode psychosis. Int. Rev. Psychiatry 2019, 31, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Allott, K.; Liu, P.; Proffitt, T.M.; Killackey, E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: Systematic review and methodological critique. Schizophr. Res. 2011, 125, 221–235. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Pillinger, T.; Efthimiou, O.; Maslej, M.; Mulsant, B.H.; Young, A.H.; Cipriani, A.; Howes, O.D. Reappraising the variability of effects of antipsychotic medication in schizophrenia: A meta-analysis. World Psychiatry 2022, 21, 287–294. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Bertolini, F.; Tedeschi, F.; Vita, G.; Brambilla, P.; Del Fabro, L.; Gastaldon, C.; Papola, D.; Purgato, M.; Nosari, G.; et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: A network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry 2022, 21, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.; Barlati, S.; Deste, G.; Rocca, P.; Rossi, A.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Biondi, M.; et al. The influence of autistic symptoms on social and non-social cognition and on real-life functioning in people with schizophrenia: Evidence from the Italian Network for Research on Psychoses multicenter study. Eur. Psychiatry 2020, 63, e98. [Google Scholar] [CrossRef] [PubMed]
- Jukic, M.; Milosavljević, F.; Molden, E.; Ingelman-Sundberg, M. Pharmacogenomics in treatment of depression and psychosis: An update. Trends Pharmacol. Sci. 2022, 43, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Glenthøj, L.B.; Kristensen, T.D.; Wenneberg, C.; Hjorthøj, C.; Nordentoft, M. Experiential negative symptoms are more predictive of real-life functional outcome than expressive negative symptoms in clinical high-risk states. Schizophr. Res. 2020, 218, 151–156. [Google Scholar] [CrossRef]
- Halverson, T.F.; Orleans-Pobee, M.; Merritt, C.; Sheeran, P.; Fett, A.K.; Penn, D.L. Pathways to functional outcomes in schizophrenia spectrum disorders: Meta-analysis of social cognitive and neurocognitive predictors. Neurosci. Biobehav. Rev. 2019, 105, 212–219. [Google Scholar] [CrossRef]
- Giuliani, L.; Giordano, G.M.; Bucci, P.; Pezzella, P.; Brando, F.; Galderisi, S. Improving Knowledge on Pathways to Functional Outcome in Schizophrenia: Main Results from the Italian Network for Research on Psychoses. Front. Psychiatry 2021, 12, 791117. [Google Scholar] [CrossRef] [PubMed]
- Rocca, P.; Rucci, P.; Montemagni, C.; Rossi, A.; Bertolino, A.; Aguglia, E.; Altamura, C.A.; Amore, M.; Andriola, I.; Bellomo, A.; et al. Does social cognition change? Evidence after 4 years from the Italian Network for Research on Psychoses. Eur. Psychiatry 2023, 66, e10. [Google Scholar] [CrossRef]
- Kaiser, S.; Lyne, J.; Agartz, I.; Clarke, M.; Mørch-Johnsen, L.; Faerden, A. Individual negative symptoms and domains—Relevance for assessment, pathomechanisms and treatment. Schizophr. Res. 2017, 186, 39–45. [Google Scholar] [CrossRef]
- Shmukler, A.B.; Gurovich, I.Y.; Agius, M.; Zaytseva, Y. Long-term trajectories of cognitive deficits in schizophrenia: A critical overview. Eur. Psychiatry 2015, 30, 1002–1010. [Google Scholar] [CrossRef]
- Hegde, S.; Thirthalli, J.; Rao, S.L.; Raguram, A.; Philip, M.; Gangadhar, B.N. Cognitive deficits and its relation with psychopathology and global functioning in first episode schizophrenia. Asian J. Psychiatry 2013, 6, 537–543. [Google Scholar] [CrossRef]
- Krukow, P.; Karakuła-Juchnowicz, H.; Juchnowicz, D.; Morylowska-Topolska, J.; Flis, M.; Jonak, K. Processing speed is associated with differences in IQ and cognitive profiles between patients with schizophrenia and their healthy siblings. Nord. J. Psychiatry 2017, 71, 33–41. [Google Scholar] [CrossRef]
- Galderisi, S.; Maj, M. Deficit schizophrenia: An overview of clinical, biological and treatment aspects. Eur. Psychiatry 2009, 24, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Merlotti, E.; Üçok, A.; Aleman, A.; Galderisi, S. Primary and persistent negative symptoms: Concepts, assessments and neurobiological bases. Schizophr. Res. 2017, 186, 19–28. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Mucci, A.; Galderisi, S. Primary, Enduring Negative Symptoms: An Update on Research. Schizophr. Bull. 2017, 43, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Rucci, P.; Kirkpatrick, B.; Mucci, A.; Gibertoni, D.; Rocca, P.; Rossi, A.; Bertolino, A.; Strauss, G.P.; Aguglia, E.; et al. Interplay Among Psychopathologic Variables, Personal Resources, Context-Related Factors, and Real-life Functioning in Individuals with Schizophrenia: A Network Analysis. JAMA Psychiatry 2018, 75, 396–404. [Google Scholar] [CrossRef]
- Bucci, P.; Galderisi, S. Categorizing and assessing negative symptoms. Curr. Opin. Psychiatry 2017, 30, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Leeson, V.C.; Robbins, T.W.; Franklin, C.; Harrison, M.; Harrison, I.; Ron, M.A.; Barnes, T.R.; Joyce, E.M. Dissociation of long-term verbal memory and fronto-executive impairment in first-episode psychosis. Psychol. Med. 2009, 39, 1799–1808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Díaz-Caneja, C.M.; Pina-Camacho, L.; Rodríguez-Quiroga, A.; Fraguas, D.; Parellada, M.; Arango, C. Predictors of outcome in early-onset psychosis: A systematic review. NPJ Schizophr. 2015, 1, 14005. [Google Scholar] [CrossRef]
- Cohen, A.S.; Kim, Y.; Najolia, G.M. Psychiatric symptom versus neurocognitive correlates of diminished expressivity in schizophrenia and mood disorders. Schizophr. Res. 2013, 146, 249–253. [Google Scholar] [CrossRef]
- Chang, W.C.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Wong, G.H.Y.; Chen, E.Y.H. Relationship between diminished expression and cognitive impairment in first-episode schizophrenia: A prospective three-year follow-up study. Schizophr. Res. 2014, 152, 146–151. [Google Scholar] [CrossRef]
- Roth, R.; Flashman, L.; Saykin, A.; McAllister, T.; Vidaver, R. Apathy in Schizophrenia: Reduced Frontal Lobe Volume and Neuropsychological Deficits. Am. J. Psychiatry 2004, 161, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Faerden, A.; Vaskinn, A.; Finset, A.; Agartz, I.; Ann Barrett, E.; Friis, S.; Simonsen, C.; Andreassen, O.A.; Melle, I. Apathy is associated with executive functioning in first episode psychosis. BMC Psychiatry 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Konstantakopoulos, G.; Ploumpidis, D.; Oulis, P.; Patrikelis, P.; Soumani, A.; Papadimitriou, G.N.; Politis, A.M. Apathy, cognitive deficits and functional impairment in schizophrenia. Schizophr. Res. 2011, 133, 193–198. [Google Scholar] [CrossRef]
- Ventura, J.; Subotnik, K.L.; Ered, A.; Gretchen-Doorly, D.; Hellemann, G.S.; Vaskinn, A.; Nuechterlein, K.H. The relationship of attitudinal beliefs to negative symptoms, neurocognition, and daily functioning in recent-onset schizophrenia. Schizophr. Bull. 2014, 40, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Rossi, A.; Rocca, P.; Bertolino, A.; Mucci, A.; Bucci, P.; Rucci, P.; Gibertoni, D.; Aguglia, E.; Amore, M.; et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 2014, 13, 275–287. [Google Scholar] [CrossRef]
- Kring, A.M.; Gur, R.E.; Blanchard, J.J.; Horan, W.P.; Reise, S.P. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. Am. J. Psychiatry 2013, 170, 165–172. [Google Scholar] [CrossRef]
- Gur, R.E.; March, M.; Calkins, M.E.; Weittenhiller, L.; Wolf, D.H.; Turetsky, B.I.; Gur, R.C. Negative symptoms in youths with psychosis spectrum features: Complementary scales in relation to neurocognitive performance and function. Schizophr. Res. 2015, 166, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Epskamp, S.; Isvoranu, A.M. New trends in network modeling of psychopathology. World Psychiatry 2022, 21, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Cascino, G.; Monteleone, A.M.; Rocca, P.; Rossi, A.; Bertolino, A.; Aguglia, E.; Amore, M.; Collantoni, E.; Corrivetti, G.; et al. Prevalence of antipsychotic-induced extrapyramidal symptoms and their association with neurocognition and social cognition in outpatients with schizophrenia in the “real-life”. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110250. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Mde, G.; Viechtbauer, W.; Simons, C.J.; van Os, J.; Krabbendam, L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol. Bull. 2009, 135, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jin, X.; He, Y.; Wu, S.; Cui, X.; Gao, X.; Huang, C.; Luo, X. Atypical frontotemporal cortical activity in first-episode adolescent-onset schizophrenia during verbal fluency task: A functional near-infrared spectroscopy study. Front. Psychiatry 2023, 14, 1126131. [Google Scholar] [CrossRef]
- Egeland, J.; Holmen, T.L.; Bang-Kittilsen, G.; Bigseth, T.T.; Engh, J.A. Category fluency in schizophrenia: Opposing effects of negative and positive symptoms? Cogn. Neuropsychiatry 2018, 23, 28–42. [Google Scholar] [CrossRef]
- Fervaha, G.; Takeuchi, H.; Foussias, G.; Agid, O.; Remington, G. Using poverty of speech as a case study to explore the overlap between negative symptoms and cognitive dysfunction. Schizophr. Res. 2016, 176, 411–416. [Google Scholar] [CrossRef]
- Kravariti, E.; Russo, M.; Vassos, E.; Morgan, K.; Fearon, P.; Zanelli, J.W.; Demjaha, A.; Lappin, J.M.; Tsakanikos, E.; Dazzan, P.; et al. Linear and non-linear associations of symptom dimensions and cognitive function in first-onset psychosis. Schizophr. Res. 2012, 140, 221–231. [Google Scholar] [CrossRef]
- Liemburg, E.J.; Enriquez-Geppert, S.; Wardenaar, K.J.; Bruggeman, R.; Aleman, A. Expressive deficits and amotivation as mediators of the associations between cognitive problems and functional outcomes: Results from two independent cohorts. Schizophr. Res. 2020, 218, 283–291. [Google Scholar] [CrossRef]
- MacBeth, A.; Gumley, A.; Schwannauer, M.; Carcione, A.; McLeod, H.J.; Dimaggio, G. Metacognition in First Episode Psychosis: Item Level Analysis of Associations with Symptoms and Engagement. Clin. Psychol. Psychother. 2016, 23, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, T.; Roesch, S.; Granholm, E. The role of dysfunctional attitudes in models of negative symptoms and functioning in schizophrenia. Schizophr. Res. 2014, 157, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Stäblein, M.; Sieprath, L.; Knöchel, C.; Landertinger, A.; Schmied, C.; Ghinea, D.; Mayer, J.S.; Bittner, R.A.; Reif, A.; Oertel-Knöchel, V. Impaired working memory for visual motion direction in schizophrenia: Absence of recency effects and association with psychopathology. Neuropsychology 2016, 30, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Torniainen, M.; Suvisaari, J.; Partonen, T.; Castaneda, A.E.; Kuha, A.; Suokas, J.; Perälä, J.; Saarni, S.I.; Lönnqvist, J.; Tuulio-Henriksson, A. Cognitive impairments in schizophrenia and schizoaffective disorder: Relationship with clinical characteristics. J. Nerv. Ment. Dis. 2012, 200, 316–322. [Google Scholar] [CrossRef]
- Cella, M.; Wykes, T. Understanding processing speed—Its subcomponents and their relationship to characteristics of people with schizophrenia. Cogn. Neuropsychiatry 2013, 18, 437–451. [Google Scholar] [CrossRef]
- Shean, G.; Murphy, A.; Meyer, J. Social cognition and symptom dimensions. J. Nerv. Ment. Dis. 2005, 193, 751–755. [Google Scholar] [CrossRef]
- Rossell, S.L. Category fluency performance in patients with schizophrenia and bipolar disorder: The influence of affective categories. Schizophr. Res. 2006, 82, 135–138. [Google Scholar] [CrossRef]
- Palmer, B.W.; Jeste, D.V. Relationship of Individual Cognitive Abilities to Specific Components of Decisional Capacity Among Middle-Aged and Older Patients with Schizophrenia. Schizophr. Bull. 2005, 32, 98–106. [Google Scholar] [CrossRef]
- Harvey, P.D.; Green, M.F.; Bowie, C.; Loebel, A. The dimensions of clinical and cognitive change in schizophrenia: Evidence for independence of improvements. Psychopharmacology 2006, 187, 356–363. [Google Scholar] [CrossRef]
- Sanz, J.H.; Karlsgodt, K.H.; Bearden, C.E.; van Erp, T.G.; Nandy, R.R.; Ventura, J.; Nuechterlein, K.; Cannon, T.D. Symptomatic and functional correlates of regional brain physiology during working memory processing in patients with recent onset schizophrenia. Psychiatry Res. 2009, 173, 177–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buck, G.; Lavigne, K.M.; Makowski, C.; Joober, R.; Malla, A.; Lepage, M. Sex Differences in Verbal Memory Predict Functioning Through Negative Symptoms in Early Psychosis. Schizophr. Bull. 2020, 46, 1587–1595. [Google Scholar] [CrossRef]
- Chan, R.C.; Chen, E.Y.; Law, C.W. Specific executive dysfunction in patients with first-episode medication-naïve schizophrenia. Schizophr. Res. 2006, 82, 51–64. [Google Scholar] [CrossRef]
- Chang, W.C.; Lau, C.F.C.; Chan, S.S.I.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Lin, J.; Chen, E.Y.H. Premorbid, clinical and cognitive correlates of primary negative symptoms in first-episode psychosis. Psychiatry Res. 2016, 242, 144–149. [Google Scholar] [CrossRef]
- Chang, W.C.; Kwong, V.W.Y.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Chen, E.Y.H. Relationship of amotivation to neurocognition, self-efficacy and functioning in first-episode psychosis: A structural equation modeling approach. Psychol. Med. 2017, 47, 755–765. [Google Scholar] [CrossRef]
- Chang, W.C.; Wong, C.S.M.; Or, P.C.F.; Chu, A.O.K.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.M.H.; Suen, Y.N.; Chen, E.Y.H. Inter-relationships among psychopathology, premorbid adjustment, cognition and psychosocial functioning in first-episode psychosis: A network analysis approach. Psychol. Med. 2020, 50, 2019–2027. [Google Scholar] [CrossRef]
- Engen, M.J.; Simonsen, C.; Melle, I.; Færden, A.; Lyngstad, S.H.; Haatveit, B.; Vaskinn, A.; Ueland, T. Cognitive functioning in patients with first-episode psychosis stratified by level of negative symptoms: A 1-year follow-up study. Psychiatry Res. 2019, 281, 112554. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.M.; Hui, C.L.M.; Chan, K.P.K.; Chan, P.Y.; Law, E.Y.L.; Chong, C.S.Y.; Chang, W.C.; Chan, S.K.W.; Lee, W.K.; Lo, A.W.F.; et al. The role of symptoms and insight in mediating cognition and functioning in first episode psychosis. Schizophr. Res. 2019, 206, 251–256. [Google Scholar] [CrossRef]
- Trampush, J.W.; Lencz, T.; DeRosse, P.; John, M.; Gallego, J.A.; Petrides, G.; Hassoun, Y.; Zhang, J.P.; Addington, J.; Kellner, C.H.; et al. Relationship of Cognition to Clinical Response in First-Episode Schizophrenia Spectrum Disorders. Schizophr. Bull. 2015, 41, 1237–1247. [Google Scholar] [CrossRef]
- Ventura, J.; Ered, A.; Gretchen-Doorly, D.; Subotnik, K.L.; Horan, W.P.; Hellemann, G.S.; Nuechterlein, K.H. Theory of mind in the early course of schizophrenia: Stability, symptom and neurocognitive correlates, and relationship with functioning. Psychol. Med. 2015, 45, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.Y.; Chang, W.C.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Suen, Y.N.; Chen, E.Y.H. Relationship of subjective quality of life with symptomatology, neurocognition and psychosocial functioning in first-episode psychosis: A structural equation modelling approach. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Bliksted, V.; Videbech, P.; Fagerlund, B.; Frith, C. The effect of positive symptoms on social cognition in first-episode schizophrenia is modified by the presence of negative symptoms. Neuropsychology 2017, 31, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ditlevsen, J.V.; Simonsen, A.; Bliksted, V.F. Predicting mentalizing deficits in first-episode schizophrenia from different subdomains of negative symptoms. Schizophr. Res. 2020, 215, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, Y.; Yu, L.; Hu, J.; Chen, J.; Jin, P.; Xu, W.; Wei, N.; Hu, S.; Qi, H.; et al. Relationship between negative symptoms and neurocognitive functions in adolescent and adult patients with first-episode schizophrenia. BMC Psychiatry 2016, 16, 344. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.M.; Crespo-Facorro, B.; González-Blanch, C.; Pérez-Iglesias, R.; Alvarez-Jiménez, M.; Martínez, O.; Vázquez-Barquero, J.L. Cognitive functioning and negative symptoms in first episode schizophrenia: Different patterns of correlates. Neurotox. Res. 2008, 14, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Stouten, L.H.; Veling, W.; Laan, W.; van der Helm, M.; van der Gaag, M. Psychosocial functioning in first-episode psychosis and associations with neurocognition, social cognition, psychotic and affective symptoms. Early Interv. Psychiatry 2017, 11, 23–36. [Google Scholar] [CrossRef]
- Ayres, A.M.; Busatto, G.F.; Menezes, P.R.; Schaufelberger, M.S.; Coutinho, L.; Murray, R.M.; McGuire, P.K.; Rushe, T.; Scazufca, M. Cognitive deficits in first-episode psychosis: A population-based study in São Paulo, Brazil. Schizophr. Res. 2007, 90, 338–343. [Google Scholar] [CrossRef]
- Mazza, M.; Pollice, R.; Pacitti, F.; Pino, M.C.; Mariano, M.; Tripaldi, S.; Casacchia, M.; Roncone, R. New evidence in theory of mind deficits in subjects with chronic schizophrenia and first episode: Correlation with symptoms, neurocognition and social function. Riv. Di Psichiatr. 2012, 47, 327–336. [Google Scholar] [CrossRef]
- Piskulic, D.; Addington, J. Social cognition and negative symptoms in psychosis. Psychiatry Res. 2011, 188, 283–285. [Google Scholar] [CrossRef]
- Saleem, M.M.; Harte, M.K.; Marshall, K.M.; Scally, A.; Brewin, A.; Neill, J.C. First episode psychosis patients show impaired cognitive function—A study of a South Asian population in the UK. J. Psychopharmacol. 2013, 27, 366–373. [Google Scholar] [CrossRef]
- Glenthøj, L.B.; Jepsen, J.R.; Hjorthøj, C.; Bak, N.; Kristensen, T.D.; Wenneberg, C.; Krakauer, K.; Nordentoft, M.; Fagerlund, B. Negative symptoms mediate the relationship between neurocognition and function in individuals at ultrahigh risk for psychosis. Acta Psychiatr. Scand. 2017, 135, 250–258. [Google Scholar] [CrossRef]
- Niendam, T.A.; Bearden, C.E.; Johnson, J.K.; McKinley, M.; Loewy, R.; O’Brien, M.; Nuechterlein, K.H.; Green, M.F.; Cannon, T.D. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr. Res. 2006, 84, 100–111. [Google Scholar] [CrossRef]
- Shin, Y.S.; Kim, S.Y.; Lee, T.Y.; Hur, J.W.; Shin, N.Y.; Kim, S.N.; Shin, M.S.; Kwon, J.S. Longitudinal change in neurocognition and its relation to symptomatic and functional changes over 2years in individuals at clinical high-risk for psychosis. Schizophr. Res. 2016, 174, 50–57. [Google Scholar] [CrossRef]
- Barbato, M.; Liu, L.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; McGlashan, T.H.; Perkins, D.O.; Seidman, L.J.; Tsuang, M.T.; Walker, E.F.; et al. Theory of Mind, Emotion Recognition and Social Perception in Individuals at Clinical High Risk for Psychosis: Findings from the NAPLS-2 cohort. Schizophr. Res. Cogn. 2015, 2, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, C.; Maheandiran, M.; Lepock, J.; Ahmed, S.; Kiang, M.; Bagby, R.M.; Mizrahi, R. Negative symptoms in the clinical high-risk state for psychosis: Connection with cognition and primacy in impacting functioning. Early Interv. Psychiatry 2020, 14, 188–195. [Google Scholar] [CrossRef]
- Lindgren, M.; Manninen, M.; Laajasalo, T.; Mustonen, U.; Kalska, H.; Suvisaari, J.; Moilanen, K.; Cannon, T.D.; Huttunen, M.; Therman, S. The relationship between psychotic-like symptoms and neurocognitive performance in a general adolescent psychiatric sample. Schizophr. Res. 2010, 123, 77–85. [Google Scholar] [CrossRef]
- Leanza, L.; Egloff, L.; Studerus, E.; Andreou, C.; Heitz, U.; Ittig, S.; Beck, K.; Uttinger, M.; Riecher-Rössler, A. The relationship between negative symptoms and cognitive functioning in patients at clinical high risk for psychosis. Psychiatry Res. 2018, 268, 21–27. [Google Scholar] [CrossRef]
- Meyer, E.C.; Carrión, R.E.; Cornblatt, B.A.; Addington, J.; Cadenhead, K.S.; Cannon, T.D.; McGlashan, T.H.; Perkins, D.O.; Tsuang, M.T.; Walker, E.F.; et al. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American Prodrome Longitudinal Study. Schizophr. Bull. 2014, 40, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Pelizza, L.; Maestri, D.; Leuci, E.; Quattrone, E.; Azzali, S.; Paulillo, G.; Pellegrini, P. Negative symptom configuration in patients with first episode affective psychosis: Findings from the 1-year follow-up of the “Parma Early Psychosis” program. Acta Bio Medica Atenei Parm. 2021, 92, e2021224. [Google Scholar] [CrossRef]
- Vargas, T.; Snyder, H.; Banich, M.; Newberry, R.; Shankman, S.A.; Strauss, G.P.; Mittal, V.A. Altered selection during language processing in individuals at high risk for psychosis. Schizophr. Res. 2018, 202, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Tyburski, E.; Pełka-Wysiecka, J.; Mak, M.; Samochowiec, A.; Bieńkowski, P.; Samochowiec, J. Neuropsychological Profile of Specific Executive Dysfunctions in Patients with Deficit and Non-deficit Schizophrenia. Front. Psychol. 2017, 8, 1459. [Google Scholar] [CrossRef]
- Bielecki, M.; Tyburski, E.; Plichta, P.; Mak, M.; Kucharska-Mazur, J.; Podwalski, P.; Rek-Owodziń, K.; Waszczuk, K.; Sagan, L.; Mueller, S.T.; et al. Executive Functions and Psychopathology Dimensions in Deficit and Non-Deficit Schizophrenia. J. Clin. Med. 2023, 12, 1998. [Google Scholar] [CrossRef]
- Plichta, P.; Tyburski, E.; Bielecki, M.; Mak, M.; Kucharska-Mazur, J.; Podwalski, P.; Rek-Owodziń, K.; Waszczuk, K.; Sagan, L.; Michalczyk, A.; et al. Cognitive Dysfunctions Measured with the MCCB in Deficit and Non-Deficit Schizophrenia. J. Clin. Med. 2023, 12, 2257. [Google Scholar] [CrossRef]
- Thibaudeau, E.; Rae, J.; Raucher-Chéné, D.; Bougeard, A.; Lepage, M. Disentangling the Relationships Between the Clinical Symptoms of Schizophrenia Spectrum Disorders and Theory of Mind: A Meta-analysis. Schizophr. Bull. 2023, 49, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M. Cognitive impairment as a diagnostic criterion and treatment target in schizophrenia. World Psychiatry 2019, 18, 171–172. [Google Scholar] [CrossRef]
- Bloomfield, M.A.P.; Chang, T.; Woodl, M.J.; Lyons, L.M.; Cheng, Z.; Bauer-Staeb, C.; Hobbs, C.; Bracke, S.; Kennerley, H.; Isham, L.; et al. Psychological processes mediating the association between developmental trauma and specific psychotic symptoms in adults: A systematic review and meta-analysis. World Psychiatry 2021, 20, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Whitehurst, T.; Shatalina, E.; Townsend, L.; Onwordi, E.C.; Mak, T.L.A.; Arumuham, A.; O’Brien, O.; Lobo, M.; Vano, L.; et al. The clinical significance of duration of untreated psychosis: An umbrella review and random-effects meta-analysis. World Psychiatry 2021, 20, 75–95. [Google Scholar] [CrossRef]
- Haddad, P.M.; Correll, C.U. Long-acting antipsychotics in the treatment of schizophrenia: Opportunities and challenges. Expert Opin. Pharmacother. 2023, 24, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Bodapati, A.S.; Jenkins, L.M.; Sharma, R.P.; Rosen, C. Visual memory uniquely predicts anhedonia in schizophrenia but not bipolar disorder. J. Neuropsychol. 2019, 13, 136–146. [Google Scholar] [CrossRef]
- Pillny, M.; Krkovic, K.; Buck, L.; Lincoln, T.M. From Memories of Past Experiences to Present Motivation? A Meta-analysis on the Association Between Episodic Memory and Negative Symptoms in People with Psychosis. Schizophr. Bull. 2022, 48, 307–324. [Google Scholar] [CrossRef]
- Strauss, G.P.; Gold, J.M. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry 2012, 169, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Toca, A.; Fernández-Aragón, C.; Madrigal, A.; Halverson, T.; Rodríguez-Jiménez, R.; Lahera, G. Social Cognition Mediates the Impact of Processing Speed and Sustained Attention on Global Functioning in Schizophrenia. Psicothema 2023, 35, 87–97. [Google Scholar] [CrossRef]
- Catalan, A.; Richter, A.; Salazar de Pablo, G.; Vaquerizo-Serrano, J.; Mancebo, G.; Pedruzo, B.; Aymerich, C.; Solmi, M.; González-Torres, M.; Gil, P.; et al. Proportion and predictors of remission and recovery in first-episode psychosis: Systematic review and meta-analysis. Eur. Psychiatry 2021, 64, e69. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.E.; Bouix, S.; Ongur, D.; Shenton, M.E. Neuroprogression across the Early Course of Psychosis. J. Psychiatry Brain Sci. 2020, 5, e200002. [Google Scholar] [CrossRef]
- Domschke, K. Prevention in psychiatry: A role for epigenetics? World Psychiatry 2021, 20, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Nordentoft, M.; Jeppesen, P.; Thorup, A.A.E. Prevention in the mental health field should be implemented synergically at different levels. World Psychiatry 2021, 20, 230–231. [Google Scholar] [CrossRef]
- Worthington, M.A.; Cannon, T.D. Prediction and Prevention in the Clinical High-Risk for Psychosis Paradigm: A Review of the Current Status and Recommendations for Future Directions of Inquiry. Front. Psychiatry 2021, 12, 770774. [Google Scholar] [CrossRef]
- Poletti, M.; Raballo, A. Developmental Psychotic Risk: Toward a Neurodevelopmentally Informed Staging of Vulnerability to Psychosis. Harv. Rev. Psychiatry 2020, 28, 271–278. [Google Scholar] [CrossRef]
- Pelletier, A.L.; Mittal, V.A. Negative symptom measurement in individuals at-risk for psychosis. Psychiatry Res. 2013, 205, 181–182. [Google Scholar] [CrossRef]
- Schultze-Lutter, F.; Michel, C.; Schmidt, S.J.; Schimmelmann, B.G.; Maric, N.P.; Salokangas, R.K.; Riecher-Rössler, A.; van der Gaag, M.; Nordentoft, M.; Raballo, A.; et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur. Psychiatry 2015, 30, 405–416. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Correll, C.U.; Arango, C.; Berk, M.; Patel, V.; Ioannidis, J.P.A. Preventive psychiatry: A blueprint for improving the mental health of young people. World Psychiatry 2021, 20, 200–221. [Google Scholar] [CrossRef]
- Addington, J.; Woods, S.W.; Yung, A.R.; Calkins, M.E.; Fusar-Poli, P. Harmonizing the structured interview for psychosis-risk syndromes (SIPS) and the comprehensive assessment of at-risk mental states (CAARMS): An initial approach. Early Interv. Psychiatry 2023, 1–7. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Schultze-Lutter, F.; Cappucciati, M.; Rutigliano, G.; Bonoldi, I.; Stahl, D.; Borgwardt, S.; Riecher-Rössler, A.; Addington, J.; Perkins, D.O.; et al. The Dark Side of the Moon: Meta-analytical Impact of Recruitment Strategies on Risk Enrichment in the Clinical High Risk State for Psychosis. Schizophr. Bull. 2016, 42, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Linscott, R.J.; van Os, J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: On the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol. Med. 2013, 43, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Papanastasiou, E.; Stahl, D.; Rocchetti, M.; Carpenter, W.; Shergill, S.; McGuire, P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 2015, 41, 892–899. [Google Scholar] [CrossRef] [PubMed]
- McGorry, P.D.; Nelson, B.; Markulev, C.; Yuen, H.P.; Schäfer, M.R.; Mossaheb, N.; Schlögelhofer, M.; Smesny, S.; Hickie, I.B.; Berger, G.E.; et al. Effect of ω-3 Polyunsaturated Fatty Acids in Young People at Ultrahigh Risk for Psychotic Disorders: The NEURAPRO Randomized Clinical Trial. JAMA Psychiatry 2017, 74, 19–27. [Google Scholar] [CrossRef]
- Morrison, A.P.; French, P.; Stewart, S.L.; Birchwood, M.; Fowler, D.; Gumley, A.I.; Jones, P.B.; Bentall, R.P.; Lewis, S.W.; Murray, G.K.; et al. Early detection and intervention evaluation for people at risk of psychosis: Multisite randomised controlled trial. BMJ 2012, 344, e2233. [Google Scholar] [CrossRef]
- Nelson, B.; Amminger, G.P.; Yuen, H.P.; Markulev, C.; Lavoie, S.; Schäfer, M.R.; Hartmann, J.A.; Mossaheb, N.; Schlögelhofer, M.; Smesny, S.; et al. NEURAPRO: A multi-centre RCT of omega-3 polyunsaturated fatty acids versus placebo in young people at ultra-high risk of psychotic disorders-medium-term follow-up and clinical course. NPJ Schizophr. 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Smit, F.; Furukawa, T.A. Most at-risk individuals will not develop a mental disorder: The limited predictive strength of risk factors. World Psychiatry 2021, 20, 224–225. [Google Scholar] [CrossRef]
- Reichenberg, A.; Levine, S.Z. Primary challenges and practical solutions in preventive psychiatry. World Psychiatry 2021, 20, 228–230. [Google Scholar] [CrossRef]
- Arango, C.; Dragioti, E.; Solmi, M.; Cortese, S.; Domschke, K.; Murray, R.M.; Jones, P.B.; Uher, R.; Carvalho, A.F.; Reichenberg, A.; et al. Risk and protective factors for mental disorders beyond genetics: An evidence-based atlas. World Psychiatry 2021, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Shioiri, T.; Shinada, K.; Kuwabara, H.; Someya, T. Early prodromal symptoms and diagnoses before first psychotic episode in 219 inpatients with schizophrenia. Psychiatry Clin. Neurosci. 2007, 61, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Rietdijk, J.; Hogerzeil, S.J.; van Hemert, A.M.; Cuijpers, P.; Linszen, D.H.; van der Gaag, M. Pathways to psychosis: Help-seeking behavior in the prodromal phase. Schizophr. Res. 2011, 132, 213–219. [Google Scholar] [CrossRef]
- Lång, U.; Ramsay, H.; Yates, K.; Veijola, J.; Gyllenberg, D.; Clarke, M.C.; Leacy, F.P.; Gissler, M.; Kelleher, I. Potential for prediction of psychosis and bipolar disorder in Child and Adolescent Mental Health Services: A longitudinal register study of all people born in Finland in 1987. World Psychiatry 2022, 21, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.W.; Addington, J.; Bearden, C.E.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; Mathalon, D.H.; Perkins, D.O.; Seidman, L.J.; Tsuang, M.T.; et al. Psychotropic medication use in youth at high risk for psychosis: Comparison of baseline data from two research cohorts 1998–2005 and 2008–2011. Schizophr. Res. 2013, 148, 99–104. [Google Scholar] [CrossRef][Green Version]
- Woodberry, K.A.; Seidman, L.J.; Bryant, C.; Addington, J.; Bearden, C.E.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; McGlashan, T.H.; Mathalon, D.H.; et al. Treatment Precedes Positive Symptoms in North American Adolescent and Young Adult Clinical High Risk Cohort. J. Clin. Child Adolesc. Psychol. 2018, 47, 69–78. [Google Scholar] [CrossRef]
- Keshavan, M.S. Characterizing transdiagnostic premorbid biotypes can help progress in selective prevention in psychiatry. World Psychiatry 2021, 20, 231–232. [Google Scholar] [CrossRef]
- Crouse, J.J.; Chitty, K.M.; Iorfino, F.; Carpenter, J.S.; White, D.; Nichles, A.; Zmicerevska, N.; Guastella, A.J.; Scott, E.M.; Lee, R.S.C.; et al. Modelling associations between neurocognition and functional course in young people with emerging mental disorders: A longitudinal cohort study. Transl. Psychiatry 2020, 10, 22. [Google Scholar] [CrossRef]
- Millman, Z.B.; Roemer, C.; Vargas, T.; Schiffman, J.; Mittal, V.A.; Gold, J.M. Neuropsychological Performance Among Individuals at Clinical High-Risk for Psychosis vs Putatively Low-Risk Peers with Other Psychopathology: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2022, 48, 999–1010. [Google Scholar] [CrossRef]
| Applied Assessment Scale | Studies | |
|---|---|---|
| PANSS | Negative Subscale—total score N1 Blunted Affect, N2 Emotional Withdrawal, N3 Poor Rapport, N4 Passive/Apathetic Social Withdrawal, N5 Difficulty in abstract thinking, N6 Lack of Spontaneity, and N7 stereotyped thinking | Ayres et al. 2007 [119] Hegde et al. 2013 [68] Lee et al. 2019 [110] Stouten et al. 2017 [118] Saleem et al. 2013 [122] Chan et al. 2006 [105] |
| Negative factor: N1 Blunted Affect, N2 Emotional Withdrawal, N3 Poor Rapport, N4 Passive/Apathetic Social Withdrawal, N6 Lack of Spontaneity and Flow of Conversation, G7 Motor Retardation, G13 Disturbance of Volition, G15 Preoccupation, and G16 Active Social Avoidance | Huang et al. 2016 [116] | |
| Negative factor: Negative subscale total score plus items G13 Disturbance of Volition and G16 Active Social Avoidance | Piskulic et Addington 2011 [121] | |
| Negative factor according to EPA guidance: N1 Blunted Affect, N2 Emotional Withdrawal, N3 Poor Rapport, N4 Passive/Apathetic Social Withdrawal, N6 Lack of Spontaneity, and Flow of Conversation | Engen et al. 2019 [109] | |
| SANS | Total score | Rodríguez-Sánchez et al. 2008 [117] Chan et al. 2006 [105] |
| Total score excluding attention scale | Bliksted et al. 2017 [114] Buck et al. 2020 [104] Ventura et al. 2015 [112] | |
| Negative factor according to EPA guidance excluding attention subscale, inappropriate affect, and poverty in content speech items | Chang et al. 2016 [106] | |
| Motivational Deficit: avolition–apathy and anhedonia–asociality subscales Expressive Deficit: Blunted affect subscale and Alogia subscale excluding the poverty of content of speech item | Chang et al. 2017 [107] Chang et al. 2020 [108] Wong et al. 2021 [113] | |
| Motivational Deficit: Avolition–Apathy and Anhedonia–asociality subscales Expressive Deficit: Blunted affect subscale and Alogia subscale | Ditlevsen et al. 2020 [115] | |
| Individual subscales global scores: Affective flattening, alogia, avolition, and anhedonia–asociality | Trampush et al. 2015 [111] | |
| BPRS | Negative affect Cluster: items 16 (Blunted affect), 17 (emotional withdrawal), and 18 (Motor retardation) | Mazza et al. 2012 [120] |
| HEN | Expressive Deficit: Affect, Behavior, and speech subscales | Chang et al. 2014 [78] |
| Applied Assessment Scale | Studies |
|---|---|
| SIPS: N1 Social Anhedonia, N2 Avolition, N3 Expression of Emotion, N4 Experience of Emotions and Self, N5 Ideational Richness, and N6 Occupational Functioning | Barbato et al. 2015 [126] Gerritsen et al. 2020 [127] Shin et al. 2016 [125] Lindgren et al. 2010 [128] Meyer et al. 2014 [130] Vargas et al. 2018 [132] Niendam et al. 2006 [124] |
| CAARMS | Pelizza et al. 2021 [131] |
| SANS Total score | Leanza et al. 2018 [129] |
| SANS Excluding attention | Üçok et al. 2021 [31] Glenthøj et al. 2017 [123] |
| Processing Speed | Verbal Fluency | Attention/ Vigilance | Working Memory | Verbal Learning and Memory | Visual Learning and Memory | Executive Functions | Social Cognition | |
|---|---|---|---|---|---|---|---|---|
| Significant correlations In parenthesis, relevant references | 0.282≤ r ≤−0.353 3/5 studies: -Average: [116,117] -Good: [109] | 0.282≤ r ≤−0.353 2/4 studies: -Average: [118] –Poor: [119] | −0.23 ≤ r ≤ −0.34 4/6 studies: -Poor: [68] -Average: [116,118] -Good: [105] | −0.191 ≤ r ≤ −2.98 5/8 studies: -Poor: [68,119] -Average [118] -Good [104,110] | r = −0.38 1/3 studies: -Poor: [68] | r = −0.56 1/5 studies: -Poor: [122] | −0.25 ≤ r ≤−0.49 5/8 studies: -Poor: [122] -Average [116] -Good: [105,106,109] | −0.29 ≤ r ≤ −0.41 4/6 studies: -Average: [114] -Poor [120] -Good [112] -Poor: [121] |
| Processing Speed | Verbal Fluency | Attention/ Vigilance | Working Memory | Verbal Learning and Memory | Visual Learning and Memory | Executive Functions | Social Cognition | |
|---|---|---|---|---|---|---|---|---|
| Significant correlations In parenthesis, relevant references | −0.21 ≤ r ≤ −0.40 3/4 studies: -Poor: [128,130] -Average: [31] | −0.40 ≤ r ≤ −0.46 2/3 studies: -Average: [125] -Poor: [129] | −0.26 ≤ r ≤−0.32 3/4 studies: -Average: [31] -Poor: [127,130] | −0.21≤ r ≤−0.38 2/4 studies: -Average: [31] -Poor: [127] | =−0.21≤ r ≤−0.38 4/6 studies: -Poor: [128,130,132] -Average: [125] | 0/3 studies | −0.21≤ r ≤ −0.33 3/6 studies: -Poor: [130] -Average: [31] -Poor: [130] | −0.38≤ r ≤ −0.40 2/3 studies: -Poor: [127,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melillo, A.; Caporusso, E.; Giordano, G.M.; Giuliani, L.; Pezzella, P.; Perrottelli, A.; Bucci, P.; Mucci, A.; Galderisi, S. Correlations between Negative Symptoms and Cognitive Deficits in Individuals at First Psychotic Episode or at High Risk of Psychosis: A Systematic Review. J. Clin. Med. 2023, 12, 7095. https://doi.org/10.3390/jcm12227095
Melillo A, Caporusso E, Giordano GM, Giuliani L, Pezzella P, Perrottelli A, Bucci P, Mucci A, Galderisi S. Correlations between Negative Symptoms and Cognitive Deficits in Individuals at First Psychotic Episode or at High Risk of Psychosis: A Systematic Review. Journal of Clinical Medicine. 2023; 12(22):7095. https://doi.org/10.3390/jcm12227095
Chicago/Turabian StyleMelillo, Antonio, Edoardo Caporusso, Giulia Maria Giordano, Luigi Giuliani, Pasquale Pezzella, Andrea Perrottelli, Paola Bucci, Armida Mucci, and Silvana Galderisi. 2023. "Correlations between Negative Symptoms and Cognitive Deficits in Individuals at First Psychotic Episode or at High Risk of Psychosis: A Systematic Review" Journal of Clinical Medicine 12, no. 22: 7095. https://doi.org/10.3390/jcm12227095
APA StyleMelillo, A., Caporusso, E., Giordano, G. M., Giuliani, L., Pezzella, P., Perrottelli, A., Bucci, P., Mucci, A., & Galderisi, S. (2023). Correlations between Negative Symptoms and Cognitive Deficits in Individuals at First Psychotic Episode or at High Risk of Psychosis: A Systematic Review. Journal of Clinical Medicine, 12(22), 7095. https://doi.org/10.3390/jcm12227095









