Minimally Invasive Strategy to Repair Mitral Valve after Repeated Coronary Revascularization: A Case Report and Literature Review
Abstract
1. Introduction
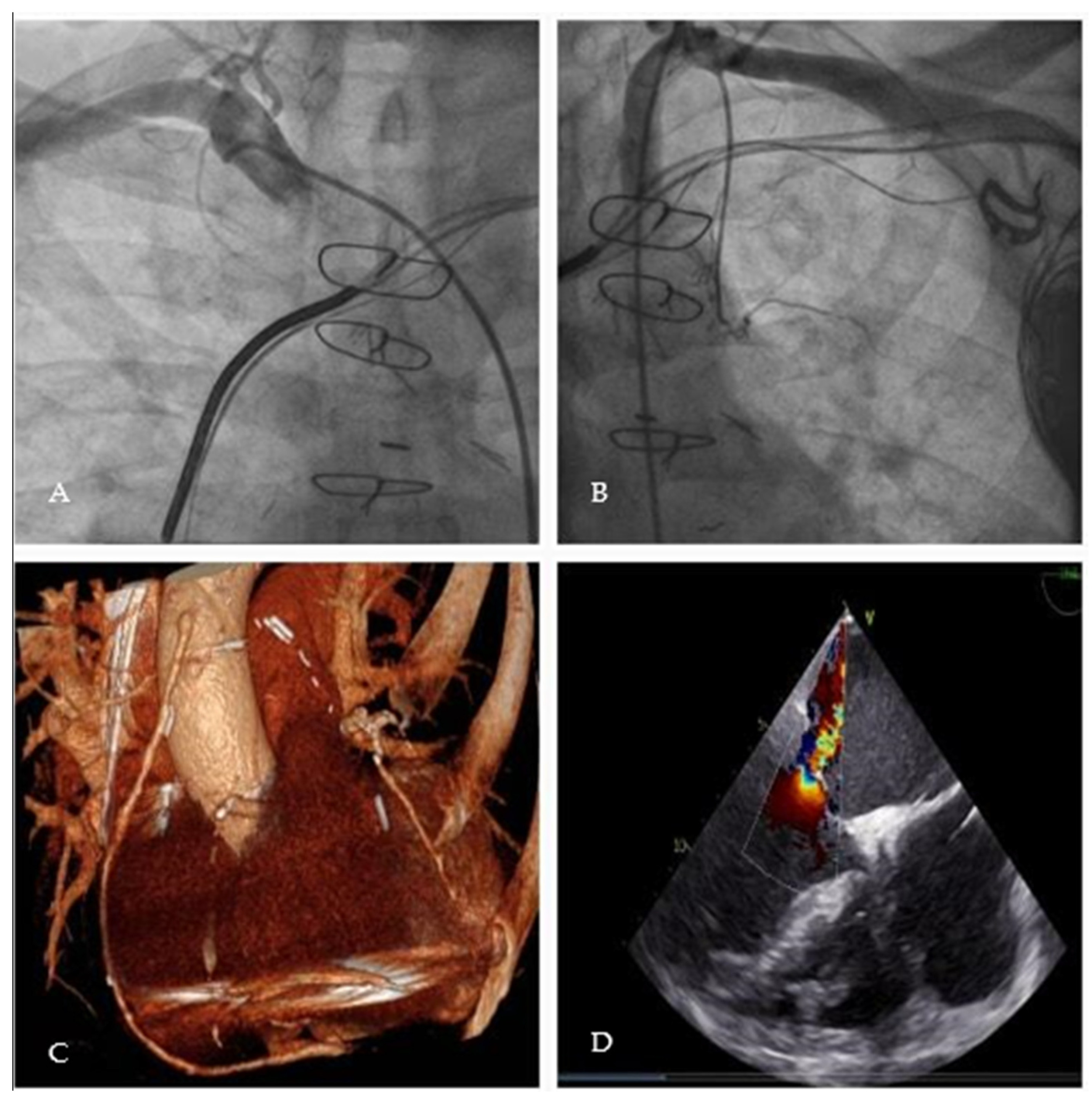
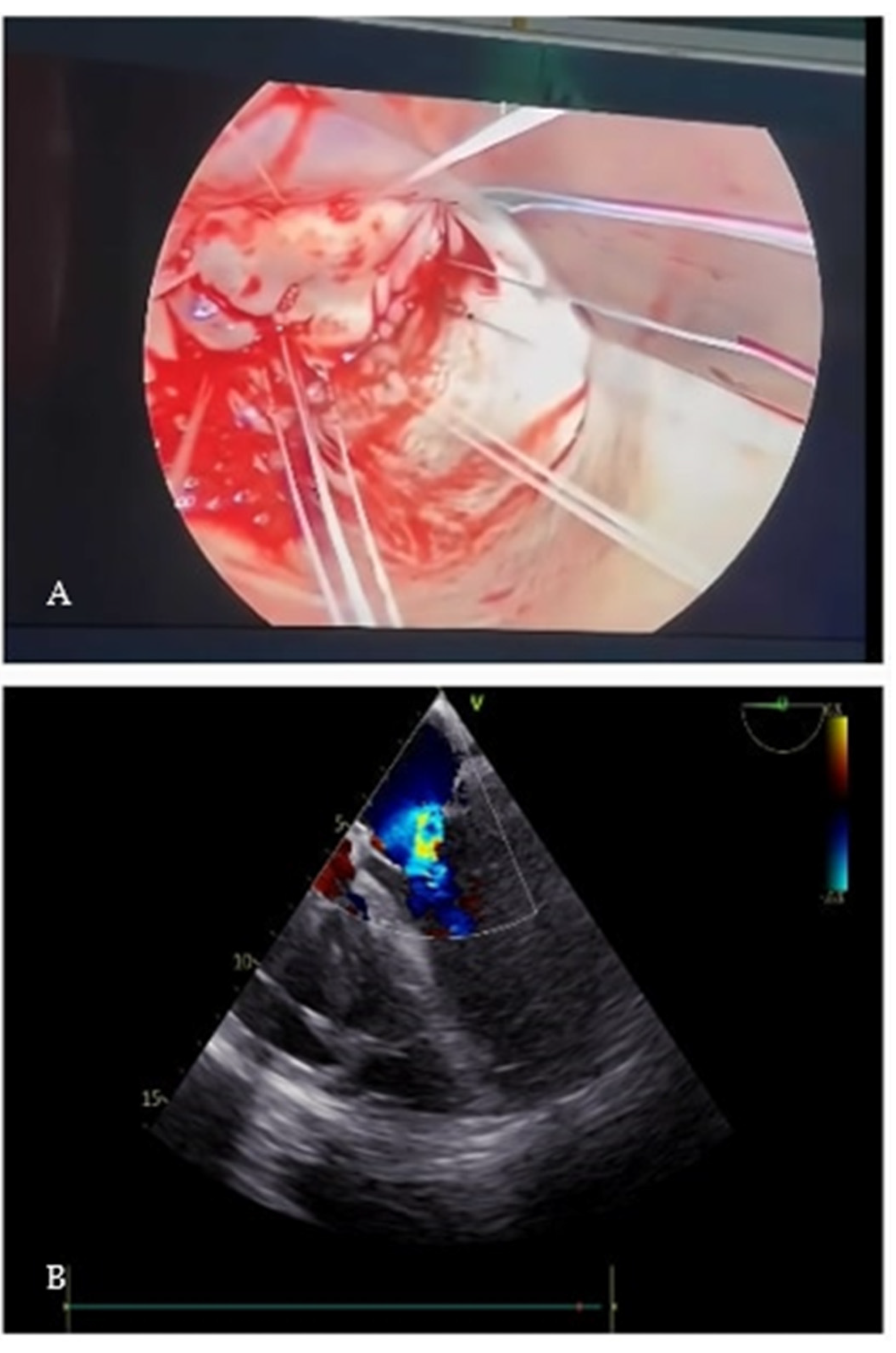
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elahi, M.; Dhannapuneni, R.; Firmin, R.; Hickey, M. Direct complications of repeat median sternotomy in adults. Asian Cardiovasc. Thorac. Ann. 2005, 13, 135–138. [Google Scholar] [CrossRef] [PubMed]
- AIFA. Luglio 2023. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_008055_023993_FI.pdf&sys=m0b1l3 (accessed on 5 July 2023).
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Clowes, G.H., Jr.; Neville, W.E.; Sancetta, S.M.; Traks, E.; Lim, K.; Barwinsky, J.; Del Guercio, L.R. Results of open surgical correction of mitral valvular insufficiency and description of technique for approach from left side. Surgery 1962, 51, 138–154. [Google Scholar]
- Carpentier, A.; Loulmet, D.; Carpentier, A.; Le Bret, E.; Haugades, B.; Dassier, P.; Guibourt, P. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. Comptes Rendus L’acad. Sci. Ser. III Sci. 1996, 319, 219–223. [Google Scholar]
- Hashim, S.W.; McMahon, S.R.; Vaitkeviciute, I.K.; Collazo, S.; Hashim, I.M.; Loya, D.S.; Takata, E.T.; Mather, J.F.; McKay, R.G. Propensity-matched comparison of right mini-thoracotomy versus median sternotomy for isolated mitral valve repair. J. Cardiovasc. Surg. 2022, 63, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; de Kroon, T.L.; Post, M.C.; Kelder, J.C.; Schut, K.F.; Saouti, N.; van Putte, B.P. Minimally invasive mitral valve surgery: A systematic safety analysis. Open Heart 2020, 7, e001393. [Google Scholar] [CrossRef]
- Grant, S.W.; Hickey, G.L.; Modi, P.; Hunter, S.; Akowuah, E.; Zacharias, J. Propensity-matched analysis of minimally invasive approach versus sternotomy for mitral valve surgery. Heart 2019, 105, 783–789. [Google Scholar] [CrossRef]
- Vo, A.T.; Nguyen, D.H.; Van Hoang, S.; Le, K.M.; Nguyen, T.T.; Nguyen, V.L.; Nguyen, B.H.; Truong, B.Q. Learning curve in minimally invasive mitral valve surgery: A single-center experience. J. Cardiothorac. Surg. 2019, 14, 213. [Google Scholar] [CrossRef]
- Monsefi, N.; Makkawi, B.; Öztürk, M.; Alirezai, H.; Alaj, E.; Bakhtiary, F. Right minithoracotomy and resternotomy approach in patients undergoing a redo mitral valve procedure. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 33–39. [Google Scholar] [CrossRef]
- Agarwal, S.; Choi, S.W.; Fletcher, S.N.; Klein, A.A.; Gill, R. The incidence and effect of resternotomy following cardiac surgery on morbidity and mortality: A 1-year national audit on behalf of the Association of Cardiothoracic Anaesthesia and Critical Care. Anaesthesia 2021, 76, 19–26. [Google Scholar] [CrossRef]
- Rappoport, N.; Aviel, G.; Shahian, D.M.; Korach, A.; Carmi, S.; Keaney, J.F., Jr.; Shapira, O.M. Resternotomy Coronary Artery Bypass 1999-2018: Insights from The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 2023, 115, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bakaeen, F.G.; Ghandour, H.; Ravichandren, K.; Pettersson, G.B.; Weiss, A.J.; Zhen-Yu Tong, M.; Soltesz, E.G.; Johnston, D.R.; Houghtaling, P.L.; Smedira, N.G.; et al. Risks and Outcomes of Reoperative Cardiac Surgery in Patients with Patent Bilateral Internal Thoracic Artery Grafts. Ann. Thorac. Surg. 2022, 114, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.G.; Karavas, A.N.; Adams, D.H.; Aklog, L.; Aranki, S.F.; Filsoufi, F.; Cohn, L.H. The preferred approach for mitral valve surgery after CABG: Right thoracotomy, hypothermia and avoidance of LIMA-LAD graft. J. Heart Valve Dis. 2001, 10, 584–590. [Google Scholar]
- Hisatomi, K.; Hashizume, K.; Tanigawa, K.; Miura, T.; Matsukuma, S.; Yokose, S.; Kitamura, T.; Shimada, T.; Eishi, K. Free-floating left atrial ball thrombus after mitral valve replacement with patent coronary artery bypass grafts: Successful removal by a right minithoracotomy approach without aortic cross-clamp. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Stoliński, J.; Musiał, R.; Plicner, D.; Andres, J. Respiratory System Function in Patients After Minimally Invasive Aortic Valve Replacement Surgery: A Case Control Study. Innovations 2017, 12, 127–136. [Google Scholar]
- Dooley, A.; Asimakopoulos, G. Does a minimally invasive approach result in better pulmonary function postoperatively when compared with median sternotomy for coronary artery bypass graft? Interact. Cardiovasc. Thorac. Surg. 2013, 16, 880–885. [Google Scholar] [CrossRef][Green Version]
- Murzi, M.; Miceli, A.; Di Stefano, G.; Cerillo, A.G.; Farneti, P.; Solinas, M.; Glauber, M. Minimally invasive right thoracotomy approach for mitral valve surgery in patients with previous sternotomy: A single institution experience with 173 patients. J. Thorac. Cardiovasc. Surg. 2014, 148, 2763–2768. [Google Scholar] [CrossRef]
- Hanafy, D.A.; Melisa, S.; Andrianto, G.A.; Suwatri, W.T.; Sugisman. Outcomes of minimally invasive versus conventional sternotomy for redo mitral valve surgery according to Mitral Valve Academic Research Consortium: A systematic review and meta-analysis. Asian J. Surg. 2023. [Google Scholar] [CrossRef]
- Daemen, J.H.T.; Heuts, S.; Olsthoorn, J.R.; Maessen, J.G.; Nia, P.S. Right minithoracotomy versus median sternotomy for reoperative mitral valve surgery: A systematic review and meta-analysis of observational studies. Eur. J. Cardiothorac. Surg. 2018, 54, 817–825. [Google Scholar] [CrossRef]
- Shirke, M.M.; Ravikumar, N.; Shawn, T.J.X.; Mutsonziwa, N.; Soh, V.; Harky, A. Mitral valve surgery via repeat median sternotomy versus right mini-thoracotomy: A systematic review and meta-analysis of clinical outcomes. J. Card. Surg. 2022, 37, 4500–4509. [Google Scholar] [CrossRef]
- Salman, J.; Fleißner, F.; Naqizadah, J.; Avsar, M.; Shrestha, M.; Warnecke, G.; Ismail, I.; Rümke, S.; Cebotari, S.; Haverich, A.; et al. Minimally Invasive Mitral Valve Surgery in Re-Do Cases-The New Standard Procedure? Thorac. Cardiovasc. Surg. 2018, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Murzi, M.; Solinas, M.; Glauber, M. Is a minimally invasive approach for re-operative mitral valve surgery superior to standard resternotomy? Interact. Cardiovasc. Thorac. Surg. 2009, 9, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Hassanabad, A.F.; Svystonyuk, D.A.; Kent, W.D.T. Minimally Invasive Beating Heart Mitral Valve Repair in a Patient with Connective Tissue Disease at Prohibitive Risk for Redo Sternotomy. Tex. Heart Inst. J. 2022, 49, e217699. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, R.; Leacche, M.; Petracek, M.R.; Kumar, S.; Solenkova, N.V.; Kaiser, C.A.; Greelish, J.P.; Balaguer, J.M.; Ahmad, R.M.; Ball, S.K.; et al. Safety of minimally invasive mitral valve surgery without aortic cross-clamp. Ann. Thorac. Surg. 2008, 85, 1544–1549; discussion 1549–1550. [Google Scholar] [CrossRef]
- Rival, P.M.; Moore, T.H.M.; McAleenan, A.; Hamilton, H.; Du Toit, Z.; Akowuah, E.; Angelini, G.D.; Vohra, H.A. Transthoracic clamp versus endoaortic balloon occlusion in minimally invasive mitral valve surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2019, 56, 643–653. [Google Scholar] [CrossRef]
- Malvindi, P.G.; Margari, V.; Mastro, F.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Labriola, C.; Carbone, C.; Paparella, D. External aortic cross-clamping and endoaortic balloon occlusion in minimally invasive mitral valve surgery. Ann. Cardiothorac. Surg. 2018, 7, 748–754. [Google Scholar]
- Kitamura, T.; Stuklis, R.G.; Edwards, J. Redo mitral valve operation via right minithoracotomy—“no touch” technique. Int. Heart J. 2011, 52, 107–109. [Google Scholar] [CrossRef]
- Grinberg, D.; Pozzi, M.; Bordet, M.; Nouhou, K.A.; Kwon, Y.J.; Obadia, J.F.; Vola, M. Minithoracotomy and Beating Heart Strategy for Mitral Surgery in Secondary Mitral Regurgitation. Thorac. Cardiovasc. Surg. 2020, 68, 462–469. [Google Scholar] [CrossRef]
- Romano, M.A.; Haft, J.W.; Pagani, F.D.; Bolling, S.F. Beating heart surgery via right thoracotomy for reoperative mitral valve surgery: A safe and effective operative alternative. J. Thorac. Cardiovasc. Surg. 2012, 144, 334–339. [Google Scholar] [CrossRef]
- Pasic, M.; Sündermann, S.; Unbehaun, A.; Kempfert, J.; Jacobs, S.; Falk, V. Beating heart mitral valve surgery: Results in 120 consecutive patients considered unsuitable for conventional mitral valve surgery. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 541–547. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.S.; Wen, B.; Zhao, W.Z.; Liu, C. Minimally invasive beating heart technique for mitral valve surgery in patients with previous sternotomy and giant left ventricle. J. Cardiothorac. Surg. 2020, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; La Canna, G.; Colombo, A.; Alfieri, O. The evolution from surgery to percutaneous mitral valve interventions: The role of the edge-to-edge technique. J. Am. Coll. Cardiol. 2011, 58, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Loulmet, D.F.; Patel, N.C.; Jennings, J.M.; Subramanian, V.A. Less invasive intracardiac surgery performed without aortic clamping. Ann. Thorac. Surg. 2008, 85, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Gössl, M.; Sorajja, P. MitraClip patient selection: Inclusion and exclusion criteria for optimal outcomes. Ann. Cardiothorac. Surg. 2018, 7, 771–775. [Google Scholar] [CrossRef]
- Debonnaire, P.; Heyning, C.M.V.; Haddad, M.E.; Coussement, P.; Paelinck, B.; de Ceuninck, M.; Timmermans, F.; De Bock, D.; Drieghe, B.; Dujardin, K.; et al. Left Ventricular End-Systolic Dimension and Outcome in Patients with Heart Failure Undergoing Percutaneous MitraClip Valve Repair for Secondary Mitral Regurgitation. Am. J. Cardiol. 2020, 126, 56–65. [Google Scholar] [CrossRef]
- Sengupta, A.; Alexis, S.L.; Zaid, S.; Tang, G.H.L.; Lerakis, S.; Martin, R.P. Imaging the mitral valve: A primer for the interventional surgeon. Ann. Cardiothorac. Surg. 2021, 10, 28–42. [Google Scholar] [CrossRef]
- Goldberg, S.L.; Meredith, I.; Marwick, T.; Haluska, B.A.; Lipiecki, J.; Siminiak, T.; Mehta, N.; Kaye, D.M.; Sievert, H.; REDUCE FMR Investigators. A randomized double-blind trial of an interventional device treatment of functional mitral regurgitation in patients with symptomatic congestive heart failure-Trial design of the REDUCE FMR study. Am. Heart J. 2017, 188, 167–174. [Google Scholar] [CrossRef]
- Rudolph, V.; Lubos, E.; Schlüter, M.; Lubs, D.; Goldmann, B.; Knap, M.; de Vries, T.; Treede, H.; Schirmer, J.; Conradi, L.; et al. Aetiology of mitral regurgitation differentially affects 2-year adverse outcomes after MitraClip therapy in high-risk patients. Eur. J. Heart Fail. 2013, 15, 796–807. [Google Scholar] [CrossRef]
- Isaacs, A.J.; Shuhaiber, J.; Salemi, A.; Isom, O.W.; Sedrakyan, A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J. Thorac. Cardiovasc. Surg. 2015, 149, 1262–1269.e3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asta, L.; Benedetto, U.; Tancredi, F.C.; Di Giammarco, G. Minimally Invasive Strategy to Repair Mitral Valve after Repeated Coronary Revascularization: A Case Report and Literature Review. J. Clin. Med. 2023, 12, 7096. https://doi.org/10.3390/jcm12227096
Asta L, Benedetto U, Tancredi FC, Di Giammarco G. Minimally Invasive Strategy to Repair Mitral Valve after Repeated Coronary Revascularization: A Case Report and Literature Review. Journal of Clinical Medicine. 2023; 12(22):7096. https://doi.org/10.3390/jcm12227096
Chicago/Turabian StyleAsta, Laura, Umberto Benedetto, Fabrizio Costantino Tancredi, and Gabriele Di Giammarco. 2023. "Minimally Invasive Strategy to Repair Mitral Valve after Repeated Coronary Revascularization: A Case Report and Literature Review" Journal of Clinical Medicine 12, no. 22: 7096. https://doi.org/10.3390/jcm12227096
APA StyleAsta, L., Benedetto, U., Tancredi, F. C., & Di Giammarco, G. (2023). Minimally Invasive Strategy to Repair Mitral Valve after Repeated Coronary Revascularization: A Case Report and Literature Review. Journal of Clinical Medicine, 12(22), 7096. https://doi.org/10.3390/jcm12227096






