Effectiveness of Lifestyle Interventions during Pregnancy on Preventing Gestational Diabetes Mellitus in High-Risk Women: A Systematic Review and Meta-Analyses of Published RCTs
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment of the Studies and Rating of Overall Evidence
2.5. Statistical Analysis
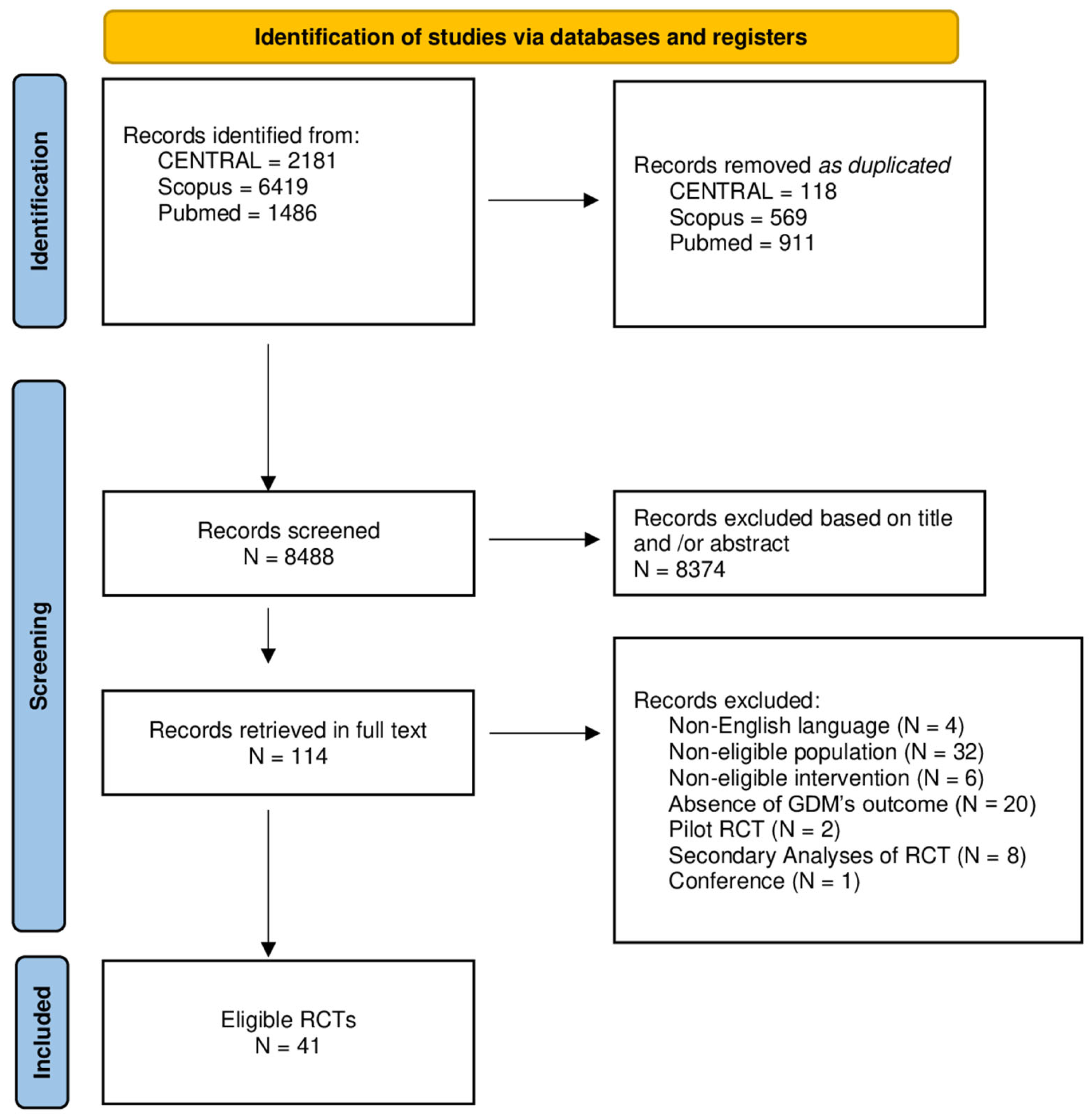
3. Results
3.1. Eligible Studies
3.2. Characteristics of Eligible Studies
4. Characteristics of Participants
4.1. Characteristics of Interventions
4.2. Description of Exercise Intervention in Eligible Trials
4.3. GDM Diagnosis
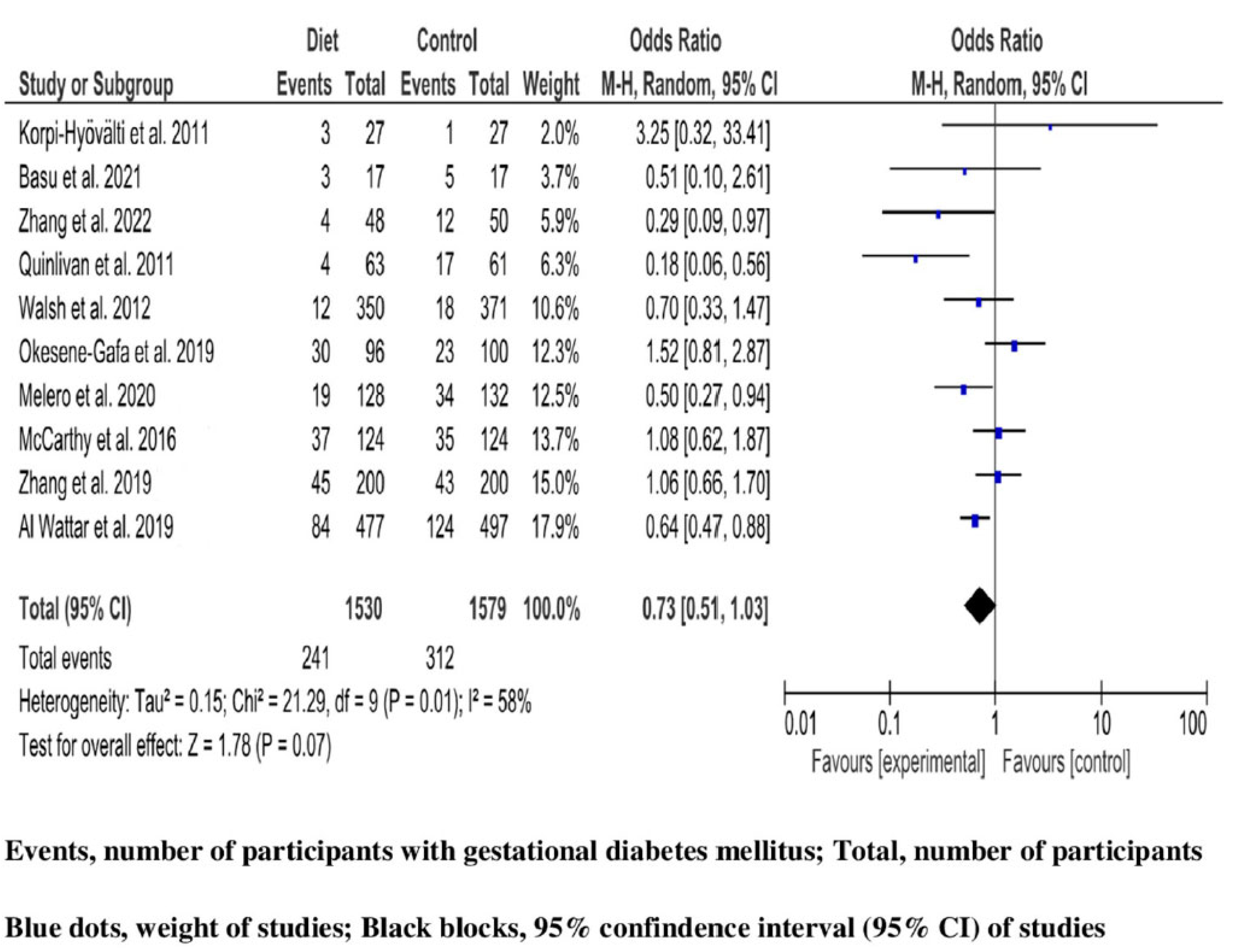
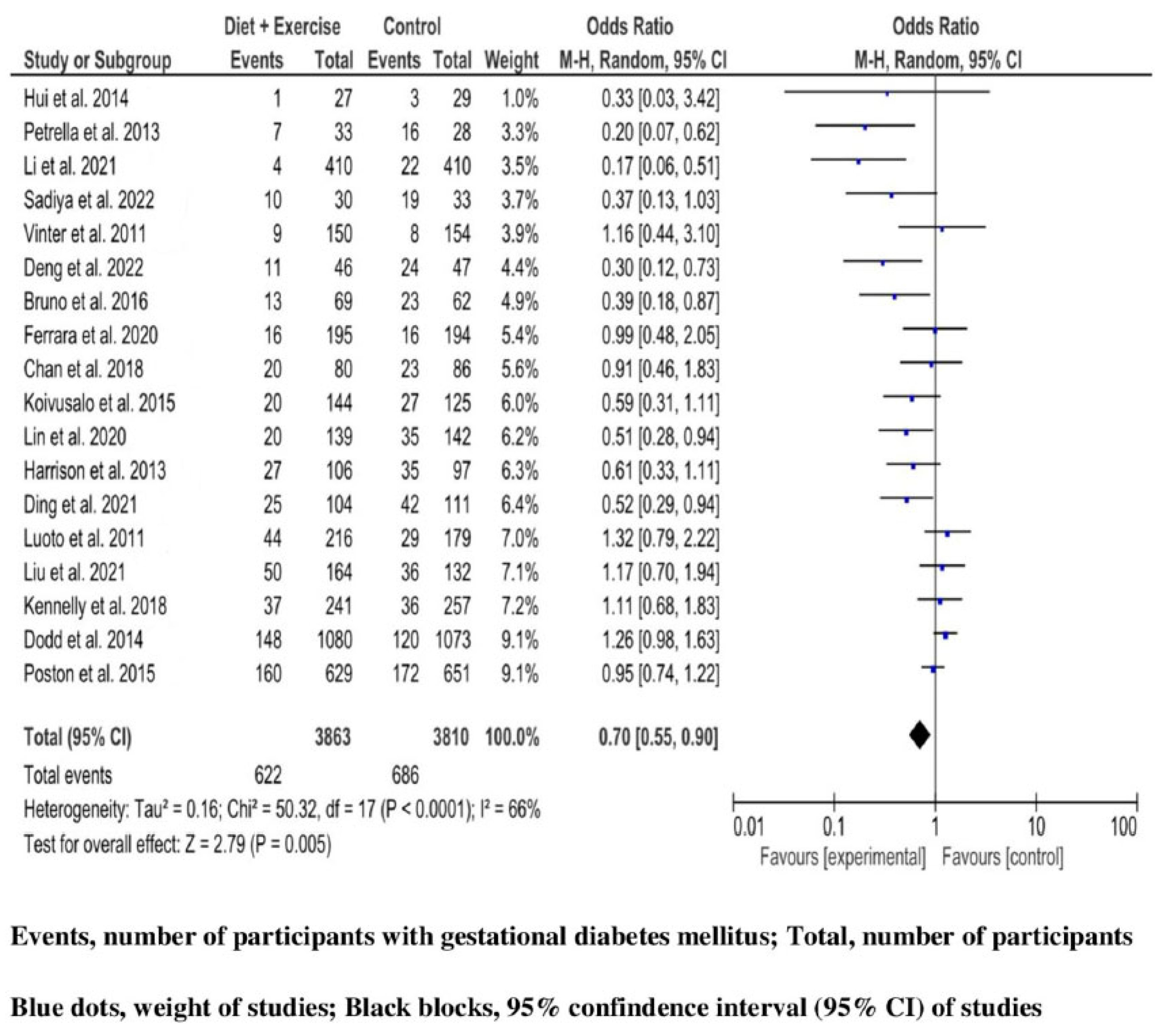
4.4. Effectiveness of Lifestyle Interventions during Pregnancy
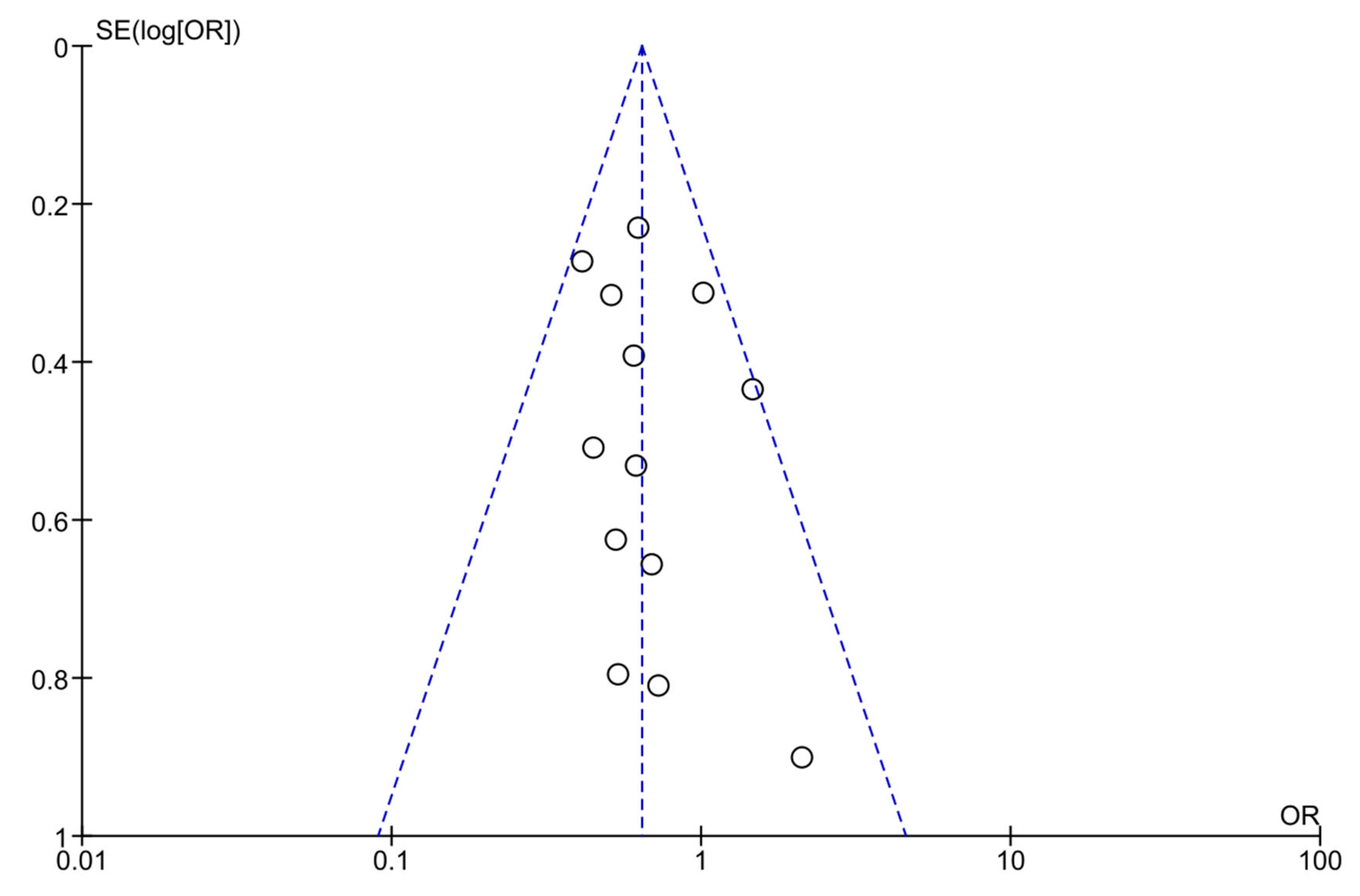
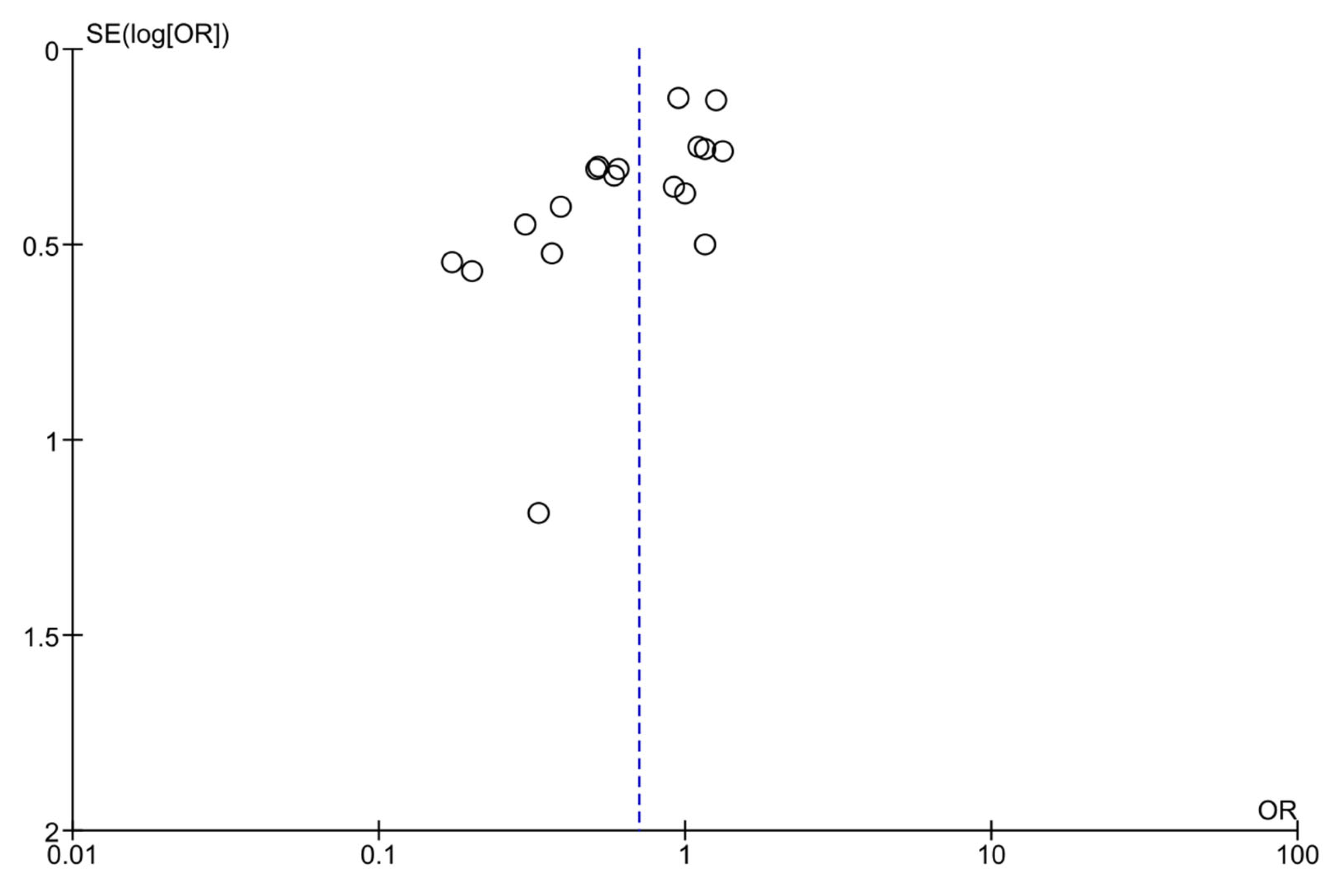
4.5. Quality of Reporting, Potential Bias, and Quality of Evidence
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Q.; Zhong, Y. Effectiveness of five interventions used for prevention of gestational diabetes: A network meta-analysis. Medicine 2022, 101, e29126. [Google Scholar] [CrossRef]
- Wei, J.; Yan, J. Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2831. [Google Scholar] [CrossRef] [PubMed]
- Melero, V.; García de la Torre, N. Effect of a Mediterranean Diet-Based Nutritional Intervention on the Risk of Developing Gestational Diabetes Mellitus and Other Maternal-Fetal Adverse Events in Hispanic Women Residents in Spain. Nutrients 2020, 12, 3505. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Feng, D. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Kinnunen, T.I. Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial. PLoS Med. 2011, 8, e1001036. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, S.B.; Rönö, K. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL). Diabetes Care 2016, 39, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Gou, B. WeChat-assisted dietary and exercise intervention for prevention of gestational diabetes mellitus in overweight/obese pregnant women: A two-arm randomized clinical trial. Arch. Gynecol. Obstet. 2021, 304, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hou, Y. Effects of Diet and Exercise Interventions to Prevent Gestational Diabetes Mellitus in Pregnant Women with High-Risk Factors in China: A Randomized Controlled Study. Clin. Nurs. Res. 2022, 31, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.M.; Nobles, C. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus. Obstet. Gynecol. 2015, 125, 576–582. [Google Scholar] [CrossRef]
- Sadiya, A.; Jakapure, V. Lifestyle intervention in early pregnancy can prevent gestational diabetes in high-risk pregnant women in the UAE: A randomized controlled trial. BMC Pregnancy Childbirth 2022, 33, 668. [Google Scholar] [CrossRef]
- Harrison, C.L.; Lombard, C.B. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: A randomized controlled trial. Obesity 2013, 21, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Tsironikos, G.I.; Perivoliotis, K. Effectiveness of exercise intervention during pregnancy on high-risk women for gestational diabetes mellitus prevention: A meta-analysis of published RCTs. PLoS ONE 2022, 17, e0272711. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.S.; Tam, W.H. Randomized trial examining effectiveness of lifestyle intervention in reducing gestational diabetes in high risk Chinese pregnant women in Hong Kong. Sci. Rep. 2018, 8, 13849. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Amiri, F.; Sepidarkish, M. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Bain, E.; Crane, M. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2015, 12, CD010443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Cheng, D.C. The effect of dietary fiber supplement on prevention of gestational diabetes mellitus in women with pre-pregnancy overweight/obesity: A randomized controlled trial. Front. Pharmacol. 2022, 13, 922015. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, Μ.A.; Ainscough, K. Pregnancy exercise and nutrition with smartphone application support. Obstet. Gynecol. 2018, 131, 818–826. [Google Scholar] [CrossRef]
- Lin, X.; Yang, T. Lifestyle intervention to prevent gestational diabetes mellitus and adverse maternal outcomes among pregnant women at high risk for gestational diabetes mellitus. J. Int. Med. Res. 2020, 48, 300060520979130. [Google Scholar] [CrossRef]
- Poel, Y.H.; Hummel, P. Vitamin D and gestational diabetes: A systematic review and meta-analysis. Eur. J. Int. Med. 2012, 23, 465–469. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Dodds, J. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Doi, S.A.R.; Furuya-Kanamori, L. Physical activity in pregnancy prevents gestational diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2020, 168, 108371. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, J. Lifestyle intervention can reduce the risk of gestational diabetes: A meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, J. Prevention of Gestational Diabetes Mellitus and Gestational Weight Gain Restriction in Overweight/Obese Pregnant Women: A Systematic Review and Network Meta-Analysis. Nutrients 2022, 14, 2383. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Sen, A. Physical activity and the risk of gestational diabetes mellitus: A systematic review and dose–response meta-analysis of epidemiological studies. Eur. J. Epidemiol. 2016, 31, 967–997. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.R.; Wu, T.W. Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials. Medicina 2018, 54, 77. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Bailey, C. Association of Antenatal Diet and Physical Activity–Based Interventions with Gestational Weight Gain and Pregnancy Outcomes a Systematic Review and Meta-analysis. JAMA Intern. Med. 2022, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Griffith, R.J.; Alsweiler, J. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 6, CD012394. [Google Scholar] [CrossRef]
- Oteng-Ntim, E.; Varma, R. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: Systematic review and meta-analysis. BMC Med. 2012, 10, 47. [Google Scholar] [CrossRef]
- Peaceman, A.M.; Clifton, R.G. Lifestyle Interventions Limit Gestational Weight Gain in Women with Overweight or Obesity: LIFE-Moms Prospective Meta-Analysis. Obesity 2018, 26, 1396–1404. [Google Scholar] [CrossRef]
- Guise, J.M.; Butler, M.E. AHRQ series on complex intervention systematic reviews—Paper 6: PRISMA-CI extension statement and checklist. J. Clin. Epidemiol. 2017, 90, 43–50. [Google Scholar] [CrossRef]
- Slade, S.C.; Dionne, C.E. Consensus on Exercise Reporting Template (CERT): Modified Delphi Study. Phys. Ther. 2016, 96, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, I4898. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.; Alhazzani, W. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Der Simonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Schmid, C.H. Heterogeneity and statistical significance in meta-analysis: An empirical study of 125 meta-analyses. Stat. Med. 2000, 19, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V. Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: West Sussex, UK, 2009; pp. 124–125, 192–200. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004, 23, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Korpi-Hyövälti, E.; Schwab, U. Effect of intensive counselling on the quality of dietary fats in pregnant women at high risk of gestational diabetes mellitus. Br. J. Nutr. 2012, 108, 910–917. [Google Scholar] [CrossRef]
- Walsh, J.M.; McGowan, C.A. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): Randomised control trial. BMJ 2012, 345, e5605. [Google Scholar] [CrossRef]
- McCarthy, E.A.; Walker, S.P. Self-weighing and simple dietary advice for overweight and obese pregnant women to reduce obstetric complications without impact on quality of life: A randomised controlled trial. BJOG 2016, 123, 965–973. [Google Scholar] [CrossRef]
- Quinlivan, J.A.; Lam, L.T. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Okesene-Gafa, K.A.M.; Li, M. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. Am. J. Obstet. Gynecol. 2019, 221, e1–e152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. Effectiveness of Low Glycemic Index Diet Consultations Through a Diet Glycemic Assessment App Tool on Maternal and Neonatal Insulin Resistance: A Randomized Controlled Trial. JMIR mHealth uHealth 2019, 7, e12081. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M. Exercise during pregnancy and gestational diabetes-related adverse effects: A randomised controlled trial. BJSM 2013, 47, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.; Farren, M. A medically supervised pregnancy exercise intervention in obese women: A randomized controlled trial. Obstet. Gynecol. 2017, 130, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.; Santos-Lozano, A. Maternal Cardiac Adaptations to a Physical Exercise Program during Pregnancy. Med. Sci. Sports Exerc. 2016, 48, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Perales, M. Supervised Exercise–Based Intervention to Prevent Excessive Gestational Weight Gain: A Randomized Controlled Trial. Mayo Clin. Proc. 2013, 88, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Garnæs, K.K.; Mørkved, S. Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial). PLoS Med. 2016, 13, e1002079. [Google Scholar] [CrossRef]
- Oostdam, N.; van Poppel, M.N. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: Results of a randomised controlled trial. BJOG 2012, 119, 1098–1107. [Google Scholar] [CrossRef]
- Nascimento, S.L.; Surita, F.G. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: A randomised clinical trial. BJOG 2011, 118, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Bisson, M.; Alméras, N. A 12-Week Exercise Program for Pregnant Women with Obesity to Improve Physical Activity Levels: An Open Randomised Preliminary Study. PLoS ONE 2015, 10, e0137742. [Google Scholar] [CrossRef] [PubMed]
- Price, B.B.; Amini, S.B. Exercise in Pregnancy: Effect on Fitness and Obstetric Outcomes—A Randomized Trial. Med. Sci. Sports Exerc. 2012, 44, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.; Marcus, B.H. Effect of an exercise intervention on gestational diabetes mellitus. Obstet. Gynecol. 2015, 125, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, K.J.; Ong, M.J. Regular Exercise to Prevent the Recurrence of Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 128, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.N.; Jiang, Y. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG 2016, 123, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, Y. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef]
- Vinter, C.A.; Jensen, D.M. The LiP (Lifestyle in Pregnancy) Study A randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011, 34, 2502–2507. [Google Scholar] [CrossRef]
- Poston, L.; Bell, R. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Petrella, E.; Malavolti, M. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J. Matern.-Fetal Neonatal Med. 2014, 27, 1348–1352. [Google Scholar] [CrossRef]
- Bruno, R.; Petrella, E. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: A randomized controlled trial. Matern. Child Nutr. 2017, 13, e12333. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L. Lifestyle Intervention for Overweight/ Obese Pregnant Women with Polycystic Ovarian Syndrome: Lessons and Challenges. Obes. Facts 2021, 14, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Wang, Y.H. Effect of individualized medical nutrition guidance on pregnancy outcomes in older pregnant women. J. Int. Med. Res. 2021, 49, 3000605211033193. [Google Scholar] [CrossRef]
- Dodd, J.M.; Turnbull, D. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014, 348, g1285. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A.; Hedderson, M.M. A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): A randomised, parallel-group, controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.L.; Back, L. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre-pregnancy Body Mass Index in a randomized control trial. BMC Pregnancy Childbirth 2014, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Tieu, J.; Shepherd, E. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2017, 1, CD006674. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Cavero-Redondo, I. Exercise versus metformin to improve pregnancy outcomes among overweight pregnant women: A systematic review and network meta-analysis. J. Clin. Med. 2021, 10, 3490. [Google Scholar] [CrossRef]
- Magro-Malosso, E.R.; Saccone, G. Exercise during pregnancy and risk of preterm birth in overweight and obese women: A systematic review and meta-analysis of randomized controlled trials. Acta Obstet. Gynecol. Scand. 2017, 96, 263–273. [Google Scholar] [CrossRef]
- Du, M.C.; Ouyang, Y.Q. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: A meta-analysis. Birth 2019, 46, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.L.; Taylor, N.F. Attitudes, barriers and enablers to physical activity in pregnant women: A systematic review. J. Physiother. 2018, 64, 24–32. [Google Scholar] [CrossRef]
- Catalano, P.; deMouzon, S.H. Maternal obesity and metabolic risk to the offspring: Why lifestyle interventions may have not achieved the desired outcomes. Int. J. Obes. 2015, 39, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Tocci, V. Maternal Preconception Body Mass Index Overtakes Age as a Risk Factor for Gestational Diabetes Mellitus. J. Clin. Med. 2023, 12, 2830. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.M.; Grivell, R.M. Systematic review of antenatal dietary and lifestyle interventions in women with a normal body mass index. Acta Obstet. Gynecol. Scand. 2016, 95, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Rogozinska, E. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; O’Brien, K.M. Gestational diabetes mellitus may be associated with increased risk of breast cancer. Br. J. Cancer 2017, 116, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Saccone, G. Incidental diagnosis of a pancreatic adenocarcinoma in a woman affected by gestational diabetes mellitus: Case report and literature review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100471. [Google Scholar] [CrossRef]
- Cade, T.J.; Polyakov, A. Implications of the introduction of new criteria for the diagnosis of gestational diabetes: A health outcome and cost of care analysis. BMJ Open 2019, 9, e023293. [Google Scholar] [CrossRef]
- Moss, J.R.; Crowther, C.A. Costs and consequences of treatment for mild gestational diabetes mellitus—Evaluation from the ACHOIS randomised trial. BMC Pregnancy Childbirth 2007, 7, 27. [Google Scholar] [CrossRef]
- Bailey, C.; Skouteris, H. Cost Effectiveness of Antenatal Lifestyle Interventions for Preventing Gestational Diabetes and Hypertensive Disease in Pregnancy. Pharmacoecon. Open 2020, 4, 499–510. [Google Scholar] [CrossRef]
- Broekhuizen, K.; Simmons, D. Cost-effectiveness of healthy eating and/or physical activity promotion in pregnant women at increased risk of gestational diabetes mellitus: Economic evaluation alongside the DALI study, a European multicenter randomized controlled trial. Int. J. Behav. Nutr. Phys. Act 2018, 15, 23. [Google Scholar] [CrossRef]
- Kolu, P.; Raitanen, J. Cost-Effectiveness of Lifestyle Counselling as Primary Prevention of Gestational Diabetes Mellitus: Findings from a Cluster-Randomised Trial. PLoS ONE 2013, 8, e56392. [Google Scholar] [CrossRef]
- Oostdam, N.; Bosmans, J. Cost-effectiveness of an exercise program during pregnancy to prevent gestational diabetes: Results of an economic evaluation alongside a randomised controlled trial. BMC Pregnancy Childbirth 2012, 12, 64. [Google Scholar] [CrossRef][Green Version]


| Name of First Author, Year of Publication | Country | Number of Participating Centers | Study Design | Study Duration (mo) | Drop-Out Rate n (%) |
|---|---|---|---|---|---|
| Korpi-Hyövälti, 2011 [40] | Finland | 1 | Parallel | ND | 6 (10.0) |
| Quinlivan, 2011 [43] | Australia | 1 | Parallel | ND | 8 (6.1) |
| Walsh, 2012 [41] | Ireland | 1 | Parallel | 48 | 41 (5.1) |
| McCarthy, 2016 [42] | Australia | 1 | Parallel | 20 | 11 (2.9) |
| Yi Zhang, 2019 [45] | China | 2 | Parallel | 40 | 31 (10.2) |
| Al Wattar, 2019 [20] | UK | 5 | Parallel | 17 | 67 (5.3) |
| Okesene-Gafa, 2019 [44] | New Zealand | 1 | 2 × 2 Factorial | 26 | 6 (2.6) |
| Melero, 2020 [3] | Spain | 1 | Parallel | 12 | 25 (8.8) |
| Basu, 2021 [4] | USA | 1 | Parallel | 23 | 11 (24.4) |
| Dong-Yao Zhang, 2022 [16] | China | 1 | Parallel | 10 | 6 (5.8) |
| Name of First Author, Year of Publication | Country | Number of Participating Centers | Study Duration (mo) | Drop-Out Rate n (%) |
|---|---|---|---|---|
| Do Nascimento, 2011 [52] | Brazil | 1 | 19 | 2 (2.4) |
| Oostdam, 2012 [51] | Netherlands | 5 | 48 | 22 (18.2) |
| Price, 2012 [54] | USA | 1 | 45 | 29 (31.9) |
| Barakat, 2013 [46] | Spain | 1 | 40 | 82 (16) |
| Ruiz, 2013 [49] | Spain | 3 | 40 | 138 (14.3) |
| Nobles, 2015 [55] | USA | 1 | 60 | 39 (13.4) |
| Bisson, 2015 [53] | Canada | 1 | 25 | 5 (10.0) |
| Seneviratne, 2015 [57] | New Zealand | 1 | 19 | 1 (1.3) |
| Perales, 2016 [48] | Spain | 1 | 49 | 99 (41.1) |
| Krohn Garnæs, 2016 [50] | Norway | 1 | 22 | 17 (18.7) |
| Guelfi, 2016 [56] | Australia | 1 | 37 | 3 (1.7) |
| Wang, 2017 [58] | China | 1 | 20 | 35 (11.7) |
| Daly, 2017 [47] | Ireland | 1 | 41 | 2 (2.3) |
| Name of First Author, Year of Publication | Country | Number of Participating Centers | Study Design | Study Duration (mo) | Drop-Out Rate n (%) |
|---|---|---|---|---|---|
| Luoto, 2011 [5] | Finland | 14 | Cluster | 14 | 43 (9.7) |
| Vinter, 2011 [59] | Denmark | 2 | Parallel | 36 | 56 (15.5) |
| Harrison, 2013 [11] | Australia | 3 | Parallel | ND | 25 (10.1) |
| Petrella, 2013 [61] | Italy | 1 | Parallel | 6 | 0 (0) |
| Dodd, 2014 [65] | Australia | 3 | Parallel | 30 | 70 (3.2) |
| Hui, 2014 [67] | Canada | 1 | Parallel | 28 | 0 (0) |
| Poston, 2015 [60] | UK | 8 | Parallel | 62 | 275 (17.7) |
| Koivusalo, 2015 [6] | Finland | 4 | Parallel | 71 | 24 (8.2) |
| Bruno, 2016 [62] | Italy | 1 | Parallel | 16 | 60 (31.4) |
| Kennelly, 2018 [17] | Ireland | 1 | Parallel | 35 | 67 (11.8) |
| Chan, 2018 [13] | China | 1 | Parallel | 24 | 63 (27.5) |
| Ferrara, 2020 [66] | USA | 5 | Parallel | 42 | 4 (1) |
| Lin, 2020 [18] | China | 1 | Parallel | 5 | 23 (7.6) |
| Li, 2021 [64] | China | 1 | Parallel | 10 | ND |
| Liu, 2021 [63] | China | 1 | Parallel | 27 | 58 (15.1) |
| Ding, 2021 [7] | China | 1 | Parallel | 10 | 15 (6.5) |
| Deng, 2022 [8] | China | 1 | Parallel | 11 | 10 (10.6) |
| Sadiya, 2022 [10] | UAE | 1 | Parallel | 22 | 7 (11.1) |
| Name of First Author, Year of Publication | Sample Size (Intervention/Control) | Mean Age (SD), yr Intervention/Control | Low Education Level, n (%) Intervention/Control | Risk Factors for GDM |
|---|---|---|---|---|
| Korpi-Hyövälti, 2011 [40] | 60 (30/30) | ND | ND | At least one of (1) advanced maternal age (>40 years), (2) overweight or obesity (BMI > 25), (3) family history of DM, (4) history of GDM or history of macrosomia |
| Quinlivan, 2011 [43] | 132 (67/65) | 28.3/29.5 | ND | Overweight (BMI 25–29.9) or obesity (BMI > 29.9) |
| Walsh, 2012 [41] | 800 (394/406) | 32.0 (4.2)/32.0 (4.2) | ND | History of macrosomia |
| McCarthy, 2016 [42] | 382 (190/192) | 31.9 (4.6)/31.8 (4.6) | 20 (10.5)/15 (7.8) | Overweight or obesity (BMI ≥ 25) |
| Yi Zhang,2019 [45] | 400 (200/200) | 28.1 (3.6)/28.0 (3.7) | ND | Overweight or obesity (BMI ≥ 24) |
| Al Wattar, 2019 [20] | 1252 (627/625) | 31.4 (5.2)/30.9 (5.2) | ND | At least one of (1) obesity (BMI ≥ 30), (2) raised serum triglycerides (≥1.7 mmol/L), (3) chronic HY (systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg) |
| Okesene-Gafa, 2019 [44] | 230 (116/114) | 29.8 (5.7)/27.8 (5.5) | 35 (30.2)/34 (29.8) | Obesity (BMI ≥ 30) |
| Melero, 2020 [3] | 285 (143/142) | 31.7 (5.4)/31.3 (5.6) | 18 (12.6)/28 (19.7) | Hispanic origin |
| Basu, 2021 [4] | 45 (22/23) | 27.0 (5.3)/27.0 (5.0) | ND | Obesity (BMI ≥ 30) AND at least one of (1) family history of DM, (2) history of GDM |
| Dong-Yao Zhang, 2022 [16] | 104 (52/52) | 31.1 (4.2)/30.0 (4.0) | ND | Overweight or obesity (BMI ≥ 24) |
| Name of First Author, Year of Publication | Sample Size (Intervention/Control) | Mean Age (SD), yr Intervention/Control | Low Education Level, n (%) Intervention/Control | Risk Factors for GDM |
|---|---|---|---|---|
| Do Nascimento, 2011 [52] | 82 (40/42) | 29.7 (6.8)/30.9 (5.9) | 6 (15)/10 (23.8) | Overweight (BMI 26.0–29.9) or obesity (BMI ≥ 30.0) |
| Oostdam, 2012 [51] | 121 (62/59) | 30.1 (4.5)/30.8 (5.2) | 16 (34)/17 (34.7) | Overweight (BMI ≥ 25) or obesity (BMI ≥ 30.0) AND at least one of (1) family history of DM, (2) history of GDM, (3) history of macrosomia |
| Price, 2012 [54] | 91 (43/48) | 30.5 (5)/27.6 (7.3) | ND | Sedentary lifestyle: no aerobic exercise more than once per week for at least the past six months |
| Barakat, 2013 [46] | 510 (255/255) | 31 (3)/31 (4) | 54 (24.7)/75 (34.4) | Sedentary lifestyle: not exercising more than 20 min on more than 3 days/week |
| Ruiz, 2013 [49] | 962 (481/481) | 31.6 (6.4)/31.9 (4) | 211 (43.9)/183 (38.3) * | Sedentary lifestyle: not exercising more than 20 min on more than three days/week |
| Nobles, 2015 [55] | 290 (143/147) | Range 18–40 | 26 (22.2)/31 (27.4) | One of overweight or obesity (BMI ≥ 25) AND family history of DM, (2) history of GDM |
| Bisson, 2015 [53] | 50 (25/25) | 30.5 (3.7)/31.0 (4.0) | 10 (40)/9 (36) | Obesity (BMI ≥ 30.0) |
| Seneviratne, 2015 [57] | 75 (38/37) | ND | ND | Overweight or obesity (BMI ≥ 25) |
| Perales, 2016 [48] | 241 (120/121) | 31 (4)/31 (4) | 29 (24)/30 (25) | Sedentary lifestyle: not exercising regularly more than 30 min on three days/week |
| Krohn Garnæs, 2016 [50] | 91 (46/45) | 31.3 (3.8)/31.4 (4.7) | 1 (2.3)/3 (7.0) | Overweight or obesity (BMI ≥ 28.0) |
| Guelfi, 2016 [56] | 172 (85/87) | 33.6 (4.1)/33.8 (3.9) | ND | History of GDM |
| Wang, 2017 [58] | 300 (150/150) | 32.1 (4.6)/32.5 (4.9) | 31 (20.7)/40 (26.7) | Overweight or obesity (BMI ≥ 24.0) |
| Daly, 2017 [47] | 88 (44/44) | 30.0 (5.1)/29.4 (4.8) | ND | Obesity (BMI ≥ 30.0) |
| Name of First Author, Year of Publication | Sample Size (Intervention/Control) | Mean Age (SD), yr Intervention/Control | Low Education Level, n (%) Intervention/Control | Risk Factors for GDM |
|---|---|---|---|---|
| Luoto, 2011 [5] | 442 (246/196) | 29.5 (4.8)/30.0 (4.7) | 73 (33.8)/59 (33.7) | At least one of (1) advanced maternal age (≥40 years), (2) overweight or obesity (BMI ≥ 25), (3) family history of DM, (4) history of GDM or history of macrosomia |
| Vinter, 2011 [59] | 360 (180/180) | 29 (Range 27–32)/29 (Range 26–31) | 39 (26.0)/54 (35.0) | Obesity (BMI 30.0–45) |
| Harrison, 2013 [11] | 228 (121/107) | 32.4 (4.6)/31.7 (4.5) | 20 (16)/12 (11) | Overweight (BMI ≥ 25 or ≥23 if high-risk ethnicity (Polynesian, Asian, African) or obesity (BMI ≥ 30.0) |
| Petrella, 2013 [61] | 61 (33/28) | 31.5 (4.2)/32.4 (5.9) | 11 (33.3)/13 (43.3) | Overweight or obesity (BMI ≥ 25) |
| Dodd, 2014 [65] | 2202 (1105/1097) | 29.3 (5.4)/29.6 (5.6) | ND | Overweight or obesity (BMI ≥ 25) |
| Hui, 2014 [67] | 113 (57/56) | 31.0 (4.0)/32.0 (5.0) | ND | Overweight or obesity (BMI ≥ 25) |
| Poston, 2015 [60] | 1555 (783/772) | 30.5 (5.5)/30.4 (5.6) | ND | Obesity (BMI ≥ 30.0) |
| Koivusalo, 2015 [6] | 293 (155/138) | 32.3 (4.9)/32.6 (4.5) | 4 (3)/3 (2) | At least one of (1) obesity (BMI ≥ 30.0), (2) history of GDM |
| Bruno, 2016 [62] | 191 (96/95) | 31.5 (5.0)/30.8 (5.5) | 39 (40.6)/35 (35.9) | Overweight or obesity (BMI ≥ 25) |
| Kennelly, 2018 [17] | 565 (278/287) | 32.8 (4.6)/32.1 (4.2) | 7 (2.7)/6 (2.2) | Overweight or obesity (BMI 25.0–39.9) |
| Chan, 2018 [13] | 229 (118/111) | 33.2 (4.4)/33.1 (4.1) | 31 (38.8)/41 (47.7) | At least one of (1) advanced maternal age (≥35 years), (2) overweight or obesity (BMI ≥ 25), (3) family history of DM, (4) history of GDM or history of macrosomia |
| Ferrara, 2020 [66] | 398 (200/198) | 32.4 (4.1)/32.6 (4.3) | 10 (5)/12 (6) | Overweight or obesity (BMI 25.0–40.0) |
| Lin, 2020 [18] | 304 (152/152) | 31.4 (4.9)/31.8 (5.1) | ND | At least one of (1) advanced maternal age (≥35 years), (2) overweight or obesity (BMI ≥ 25), (3) family history of DM, (4) history of GDM (5) history of PCOS |
| Li, 2021 [64] | 820 (410/410) | 37.5 (3.5)/38 (2.5) | ND | Older pregnant women |
| Liu, 2021 [63] | 384 (192/192) | 31.8 (3.4)/32.3 (3.8) | ND | Overweight or obesity (BMI ≥ 25) AND history of PCOS |
| Ding, 2021 [7] | 230 (114/116) | 30.6 (2.8)/30.1 (2.7) | ND | Overweight or obesity (BMI ≥ 24) |
| Deng, 2022 [8] | 94 (47/47) | 29.6 (3.9)/29.6 (3.4) | ND | At least one of (1) advanced maternal age (≥35 years), (2) overweight or obesity, (3) family history of DM, (4) history of GDM, (5) history of macrosomia, (6) history of PCOS, (7) history of abnormal lipid metabolism, (8) elevated FPG in early pregnancy (FPG ≥ 5.1 mmol/L) |
| Sadiya, 2022 [10] | 63 (30/33) | 32.8 (4.1)/30.8 (5.2) | 3 (10)/6 (18) | High-risk ethnic group (Middle Eastern, Southern Asian) AND at least two of (1) obesity (BMI ≥ 30), (2) family history of DM, (3) history of GDM, (4) history of macrosomia, (5) history of PCOS |
| Name of First Author, Year of Publication | Provider | Duration of Intervention | Description of Dietary Intervention | Side Effects/Adverse Events (Intervention/Control) |
|---|---|---|---|---|
| Korpi-Hyövälti, 2011 [40] | Clinical nutritionist | From GW 8–12 until GW 36–40 | Delivery: Individual dietary advice, four times in the first and second trimesters, and two times in the third trimester. Components: Food record before the first appointment at GW 8–12. Repetition of food record procedure before fifth and sixth appointment at GW 26–28, and 36–40, respectively. Nutrition rich in fruits, vegetables, and berries, consumption of fat-free and low-fat dairy products, vegetable oils, and wholegrain products. Daily energy intake of 126 kJ/kg and 105 kJ/kg for normal-weight, and for overweight women, respectively, divided into carbohydrate 50–55 E%, fat 30 E%, saturated fat < 10 E%, protein 15–20 E%, and dietary fiber at least 15 g/4184 kJ (1000 kcal). Motivation: Informative. Assessment: Questionnaires, nutrient calculation software, food records analyses. | ND |
| Quinlivan, 2011 [43] | Food technologist | ND | Delivery: Dietary intervention at antenatal visits with weighing, and continuous care provider. Components: Increasing consumption of fresh vegetables, fresh fruit, home-cooked main meal, and water; decreasing consumption of carbonated drinks, sports drinks, commercial fruit juices, and fresh fast foods. Motivation: Psychological assessment and intervention if necessary. Assessment: ND | Preterm delivery (1/1), acute polyhydramnios (0/1) |
| Walsh, 2012 [41] | Dietitian | From GW 15 ± 3 until GW 34 | Delivery: Dietary sessions in a group of two to six women, lasting 2 h. Components: Firstly, general advice on guidelines of healthy eating during pregnancy. Recommended low-GI eucaloric diet, and not reducing total caloric intake. Focus of education sessions on the GI. Encouragement to choose many low-GI foods instead high-GI foods. Motivation: Answers to any dietary queries of participants, written information regarding low-GI nutrition. Assessment: Food diaries for estimation of the GI, questionnaires for assessment of adherence to the low-GI diet. | Stillbirths (1/0) |
| McCarthy, 2016 [42] | Midwives | From GW < 20 until GW 36 | Delivery: Individual sessions provided by research midwife of approximately 30 min at subsequent antenatal appointments. Components: Nutrition advice, encouragement to record weight, and discussion of weight gain with doctors and/or midwives. Motivation: N/a Assessment: Self-weight record in light indoor clothing, questionnaires | Early pregnancy loss (2/1) |
| Yi Zhang,2019 [45] | Dietitians | From GW ≤ 16 until GW 34–36 | Delivery: Individualized dietary consultation by separate dietitians on a different day. Components: Individualized diet planning for achievement of a low-GI goal with consideration of individual food preference. Participants nutrition assessment through 24 h food recall intake of the nearest working day. Also, diet GI and GL calculations in the diet assessment. Equipment of a mobile phone app DietGI with the function of calculating GI and GL for every selected food. Motivation: Counseling about substituting high-GI with low-GI foods, cooking techniques, and combining foods in meals. Assessment: Worksheet for diet records, and customized excel worksheet for calculation of GI and GL. | Miscarriage (5/4) |
| Al Wattar, 2019 [20] | Dietitian, trained researchers | From GW 18 until GW 28 | Delivery: Three sessions: a personalised session at GW 18, and two sessions in a group at GW 20 and 28, using presentations. In addition, permission for presence women’s spouses. Components: Firstly, 24 h food recall and consumption needed to follow a Mediterranean pattern of diet. A book providing cooking advice on Mediterranean diet. Diet with increased consumption of fruit, vegetables, non-refined grains, legumes, nuts, and EVOO; moderate to high fish intake; low to moderate poultry and dairy products consumption; low red and processed meat intake; and avoidance of sugary drinks, fast food, and animal fat. Provision of mixed nuts and EVOO as main sources of fat. Motivation: Follow-up telephone calls Assessment: Number of attended sessions, questionnaires | None reported |
| Okesene-Gafa, 2019 [44] | Dietitian, community health workers | From GW 12+0–17+6 until delivery | Delivery: Four education sessions at home. Development of a manual provided by a dietitian. Components: A HUMBA handbook with advice on healthy nutritious foods, unhealthy drinks, and recipes. Behavioural change techniques promoting healthy diet, and setting SMARTER goals. Motivation: Feedback, positive reinforcement for goals achieved, motivational text messages Assessment: A personal pregnancy weight-gain chart, number of attended sessions, questionnaires. | Fetal death (1/3) |
| Melero, 2020 [3] | Dietitian | From GW 8–12 until delivery | Delivery: Four clinic-based visits of participants. Components: Counseling on Mediterranean diet, and increased consumption of EVOO and nuts; daily consumption of EVOO ≥ 40 mL, and pistachios 25–30 g for at least 3 days weekly. Free provision of 10 L of EVOO, and 2 kg of pistachios during the first and second visit. Advice against consumption of alcohol and juices. Motivation: Free of cost supplies Assessment: Questionnaires, MEDAS for assessment adoption of Mediterranean Diet; MEDAS score 0–12 points, without consideration of alcohol and juice. | ND |
| Basu, 2021 [4] | Dietitian, nurse practitioner | From GW < 20 until GW 32–36 | Delivery: Three clinic-based visits of participants: one at baseline, GW < 20, and two subsequent visits in the second, and third trimesters at GW 24–28 and 32–36, respectively. Components: Maintenance of habitual diet throughout the study (280 g daily food intake of 160 kcal divided into 38 g total carbohydrates, 8 g total fiber, 8 mg vit C, 3 mg sodium, 168 mg K, 1600 mg total polyphenols, and 700 mg anthocyanins). Consumption of two cups of frozen blueberries in mid-morning, or afternoon, or evening as a snack by itself and not in combination with any other food items. Intake of 12 g soluble fiber daily from meals. No consumption of fruit juice. Motivation: Free of cost food supplies, education, telephone calls Assessment: Return of any unconsumed supplementation, 24 h food recalls, nutrition calculation software. | Miscarriage (1/1) |
| Dong-Yao Zhang, 2022 [16] | Νutritionists | From GW 20+0–24+6 until delivery | Delivery: Dietary education and advice according the Chinese Dietary Guidelines for pregnancy. Components: Participants recalls of amount and frequency consumption of each food in the last five weeks, at GW 20 and 25. In addition to the above guidelines, consumption of soluble powder of fiber twice daily at the total amount of 12 g (energy, 51.93 kcal; carbohydrates, 3.31 g; fiber, 9.78 g). Motivation: Presentations Assessment: Questionnaires, Chinese Food Composition Table for evaluation of mean daily total energy intake | Dietary fiber intolerance (2/0), loss of pregnancy (0/1) |
| Name of First Author, Year of Publication | Provider | Duration of Intervention | Description of Exercise Intervention | Side Effects/Adverse Events (Intervention/Control) |
|---|---|---|---|---|
| Do Nascimento, 2011 [52] | Physiotherapist | From GW 14–24 until GW 36 | Delivery: Individualized or in a group, supervised exercise program of weekly classes. Components: PA of light to moderate intensity with HR less than 140 beats per min, according to the ACOG recommendations (2002). Total duration of 40 min: stretching for 10 min, strengthening exercises including lower and upper limb muscles for 22 min, and relaxing for 10 min. In addition, home-based exercise five times weekly, or walking. Motivation: Counseling Assessment: Self-reported exercise journal | None reported |
| Oostdam, 2012 [51] | Physiotherapist | From GW 15 until delivery | Delivery: Individualized, supervised exercise program. Components: Light intensity beginning with a warm-up period for 5–10 min. Next, program’s core for 40 min consisting of aerobic and strength exercises. Finally, a cool-down period for 5–10 min. Motivation: Information about the benefits of exercise in pregnancy. Assessment: Accelerometer for moderate and vigorous activity, METs with cut-off values from the ACSM statement | None reported |
| Price, 2012 [54] | Researchers | From GW 12–14 until GW 36 or until delivery if participants wished | Delivery: Both individualized and in a group, supervised exercise program performed four times per week. Components: Aerobic PA of moderate intensity for of 45–60 min, according to the ACOG guidelines. Activities: performance of step aerobics (first day), walking in a group over hilly terrain (second day), training circuit (third day), and individual walking for 30 to 60 min (fourth day). Substitution with weight training for 1–10 min, after performing aerobic exercise with an equal time interval. End of sessions with stretches of lower limb muscles for 5 min. Motivation: N/a Assessment: Questionnaires, RPE on the Borg Scale, produced power during the timed walking, documentation of temperature, sit-and-reach test. | Anxiety with exercise (1/0), history of preterm pregnancy (1/0), pain from leiomyomas (1/0) |
| Barakat, 2013 [46] | Fitness specialist; obstetrician | From GW 10–12 until GW 38–39 | Delivery: In a group of 10–12 women, supervised exercise program for three sessions per week (Monday, Wednesday, and Friday). Components: Aerobics, strength and flexibility exercises for 50–55 min, according to the ACOG guidelines. A gradual warm-up and a cool-down period preceding and following main part, respectively, both for 10–12 min. Main session of 25–30 min, including resistance exercises of moderate intensity. In addition, aerobic dance at one session per week, in sections of three to four min with one min breaks including stretching and relaxation activities. Motivation: Sessions accompanied with music, and performance in an airy, well-lighted exercise room at the Hospital. Assessment: HR monitors during the training sessions, Borg’s Scale. | Premature labour (5/3), pregnancy-induced HY (5/4), persistent bleeding (3/0), molar pregnancy (0/3) |
| Ruiz, 2013 [49] | Researchers | From GW 9 until GW 38–39 | Delivery: In a group of 8–10 women, supervised exercise program of three times a week (Monday, Wednesday, and Friday). Components: Aerobic and unaerobic PA of light to moderate intensity for 50–55 min per session. In the beginning, a warm-up period of light intensity for 10-min. Afterward, main part for 25 to 30 min, including aerobic exercises once a week (usually on Friday), and resistance exercises twice a week (usually on Monday and Wednesday). In the end, a cool-down period of light intensity for 10-min. Motivation: N/a Assessment: HR monitors, RPE on the Borg Scale | Threat of premature delivery (14/11), persistent bleeding (7/9) |
| Nobles, 2015 [55] | Health educators | 10 GW on average | Delivery: N/a both design (individualized or in a group), and guidance. Performance on most days of the week. Components: Self-selected specific activities (i.e., dancing, walking, and yard work) of moderate-intensity, for 30 min at least. Motivation: Telephone calls, emails Assessment: Questionnaires, and responses-based individual manual. | Medical contraindication (3/1), miscarriage or termination (1/2) |
| Bisson, 2015 [53] | Kinesiologists | From GW 15 until GW 27 | Delivery: Individualized, supervised exercise program, three times weekly. Components: Consistent with the ACSM Guidelines, moderate-intensity exercise program, and duration of 1 h per session. Session’s content: a warm-up period on a stationary ergocycle for 5–10 min, treadmill walk for 15–30 min, strength PA for 20 min, and finally, relaxing. Motivation: Pamphlet, kinesiologists always available for counseling, modification of the resistance exercises, counseling Assessment: Number of completed sessions, HR monitors, exercise log, RPE on the Borg Scale, accelerometer, questionnaires, METs | None reported |
| Seneviratne, 2015 [57] | Exercise physiologist | From GW 20 until GW 35 | Delivery: Individualized, unsupervised home-based exercise program. Varied frequency and duration, according to stage of pregnancy, between three and five sessions per week, and 15 and 30 min per session, respectively. Components: Home-based exercise sessions of moderate intensity. A warm-up and a cool-down period of low intensity for 5 min, and stationary cycling as main session’s part of moderate intensity. Motivation: Support by an available exercise physiologist. Assessment: HR monitors | None reported |
| Perales, 2016 [48] | Fitness instructors | From GW 9–11 until GW 38–39 | Delivery: In a group of 8–10 women, supervised exercise program, sessions thrice weekly (Monday, Wednesday, and Friday). Components: Session’s duration of 55–60 min. Same structure of all sessions; in the beginning a warm-up period for 5 to 7 min, next aerobic and resistance PA of moderate intensity for 25–30 min, and finally a cool-down period for 5 to 10 min. Motivation: N/a Assessment: HR monitors, resting HR values. | Risk of preterm delivery (4/5), obstetric complications (3/4) |
| Krohn Garnæs, 2016 [50] | Physiotherapist | From GW 12–19 until delivery | Delivery: In a group, supervised exercise sessions, thrice weekly. Components: PA program for 60 min; aerobic PA of moderate intensity for 35 min, and strength PA for 25 min. Additionally, home-based exercise program at least once weekly for 50 min; endurance training for 35 min, and strength exercises for 15 min. Motivation: Encouragement, and motivational interview individually or in a group. Assessment: Self-reporting in a training diary, Borg Scale, and individually adjusted resistance training. | None reported |
| Guelfi, 2016 [56] | Exercise physiologist | From GW 14 until GW 28 | Delivery: Individualized, supervised sessions of stationary cycling at home, three times per week. Components: Beginning with a warm up period for 5 min. Main part of session with periods of continuous cycling of moderate intensity for 5 min, and periods of interval cycling for 5 min; two types of intervals: an increase in pedaling rate for 15 sec, and an increase in cycling resistance for 30 sec repeated every 2 min. Finish with a cool-down period for 5 min. Motivation: N/a Assessment: Accelerometer, questionnaires | Pregnancy loss (1/2) |
| Wang, 2017 [58] | Researchers | From GW < 12+6 until GW 36–37 (27 ± 2 weeks) | Delivery: Supervised cycling program, at least three times per week. Components: Progressively increased PA according to individual ability. Exercise of low intensity in the beginning of the intervention, cycling for 30 min. Continuous increase in the duration until 45–60 min by adding 5 min to the cycling phases of moderate intensity or to the intervals of cycling. A warm-up and cool-down period for 5 min of each one, in the beginning, and in the end of sessions, respectively. Motivation: N/a Assessment: Questionnaires, RPE on the Borg Scale, records | Cervical length < 25 mm (1/5), low-lying placenta (1/0), ankle sprain (1/0), malformation (0/1), fetal death in utero (0/1) |
| Daly, 2017 [47] | Researchers | From GW 13+4/7 ± 1+2/7 until 6 weeks postpartum | Delivery: In a group, supervised exercise program, on a choice of performance day and h. Components: Exercise program for 50–60 min with a warm-up period for 10 min, aerobic resistance exercise both for 15–20 min, and a cool-down period for 10 min. Motivation: Secret Facebook group, SMART goal-setting, classes in a group, journaling, choice of day and h, variance of classes each day, and assurance of childcare during classes. Assessment: HR monitoring, and Borg Scale, according to the ACOG guidelines. | None reported |
| First Author, Publication Year | Provider | Duration of Intervention | Description of Diet Intervention | Description of Exercise Intervention | Side Effects/Adverse Events (Intervention/Control) |
|---|---|---|---|---|---|
| Luoto, 2011 [5] | Nurses | From GW 8–12 until GW 37 | Delivery: Dietary counseling session at four visits. Components: Advice on consuming vegetables, fruits, and berries, preferably at least five portions (400 g) a day; selecting mostly high-fiber bread (>6 g fiber/100 g) and other wholemeal products; selecting mostly fat-free or low-fat versions of milk and milk products and of meat and meat products; eating fish at least twice per week (excluding the fish species not recommended for pregnant women); using moderate amounts of soft table spreads on bread, oil-based salad dressing in salad, and oil in cooking and baking; consuming seldom and only in small portions foods high in fat; and consuming seldom and only in small-portions snacks containing high levels of sugar and/or fat Motivation: Counseling cards Assessment: Participants’ notebooks, questionnaires | Delivery: Monthly thematic exercise program in a group Components: Progressive increase in minimum weekly resting time PA dose at 800 MET min, including light-intensity PA. Maximum of 750 MET min of moderate-intensity PA. Motivation: PA counseling Assessment: Self-reports, questionnaires, METs | Miscarriages (6/8) |
| Vinter, 2011 [59] | Dietitians, physiotherapists | From GW 10–14 until GW 34–36 | Delivery: Dietary counseling on four sessions at GW 15, 20, 28, and 35. Components: Dietary advice based on the official Danish recommendations; participants’ individually estimated energy requirements, according to weight and level of activity. Motivation: Encouragement Assessment: Online computer program | Delivery: In a group, supervised exercise program of 1 h weekly. Components: Aerobic; low-step, resistance; light weights, elastic bands, and balance exercises. In addition, advice for being moderately physically active 30–60 min daily. Motivation: Encouragement, free full-time membership in a fitness center Assessment: Bench platform, pedometer, online computer program | Missed abortion (1/4) |
| Harrison, 2013 [11] | Health trainer | From GW 14–16 until GW 28 | Delivery: Four sessions of participants’ individually determined goals. Components: Reduced consumption of high-fat or convenience foods, and increased consumption of fruit and vegetables Motivation: Lifestyle messages Assessment: Self-monitoring based on IOM recommendations | Delivery: Individualised exercise program. Components: Moderate and vigorous activity of daily walking. Motivation: Lifestyle messages Assessment: Self-monitoring, pedometers, questionnaires, METs | Miscarriages or termination (1/2), premature delivery (3/1) |
| Petrella, 2013 [61] | Dietitian | From GW 16 until GW 36 | Delivery: 1 h counseling session Components:1500 kcal/day, divided into three main meals, and three snacks; due to PA program, an addition of 200 kcal/day for obese, and 300 kcal/day for overweight women. Macronutrient diet target composition of 55% carbohydrates of at least of 225 g/day to prevent ketosis (80% complex low GI, 20% simplex), 20% protein (50% animal, 50% vegetable), and 25% fat (12% mono-unsaturated, 7% polyunsaturated, and 6% saturated) with moderately low saturated fat levels. Motivation: Counseling Assessment: Questionnaires, urine exams (ketonuria) | Delivery: PA program at least thrice a week Components: 30 min of moderate intensity activity Motivation: Exercise and lifestyle surveys Assessment: Pedometers, talk test | ND |
| Dodd, 2014 [65] | Dietitian, trained research assistants | From GW 10–16 until GW 36 | Delivery: A planning session with a research dietitian Components: Nutrition counseling according to Australian standards for a balanced consumption of carbohydrates, fat, and protein. Consumption of two servings of fruit, five servings of vegetables, and three servings of dairy each day, and reduced intake of foods high in refined carbohydrates and saturated fats, while increasing intake of fiber. Motivation: Encouragement, support, individualized facilitators, telephone calls Assessment: Self-monitoring, workbook | Delivery: Generic Components: PA advice primarily on increasing walking and incidental activity. Motivation: Encouragement, support, individualized facilitators, telephone calls Assessment: Self-monitoring, workbook | Stillbirths (5/5), miscarriages (25/25); neonatal deaths (4/1); maternal deaths (1, motor vehicle collision)/(1, ruptured maternal splenic artery aneurysm) |
| Hui, 2014 [67] | Dietitians | From GW 20–26 until GW 36 | Delivery: A personalized, individualized nutrition consultation, with the assistance of FCM interview tool (possibility of receiving a complete weekly intake and decisions for food choices). Components: Nutritional recommendations based on the dietary intake analysis and Health Canada guidelines for food intake in pregnancy with considerations for personal food preference, food beliefs, and food budgeting. Motivation: Food stickers on a magnetic board, nutritional information on the food sticker scanned into the computer, instant analysis of daily calorie intake and macronutrients. Assessment: Self-reported food intake records, software containing Canadian Food Database | Delivery: Group or individualized PA program or at home, 3–5 times weekly. Components: Aerobic exercise, strength exercise, and stretching of mild to moderate intensity, in a group or at home according to an exercise DVD instruction, for 30–45 min. Motivation: DVD, exercise logbook Assessment: Questionnaires | ND |
| Poston, 2015 [60] | Health trainer | From GW 20+6/7 until GW 28+6/7 | Delivery: Eight health trainer-led group or individual sessions of 1 h duration once a week for 8 weeks. Components: Promotion of a healthy pattern of eating but not necessarily restriction of energy intake. Recommendations tailored to the woman’s habitual diet and cultural preference. Suggested exchanging carbohydrate-rich foods with a medium-to-high GI for those with a lower GI to reduce the GL, and restricting dietary intake of saturated fat. Motivation: Telephone calls, emails, SMART goals, handbook counseling, encouragement Assessment: Self-monitoring, log book, dietary data, GWG, anthropometry, questionnaires, and nutrition calculation software. | Delivery: Eight health trainer-led group or individual sessions of 1 h duration once a week for 8 weeks Components: Walking at a moderate, progressively increased intensity, tailored to women’s pre-existing activities. Additional PA options for previously active women. Motivation: SMART goals, handbook, counseling, encouragement, DVD Assessment: Pedometer, log book, PA scores, GWG, anthropometry, questionnaires, METs | Loss of pregnancy (14/14), miscarriages (6/2), fetal deaths in utero (2/4), termination (1/3) |
| Koivusalo, 2015 [6] | Dietitians, study nurses | From GW 13.3 (12–14.6) until GW 35.1 (34.4–35.6) | Delivery: In the beginning, counseling session in a group led by a dietitian. After enrollment, three sessions of individualized counseling according to the stage of the pregnancy, led by the study nurses. Components: Dietary advice based on contemporary Nordic Nutrition Recommendations 2004. Focus on optimizing participants’ consumption of vegetables, fruits and berries, wholegrain products rich in fiber, low-fat dairy products, vegetable fats high in unsaturated fatty acids, fish, and low-fat meat products, and a lower intake of sugar-rich foods. Motivation: Counseling Assessment: Questionnaires, questionnaire-based dietary index | Delivery: In a group or individualized, supervised exercise program. Components: PA of moderate-intensity, at least for 150 min weekly. Motivation: Free access to public swimming pools. Assessment: Self-reporting | Miscarriages or termination of pregnancy (7/6) |
| Bruno, 2016 [62] | Dietitian | From GW 9–12 until GW 36 | Delivery: At enrollment, a counseling session lasting approximately 1 h with a dietitian prescribing a personalized dietary intervention. Four additional follow-up sessions. Components: Decreased intake of foods with a high GI and a high saturated fat content and replacement of them with healthier nutrition based on taste and preferences. A diet based on the high consumption of plant foods, cereals, legumes and fish, with olive oil as the main source of fat, and moderate to no consumption of red wine. A low-glycaemic, low-saturated fat diet with a total intake of 1500 kcal/day divided into three main meals, and three snacks; additionally, daily intake of 300 kcal, and 200 kcal for overweight and obese participants, respectively, due to PA program. Targets for daily dietary macronutrients composition: 55% carbohydrates at least 225 g (ketosis prevention) consisting of 80% complex carbohydrates with low GI, and 20% simple carbohydrates with high GI; 20% protein consisting of 50% animal, and 50% vegetable; 25% fat, consisting of 12% mono-unsaturated, 7% polyunsaturated, 6% saturated; and low intake of saturated fat. Motivation: Telephone calls, counseling Assessment: Questionnaires | Delivery: Group exercise program, at least three times a week. Components: PA of moderate intensity for 30 min each time, according to the ACOG 2002 and the ACSM guidelines. Motivation: Telephone calls, counseling Assessment: Pedometer, talk test | Miscarriages (7/6) |
| Kennelly, 2018 [17] | Researchers | From GW 12–17 until GW 34 | Delivery: Face-to-face education sessions individually or in pairs, smartphone application, emails every two weeks with SMART goals. Components: Healthy recommended approximately eucaloric diet in pregnancy, replacing high-GI with low-GI foods. Motivation: Food diaries quantifying GI and GL, smartphone application, encouragement. Assessment: Self-reported, food diaries, and nutrition calculation software. | Delivery: Exercise program between five and seven days per week. Components: Exercise of moderate intensity for 30 min divided into three or two periods of 10 or 15 min, respectively, according to the ACOG guidelines. Motivation: Smartphone application Assessment: Self-reported, pregnancy exercise and lifestyle surveys | Miscarriages (1/0), congenital anomaly (3/1), pretern delivery and neonatal death (1/0), maternal parvovirus infection (0/1), hyperemesis gravidarum (0/1) |
| Chan, 2018 [13] | Dietitian, exercise instructor | From GW ≤ 12 until GW 24 | Delivery: Bi-weekly face-to-face or phone consultations in the first two months, and monthly face-to-face consultations afterwards till the end of the intervention. Components: An individualized menu plan aimed at achieving a varied balanced diet with an emphasis on fruit and vegetables consumption, and intake of moderate-carbohydrate, low-fat, low-GI and low-caloric products in appropriate portions. Also, advice on the use of dietary supplements and managing pregnancy discomforts. Motivation: Telephone calls, advice, booklet, emails Assessment: Food records, questionnaires, nutrition calculation software, diet adherence score. | Delivery: A supervised exercise program at least three times a week. Components: Based on international guidelines; a session of 30 min of easy to moderate intensity of low-impact aerobics PA. Motivation: Support Assessment: Questionnaires, number of accomplished exercise sessions, PA adherence score | Miscarriages (13/13), pretern delivery (1/1) |
| Ferrara, 2020 [66] | Dietitians | From 14.3 GW (13.3–15.1) until GW 27.3 | Delivery: Core intervention of 13 weekly individual sessions. First and last sessions in person, and 11 remaining sessions by telephone. Components: Eating healthy foods in appropriate portion sizes, total caloric intake, and calories from fat. Motivation: Motivational interviewing techniques, a step-wise, phased approach to behaviour change. Assessment: Workbook, personalised graphs, 24 h dietary recalls | Delivery: Exercise program weekly. Components:150 min per week of moderate-intensity to vigorous intensity PA Motivation: Motivational interviewing techniques, a step-wise, phased approach to behaviour change. Assessment: Accelerometer | Pregnancy loss (5/4) |
| Lin, 2020 [18] | Researchers | From GW < 8 until GW 24–28 | Delivery: One face-to-face education session with an interventionist at the onset of intervention and continuous educational messages delivered via a WeChat public account at a frequency of twice per week. Components: Encouragement to consume vegetables, fruits, high-fiber wholegrain products, low-fat dairy products, and avoid foods rich in sugar and saturated fatty acids, among other guidance. Motivation: Encouragement, messages Assessment: N/a | Delivery: Exercise program at a frequency of three to four times per week. Components: Approximately 30 min of moderate-intensity PA Motivation: Encouragement, messages Assessment: N/a | Early pregnancy loss (5/4) |
| Li, 2021 [64] | Researchers | ND | Delivery: Individualized medical nutrition guidance Components: Instruction in choosing appropriate foods, and preparation methods for each meal (including vegetables, fruits, and meat). Recommendation to eat less at each meal, and more often at a frequency of six daily meals (three main meals and three additional meals). Energy distribution per each meal category: 15% to 20% for breakfast, 20% to 30% for lunch, 20% to 30% for dinner, and 15% to 30% for extra meals. Calculation of total energy intake of each participant according to pre-pregnancy BMI; increase in the average daily energy intake by 200 kcal on this basis during the second and third trimester of pregnancy, including 50% to 55% carbohydrate, 25% to 30% fat, and 15% to 20% protein. Motivation: Choice of foods Assessment: Food record, anthropometry | Delivery: Individualized exercise program Components: Walking for 15–20 min after meals. Continued engaged pre-pregnancy aerobic activities (i.e., swimming, yoga), according to individual’s preferences and physical condition for 30–40 min. Motivation: Continuation of preferable activities Assessment: Anthropometry | ND |
| Liu, 2021 [63] | Dietitian | From GW 8–12 until delivery | Delivery: Individual sessions of face-to face instruction and video teaching for 1 h every 2–4 weeks from the first intervention until the end of pregnancy. Components: A hypocaloric, low-glycaemic, low-saturated fat diet with a sample meal plan and recipes. Individualized diet according to pre-pregnancy BMI and PA. For overweight with light PA, recommended 25–30 kcal/kg of ideal body weight (IBW); for obese women with light PA, recommended 20–25 kcal/kg of ideal body weight. Distribution of the daily energy intake as carbohydrate 50–55%, protein 15–20%, and fat 25–30%. Suggested energy for the first trimester not below 1500 kcal/day, and for the second trimester not less than 1600 kcal/day. Motivation: Follow-up through WeChat for participants unavailable for meetings with the dietitian. Assessment: Dietary log | Delivery: Individualized exercise guidance, at least five days each week. Components: Moderate-intensity PA with brisk walking, swimming, dancing, pregnancy yoga, or other aerobic exercises for at least 30 min a day. Motivation: WeChat Assessment: Exercise log, HR monitoring by talking pulse | Miscarriages (19/32) |
| Ding, 2021 [7] | Dietitian, clinical nutritionist | From GW < 12 until GW 24–28 | Delivery: Personalized dietary and exercise guidelines based on the guide for diagnosis and treatment of GDM2014, social media platform, personalized nutrition care Components: IBW energy requirement calculator, and consideration of pregnancy stage. For BMI 24–27.9 and early pregnancy, EER 25–30 kcal/kg; for BMI 24–27.9 and mid-pregnancy, EER 200 kcal in addition to previous intake; for BMI ≥ 28 and early pregnancy EER 20–25 kcal/kg; and for BMI ≥ 28 and mid-pregnancy, EER 200 kcal in addition to previous intake. Minimum energy intake not less than 1500 kcal at early pregnancy, and 1800 kcal at mid-pregnancy. Daily macronutrient components of healthy recommended diet: 50–60% carbohydrate, <30% fat, and 1.0–1.3 g/kg protein. Motivation: An available dietitian, counseling, social media platform Assessment: Dietary surveys, questionnaires | Delivery: Daily exercise program Components: Walk for at least 6000 steps per day Motivation: An available dietitian, counseling, social media platform Assessment: Smartphone data, questionnaires | Early spontaneous abortion (6/5) |
| Deng, 2022 [8] | Nutritionist, exercise experts, nurses | From GW 14 until GW 24–28 | Delivery: One-on-one explanation with an average of 20 min per person, educational manuals Components: Per capita calculation of daily intake of various foods, daily energy intake, daily intake of the three major nutrients, and energy ratio. Energy requirements individually calculated, with 50% to 60% of E% coming from carbohydrates, 20 to 30 E% from fats and 15 to 20 E% from protein. Encouragement to select high-fiber bread, wholemeal products or multigrain rice as staple food; to consume more vegetables and fewer high-sugar fruits; to select fat-free or low-fat versions of milk and milk products; and to use moderate amounts of oil in cooking and baking. Presentation of sample meals and food model samples provided to the women at enrollment. Advice on choosing the favorite food of the same kind through food exchange. Motivation: Advice, social media platform Assessment: Diaries, questionnaires | Delivery: Exercise program at least five days a week. Components: According to the recommendations of ACOG (2015); 20–30 min of moderate-intensity exercise including walking, yoga, and gestational gymnastics designed by exercise experts; in situ steps, upper limb and shoulder back training, and hip 3D exercises. Motivation: Advice, social media platform Assessment: Diaries, questionnaires | None reported |
| Sadiya, 2022 [10] | Dietitians | From GW ≤ 12; 12 GW | Delivery: Face-to-face individualized dietary consultations. Components: Increased consumption of whole grains, vegetables, fruits, and lower intake of processed food, and simple carboxylates, according to ADA recommendations. Target macronutrient composition: 50–55% carbohydrates, 25–30% fat, and 20% protein. Motivation: Telephone calls, motivational interviewing, SMART goals Assessment: Food recalls, questionnaires, food log | Delivery: A choice of a weekly or daily exercise program. Components: Weekly moderate-intensity PA of 150 min or a daily minimum of 10,000 steps; 1 h and 40min daily activity. Motivation: Telephone calls, motivational interviewing, SMART goals Assessment: Self-monitoring, smartphone pedometer application | Miscarriages (2/2) |
| First Author, Publication Year | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Korpi-Hyövälti, 2011 [40] | L | L | ? | L | L | L | L |
| Quinlivan, 2011 [43] | L | L | ? | L | L | L | L |
| Walsh, 2012 [41] | L | L | ? | L | L | L | L |
| McCarthy, 2016 [42] | L | L | ? | L | L | L | L |
| Yi Zhang, 2019 [45] | L | L | H | L | L | L | L |
| Al Wattar, 2019 [20] | L | L | H | L | L | L | L |
| Okesene-Gafa, 2019 [44] | L | L | H | L | L | L | L |
| Melero, 2020 [3] | L | ? | ? | L | L | L | L |
| Basu, 2021 [4] | L | L | H | L | L | L | L |
| Dong-Yao Zhang, 2022 [16] | L | L | ? | L | L | L | L |
| First Author, Publication Year | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Do Nascimento, 2011 [52] | L | L | ? | L | L | L | L |
| Oostdam, 2012 [51] | L | L | ? | L | L | L | L |
| Price, 2012 [54] | L | L | ? | ? | H | L | L |
| Barakat, 2013 [46] | L | L | H | L | L | L | L |
| Ruiz, 2013 [49] | L | L | ? | L | L | L | L |
| Nobles, 2015 [55] | L | L | ? | L | L | L | L |
| Bisson, 2015 [53] | L | L | ? | L | L | L | L |
| Seneviratne, 2015 [57] | L | L | H | L | L | L | L |
| Perales, 2016 [48] | L | L | H | L | H | L | L |
| Krohn Garnæs, 2016 [50] | L | L | ? | L | L | L | L |
| Guelfi, 2016 [56] | L | L | ? | ? | L | L | L |
| Wang, 2017 [58] | L | L | H | L | L | L | L |
| Daly, 2017 [47] | L | L | H | L | L | L | L |
| First Author, Publication Year | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Luoto, 2011 [5] | L | L | H | L | L | L | L |
| Vinter, 2011 [59] | L | L | ? | L | L | L | L |
| Harrison, 2013 [11] | L | L | ? | L | L | L | L |
| Petrella, 2013 [61] | L | L | ? | L | L | L | L |
| Dodd, 2014 [65] | L | L | ? | L | L | L | L |
| Hui, 2014 [67] | L | L | H | L | L | L | L |
| Poston, 2015 [60] | L | L | H | L | L | L | L |
| Koivusalo, 2015 [6] | L | L | ? | L | L | L | L |
| Bruno, 2016 [62] | L | L | ? | L | H | L | L |
| Kennelly, 2018 [17] | L | L | H | L | L | L | L |
| Chan, 2018 [13] | L | L | H | L | L | L | L |
| Ferrara, 2020 [66] | L | L | ? | L | L | L | L |
| Lin, 2020 [18] | L | L | ? | L | L | L | L |
| Li, 2021 [64] | L | L | ? | L | L | ? | L |
| Liu, 2021 [63] | L | L | H | L | L | L | L |
| Ding, 2021 [7] | L | L | H | L | L | L | L |
| Deng, 2022 [8] | L | L | H | L | L | L | L |
| Sadiya, 2022 [10] | L | L | H | L | L | L | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsironikos, G.I.; Potamianos, P.; Zakynthinos, G.E.; Tsolaki, V.; Tatsioni, A.; Bargiota, A. Effectiveness of Lifestyle Interventions during Pregnancy on Preventing Gestational Diabetes Mellitus in High-Risk Women: A Systematic Review and Meta-Analyses of Published RCTs. J. Clin. Med. 2023, 12, 7038. https://doi.org/10.3390/jcm12227038
Tsironikos GI, Potamianos P, Zakynthinos GE, Tsolaki V, Tatsioni A, Bargiota A. Effectiveness of Lifestyle Interventions during Pregnancy on Preventing Gestational Diabetes Mellitus in High-Risk Women: A Systematic Review and Meta-Analyses of Published RCTs. Journal of Clinical Medicine. 2023; 12(22):7038. https://doi.org/10.3390/jcm12227038
Chicago/Turabian StyleTsironikos, Georgios I., Petros Potamianos, George E. Zakynthinos, Vasiliki Tsolaki, Athina Tatsioni, and Alexandra Bargiota. 2023. "Effectiveness of Lifestyle Interventions during Pregnancy on Preventing Gestational Diabetes Mellitus in High-Risk Women: A Systematic Review and Meta-Analyses of Published RCTs" Journal of Clinical Medicine 12, no. 22: 7038. https://doi.org/10.3390/jcm12227038
APA StyleTsironikos, G. I., Potamianos, P., Zakynthinos, G. E., Tsolaki, V., Tatsioni, A., & Bargiota, A. (2023). Effectiveness of Lifestyle Interventions during Pregnancy on Preventing Gestational Diabetes Mellitus in High-Risk Women: A Systematic Review and Meta-Analyses of Published RCTs. Journal of Clinical Medicine, 12(22), 7038. https://doi.org/10.3390/jcm12227038








