Adherence to the GOLD Guidelines in Primary Care: Data from the Swiss COPD Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Study Population
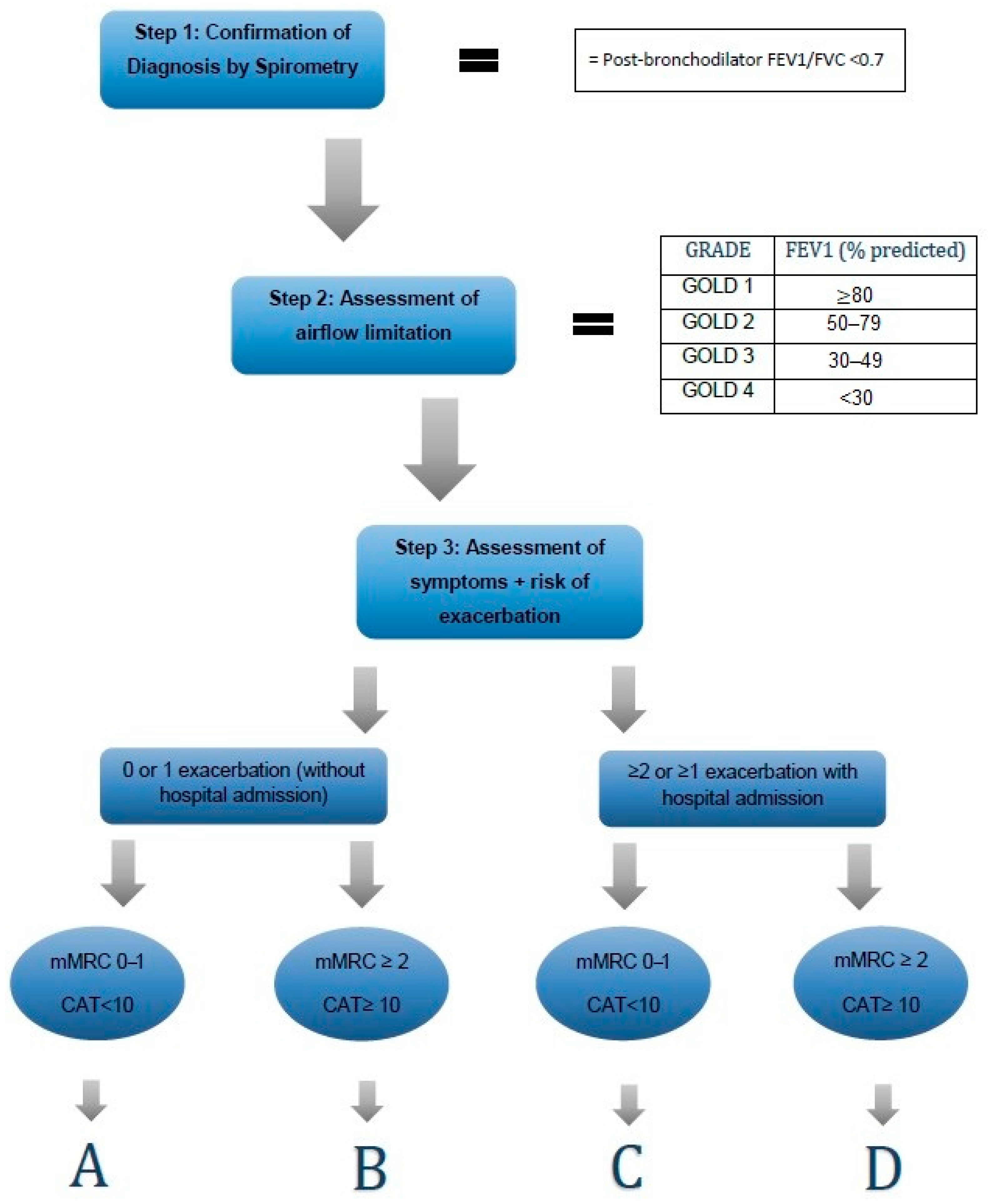
- Confirmed COPD diagnosis (FEV1/FVC < 70%, before and after inhalation of a bronchodilator);
- Age ≥ 40 years;
- Current smokers or former smokers of at least 20 pack-years;
- Informed consent.
2.3. Study Procedures
2.4. Outcomes
2.5. Statistical Methods and Analysis
3. Results
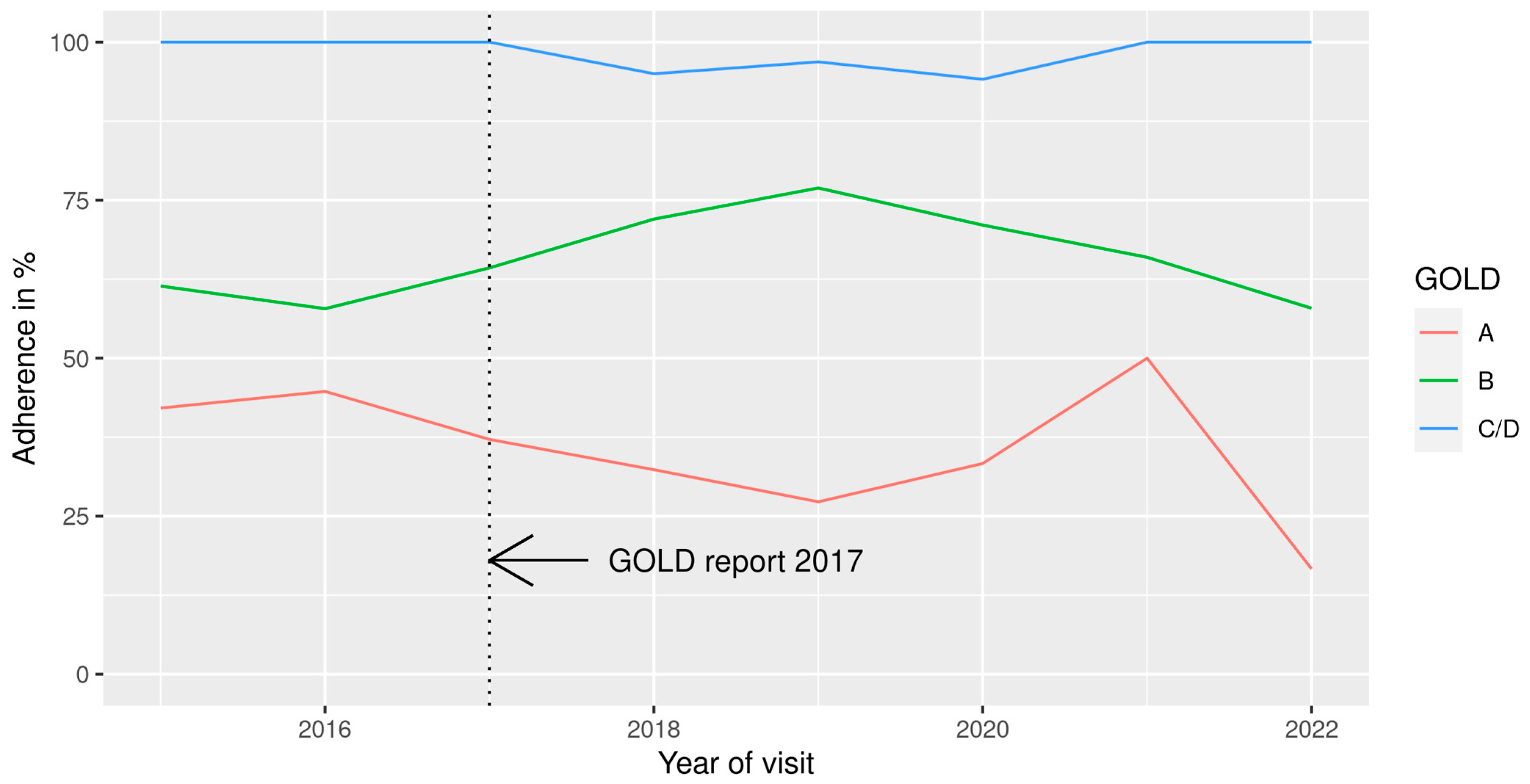
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Exacerbations/Year | Clinical Symptoms | |
|---|---|---|
| Group A | ≤1 moderate exacerbations (not leading to hospital admission) | mMRC < 2 CAT < 10 |
| Group B | mMRC ≥ 2 CAT ≥ 10 | |
| Group E | ≥2 moderate exacerbations or ≥1 exacerbation leading to hospital admission | independent from the clinical symptoms |
References
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.; MacNee, W.; Agusti, A.; Anzueto, A.; Berg, B.; Buist, A.; Calverley, P.; Chavannes, N.; Dillard, T.; Fahy, B.; et al. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur. Respir. J. 2004, 23, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Russi, E.W.; Leuenberger, P.; Brändli, O.; Frey, J.G.; Grebski, E.; Gugger, M.; Paky, A.; Pons, M.; Karrer, W.; Kuhn, M.; et al. Management of chronic obstructive pulmonary disease: The Swiss guidelines. Official Guidelines of the Swiss Respiratory Society. Swiss Med. Wkly. 2002, 132, 67–78. [Google Scholar] [PubMed]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef]
- Tabyshova, A.; Hurst, J.R.; Soriano, J.B.; Checkley, W.; Huang, E.W.C.; Trofor, A.C.; Flores-Flores, O.; Alupo, P.; Gianella, G.; Ferdous, T. Gaps in COPD Guidelines of Low- and Middle-Income Countries: A Systematic Scoping Review. Chest 2021, 159, 575–584. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease—Global Initiative for Chronic Obstructive Lung Disease—GOLD. Available online: https://goldcopd.org (accessed on 19 February 2023).
- Sehl, J.; O’doherty, J.; O’connor, R.; O’sullivan, B.; O’regan, A. Adherence to COPD management guidelines in general practice? A review of the literature. Ir. J. Med. Sci. 2017, 187, 403–407. [Google Scholar] [CrossRef]
- Hurd, S. 2017 GOLD Report. Available online: https://goldcopd.org/wp-content/uploads/2017/02/wms-GOLD-2017-FINAL.pdf (accessed on 3 September 2023).
- Hsieh, M.-J.; Huang, S.-Y.; Yang, T.-M.; Tao, C.-W.; Cheng, S.-L.; Lee, C.-H.; Kuo, P.-H.; Wu, Y.-K.; Chen, N.-H.; Hsu, W.-H.; et al. The impact of 2011 and 2017 Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) guidelines on allocation and pharmacological management of patients with COPD in Taiwan: Taiwan Obstructive Lung Disease (TOLD) study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2949–2959. [Google Scholar] [CrossRef]
- Jochmann, A.; Neubauer, F.; Miedinger, D.; Török, S.S.; Tamm, M.; Leuppi, J. General practitioner’s adherence to the COPD GOLD guidelines: Baseline data of the Swiss COPD Cohort Study. Swiss Med. Wkly. 2010, 140, w13053. [Google Scholar] [CrossRef]
- Jochmann, A.; Scherr, A.; Jochmann, D.; Miedinger, D.; Schafroth, T.; Chhajed, P.; Tamm, M.; Leuppi, J. Impact of adherence to the GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in the Swiss COPD cohort. Swiss Med. Wkly. 2012, 142, w13567. [Google Scholar] [CrossRef] [PubMed]
- Grewe, F.A.; Sievi, N.A.; Bradicich, M.; Roeder, M.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; et al. Compliance of Pharmacotherapy with GOLD Guidelines: A Longitudinal Study in Patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 627–635. [Google Scholar] [CrossRef]
- Palmiotti, G.A.; Lacedonia, D.; Liotino, V.; Schino, P.; Satriano, F.; Di Napoli, P.L.; Sabato, E.; Mastrosimone, V.; Scoditti, A.; Carone, M.; et al. Adherence to GOLD guidelines in real-life COPD management in the Puglia region of Italy. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Alfaro, T.; Fernandes, L.; Ferreira, P.; Silva, S.; Costa, J.; Seixas, E.; Viana, R. Does practice follow evidence-based guidelines? Adherence to GOLD guidelines in Portugal. Pulmonology 2019, 25, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Buffels, J.; Degryse, J.; Heyrman, J.; Decramer, M. DIDASCO Study. Office spirometry significantly improves early detection of COPD in general practice: The DIDASCO Study. Chest 2004, 125, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Urwyler, P.; Abu Hussein, N.; Bridevaux, P.O.; Chhajed, P.N.; Geiser, T.; Grendelmeier, P.; Zellweger, L.J.; Kohler, M.; Maier, S.; Miedinger, D.; et al. Predictive factors for exacerbation and re-exacerbation in chronic obstructive pulmonary disease: An extension of the Cox model to analyze data from the Swiss COPD cohort. Multidiscip. Respir. Med. 2019, 14, 7. [Google Scholar] [CrossRef]
- Ulrik, C.S.; Hansen, E.F.; Jensen, M.S.; Rasmussen, F.V.; Dollerup, J.; Hansen, G.; Andersen, K.K. Management of COPD in general practice in Denmark—Participating in an educational program substantially improves adherence to guidelines. Int. J. Chron. Obstruct. Pulmon. Dis. 2010, 5, 73–79. [Google Scholar] [CrossRef]
- Román-Rodríguez, M.; Pardo, M.G.; López, L.G.; Ruiz, A.U.; van Boven, J.F. Enhancing the use of Asthma and COPD Assessment Tools in Balearic Primary Care (ACATIB): A region-wide cluster-controlled implementation trial. NPJ Prim. Care Respir. Med. 2016, 26, 16003. [Google Scholar] [CrossRef]
- Price, D.; West, D.; Brusselle, G.; Gruffydd-Jones, K.; Jones, R.; Miravitlles, M.; Rossi, A.; Hutton, C.; Ashton, V.L.; Stewart, R.; et al. Faculty Opinions recommendation of Management of COPD in the UK primary-care setting: An analysis of real-life prescribing patterns. Int. J. Chronic Obstr. Pulm. Dis. 2017, 9, 889–904. [Google Scholar]
- Marmy, J.L.; Diedrich, J.P.; Cadus, C.; Grendelmeier, P.; Tschacher, A.; Dieterle, T.; Chhajed, P.N.; Leuppi, J.D. Adherence to GOLD Recommendations among Swiss Pulmonologists and General Practitioners. COPD J. Chronic Obstr. Pulm. Dis. 2020, 18, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S.; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Gruffydd-Jones, K. GOLD guidelines 2011: What are the implications for primary care? Prim. Care Respir. J. 2012, 21, 437–441. [Google Scholar] [CrossRef]
- Mularski, R.A.; Asch, S.M.; Shrank, W.H.; Kerr, E.A.; Setodji, C.M.; Adams, J.L.; Keesey, J.; McGlynn, E.A. The Quality of Obstructive Lung Disease Care for Adults in the United States as Measured by Adherence to Recommended Processes. Chest 2006, 130, 1844–1850. [Google Scholar] [CrossRef]
- Levy, M.L.; Bacharier, L.B.; Bateman, E.; Boulet, L.-P.; Brightling, C.; Buhl, R.; Brusselle, G.; Cruz, A.A.; Drazen, J.M.; Duijts, L.; et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim. Care Respir. Med. 2023, 33, 7. [Google Scholar] [CrossRef]
- Ernst, P.; Saad, N.; Suissa, S. Inhaled corticosteroids in COPD: The clinical evidence. Eur. Respir. J. 2014, 45, 525–537. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease—GOLD. 2023 GOLD Report. Available online: https://goldcopd.org/2023-gold-report-2 (accessed on 7 August 2023).
- Bourbeau, J.; Sebaldt, R.J.; Day, A.; Bouchard, J.; Kaplan, A.; Hernandez, P.; Rouleau, M.; Petrie, A.; Foster, G.; Thabane, L.; et al. Practice Patterns in the Management of Chronic Obstructive Pulmonary Disease in Primary Practice: The Cage Study. Can. Respir. J. 2008, 15, 13–19. [Google Scholar] [CrossRef]
- Calzetta, L.; Ritondo, B.L.; Zappa, M.C.; Manzetti, G.M.; Perduno, A.; Shute, J.; Rogliani, P. The impact of long-acting muscarinic antagonists on mucus hypersecretion and cough in chronic obstructive pulmonary disease: A systematic review. Eur. Respir. Rev. 2022, 31, 210196. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.; Chong, J.; Poole, P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014, 2014, CD009285. [Google Scholar] [CrossRef]
- Sestini, P.; Renzoni, E.; Robinson, S.; Poole, P.; Ram, F.S. Short-acting beta2-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2002, 2010, CD001495. [Google Scholar] [CrossRef]
- Alabi, F.O.; Alkhateeb, H.A.; Zibanayi, M.T.; Garces, J.; DeBarros, K.M.; Barletti, P.S.B.; Garcia, K.; James, R.K. The adherence to and utility of the Global Initiative for Chronic Obstructive Lung Disease guidelines for treating COPD among pulmonary specialists: A retrospective analysis. BMC Pulm. Med. 2023, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Bettoncelli, G.; Sessa, E.; Cricelli, C.; Biscione, G. Prevalence of Comorbidities in Patients with Chronic Obstructive Pulmonary Disease. Respiration 2010, 80, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-W.; Huang, C.-T.; Ruan, S.-Y.; Tsai, Y.-J.; Lai, F.; Yu, C.-J. Diabetes mellitus in patients with chronic obstructive pulmonary disease-The impact on mortality. PLoS ONE 2017, 12, e0175794. [Google Scholar] [CrossRef]
- Janjua, S.; Pike, K.C.; Carr, R.; Coles, A.; Fortescue, R.; Batavia, M. Interventions to improve adherence to pharmacological therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2021, 9, CD013381. [Google Scholar] [CrossRef] [PubMed]
- Gallefoss, F.; Bakke, P.S. Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respir. Med. 2000, 94, 279–287. [Google Scholar] [CrossRef]
| Guidelines | GOLD 2011 | GOLD 2017 | |
|---|---|---|---|
| GOLD Group | First Choice | Second Choice | Recommended |
| A | SABA or SAMA, or SABA/SAMA | LABA or LAMA | SABA or SAMA, or SABA/SAMA or LABA, or LAMA |
| B | LABA or LAMA | LABA/LAMA | LABA or LAMA or LABA/LAMA |
| C | ICS/LABA or LAMA | LABA/LAMA | LAMA or LABA/LAMA or ICS/LABA |
| D | ICS/LABA or LAMA | ICS/LABA/LAMA, LABA/LAMA, or LAMA/ICS | LAMA or LABA/LAMA or ICS/LABA, or ICS/LABA/LAMA, or LAMA/ICS |
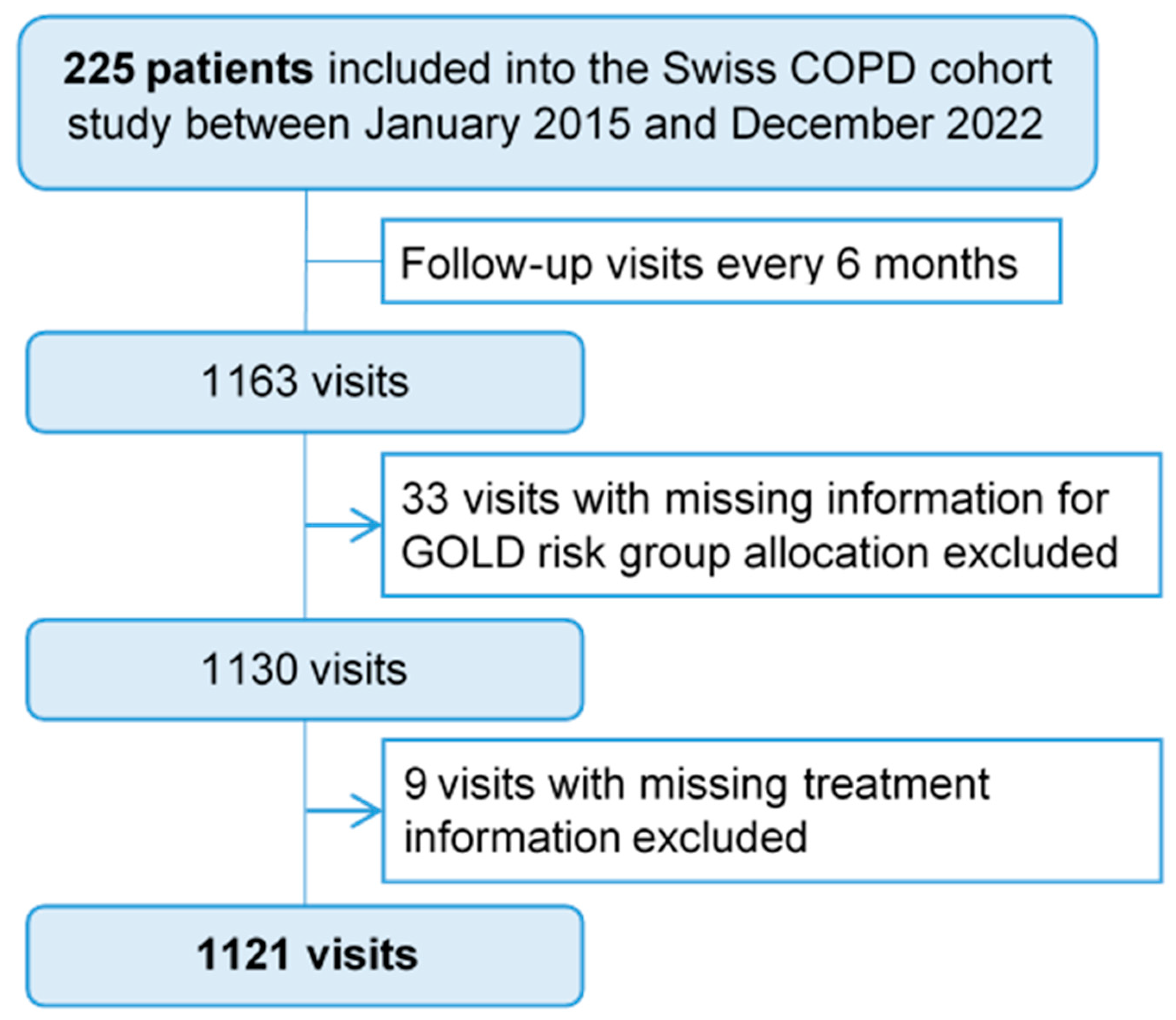
| Subjects, n (%) | Overall | GOLD A | GOLD B | GOLD C | GOLD D | Missing (%) |
|---|---|---|---|---|---|---|
| 225 (100) | 39 (17.33) | 155 (68.89) | 6 (2.67) | 25 (11.11) | ||
| Age (years), mean ± SD | 66.84 ± 9.44 | 66.41 ± 10.00 | 66.54 ± 9.23 | 70.17 ± 9.75 | 68.56 ± 10.01 | 0.0 |
| Gender, n (%) | 0.0 | |||||
| Male | 144 (64.0) | 32 (82.1) | 91 (58.7) | 6 (100.0) | 15 (60.0) | |
| Female | 81 (36.0) | 7 (17.9) | 64 (41.3) | 0 (0.0) | 10 (40.0) | |
| BMI, mean ± SD | 26.64 ± 5.79 | 28.46 ± 6.06 | 26.32 ± 5.77 | 25.48 ± 3.88 | 26.09 ± 5.52 | 0.4 |
| Smoking status | 0.0 | |||||
| Current smokers, n (%) | 129 (57.3) | 21 (53.8) | 92 (59.4) | 3 (50.0) | 13 (52.0) | |
| Ex-smokers, n (%) | 96 (42.7) | 18 (46.2) | 63 (40.6) | 3 (50.0) | 12 (48.0) | |
| Pack-years, mean ± SD | 47.98 ± 20.03 | 44.85 ± 15.57 | 47.36 ± 20.30 | 64.83 ± 17.78 | 52.64 ± 23.15 | |
| Spirometry | ||||||
| FEV1/FVC in %, mean ± SD | 54.94 ± 10.01 | 59.47 ± 8.46 | 54.70 ± 9.95 | 51.14 ± 6.93 | 50.33 ± 10.89 | 0.0 |
| Respiratory symptoms, n (%) | ||||||
| Sputum | 150 (67.0) | 13 (33.3) | 114 (74.0) | 5 (83.3) | 18 (72.0) | 0.4 |
| Cough | 174 (78.0) | 23 (59.0) | 129 (83.8) | 4 (80.0) | 18 (72.0) | 0.9 |
| CAT, mean ± SD | 15.82 ± 7.86 | 6.44 ± 2.16 | 18.08 ± 6.82 | 5.20 ± 2.28 | 18.76 ± 7.55 | 1.3 |
| Dyspnoea mMRC 0 | 16 (7.2) | 12 (30.8) | 3 (1.9) | 1 (16.7) | 0 (0.0) | 1.3 |
| Dyspnoea mMRC 1 | 88 (39.6) | 27 (69.2) | 51 (32.9) | 5 (83.3) | 5 (22.7) | |
| Dyspnoea mMRC 2 | 81 (36.5) | 0 (0.0) | 71 (45.8) | 0 (0.0) | 10 (45.5) | |
| Dyspnoea mMRC 3 | 32 (14.4) | 0 (0.0) | 26 (16.8) | 0 (0.0) | 6 (27.3) | |
| Dyspnoea mMRC 4 | 5 (2.3) | 0 (0.0) | 4 (2.6) | 0 (0.0) | 1 (4.5) | |
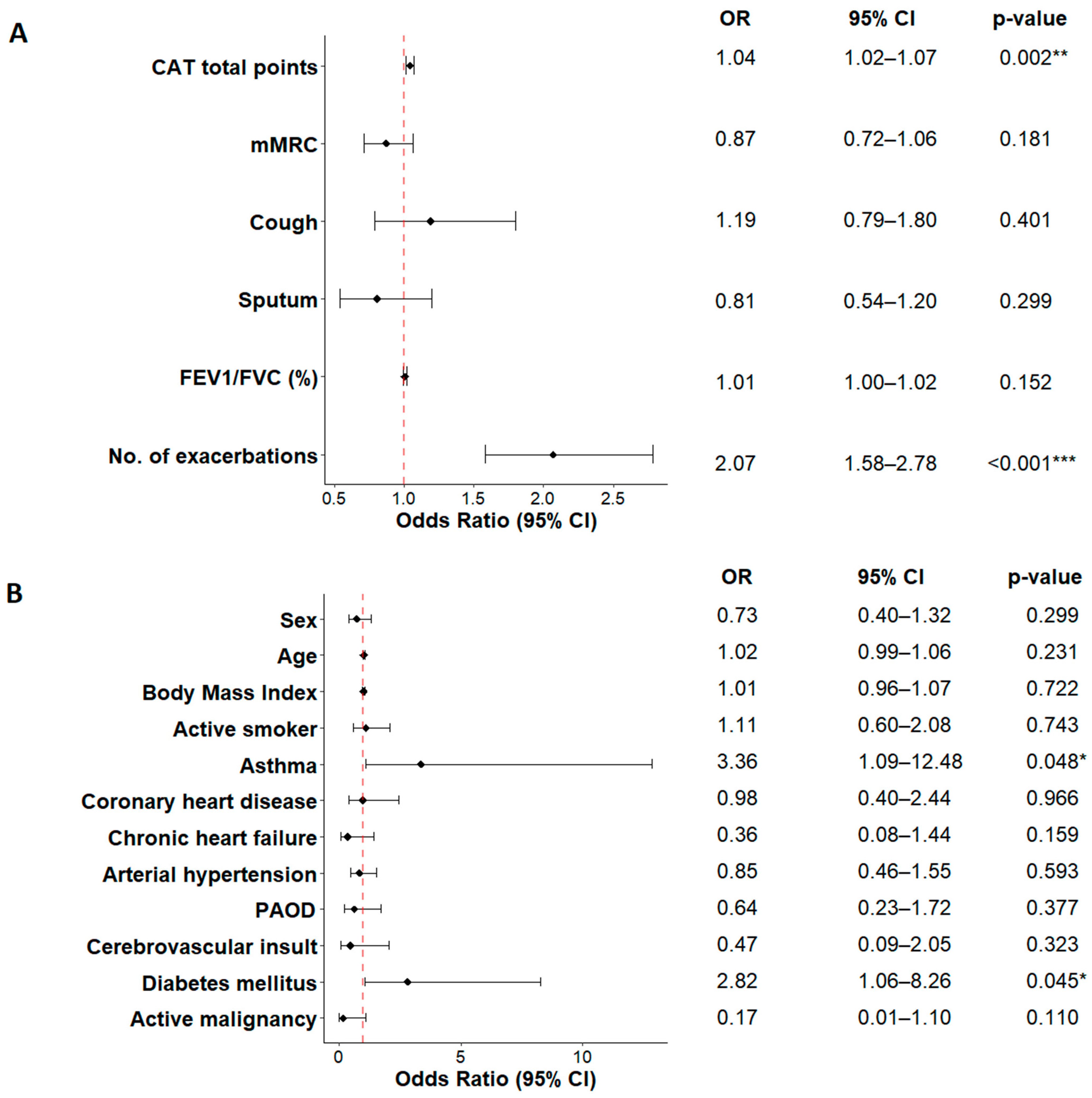
| Comorbidities, n (%) | 0.0 | |||||
| Asthma, n (%) | 18 (8.0) | 2 (5.1) | 15 (9.7) | 0 (0.0) | 1 (4.0) | |
| CHD, n (%) | 31 (13.8) | 3 (7.7) | 26 (16.8) | 1 (16.7) | 1 (4.0) | |
| Chronic heart failure, n (%) | 10 (4.4) | 1 (2.6) | 9 (5.8) | 0 (0.0) | 0 (0.0) | |
| Hypertension, n (%) | 117 (52.0) | 19 (48.7) | 82 (52.9) | 2 (33.3) | 14 (56.0) | |
| PAOD, n (%) | 21 (9.3) | 4 (10.3) | 13 (8.4) | 2 (33.3) | 2 (8.0) | |
| CVI, n (%) | 8 (3.6) | 1 (2.6) | 6 (3.9) | 0 (0.0) | 1 (4.0) | |
| Diabetes, n (%) | 27 (12.0) | 4 (10.3) | 19 (12.3) | 0 (0.0) | 4 (16.0) | |
| Malignancy, n (%) | 6 (2.7) | 0 (0.0) | 5 (3.2) | 0 (0.0) | 1 (4.0) | |
| Lung cancer, n (%) | 4 (1.8) | 0 (0.0) | 3 (1.9) | 1 (16.7) | 0 (0.0) |
| Overall | GOLD A | GOLD B | GOLD C | GOLD D | Missing (%) | |
|---|---|---|---|---|---|---|
| 1130 | 196 | 815 | 10 | 109 | ||
| Systemic steroids | 13 (1.1) | 0 (0.0) | 8 (1.0) | 0 (0.0) | 5 (4.7) | 1.7 |
| Oxygen therapy | 76 (6.6) | 1 (0.5) | 52 (6.4) | 0 (0.0) | 23 (21.1) | 1.6 |
| Non-pharmacological treatment | ||||||
| Sport exercise (≥twice/week) | 357 (31.3) | 84 (43.5) | 227 (28.0) | 4 (40.0) | 42 (38.5) | 1.8 |
| Pulmonary rehabilitation | 54 (4.7) | 3 (1.5) | 32 (4.0) | 0 (0.0) | 18 (16.5) | 1.7 |
| Vaccination (Influenza) | 877 (77.3) | 145 (75.1) | 631 (78.4) | 4 (40.0) | 83 (77.6) | 2.4 |
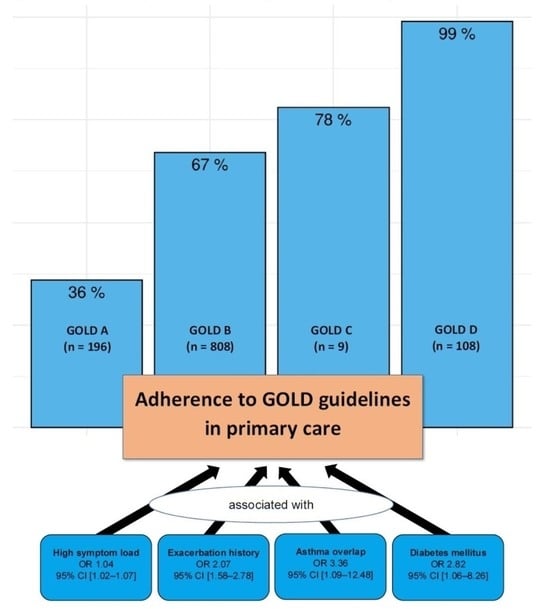
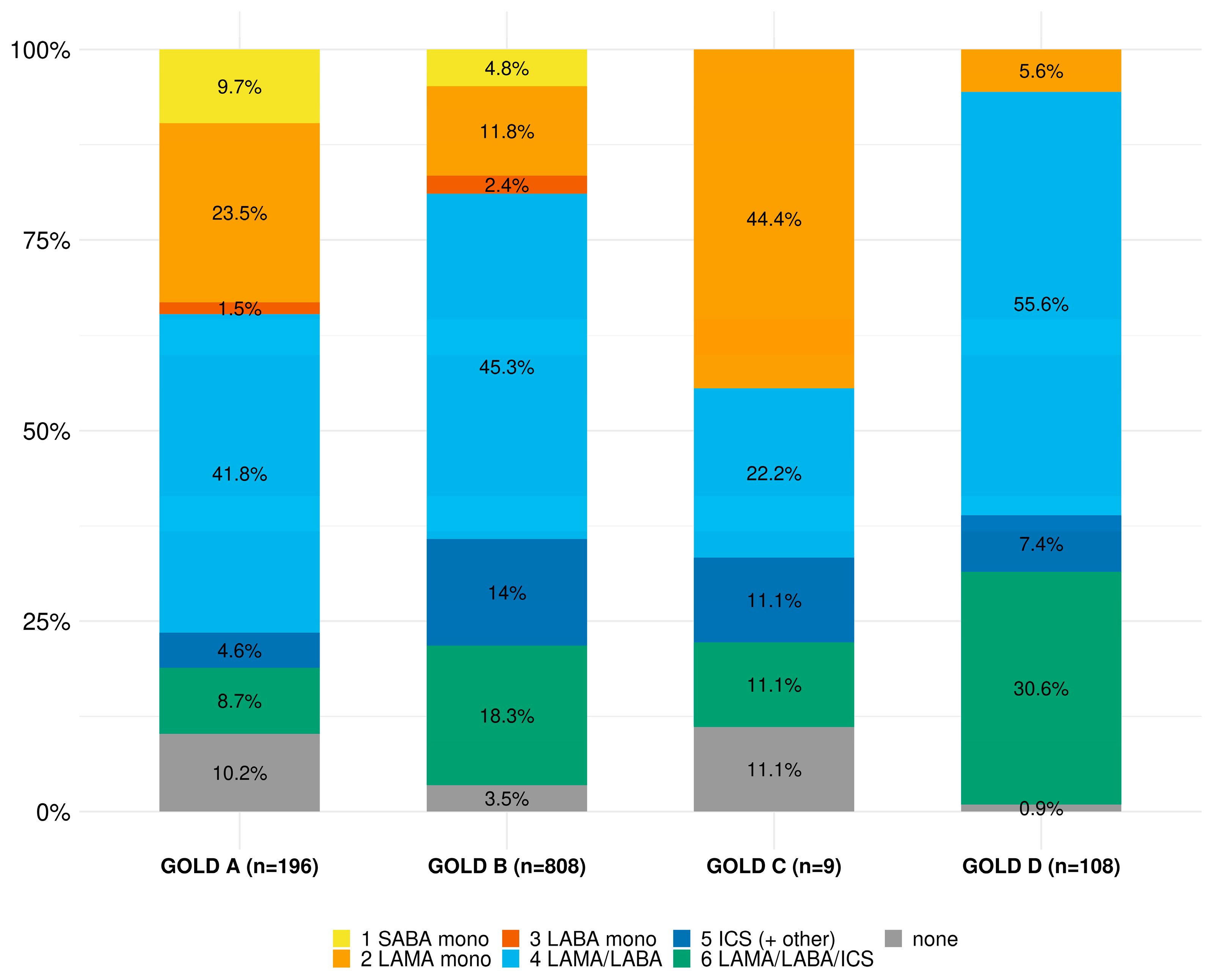
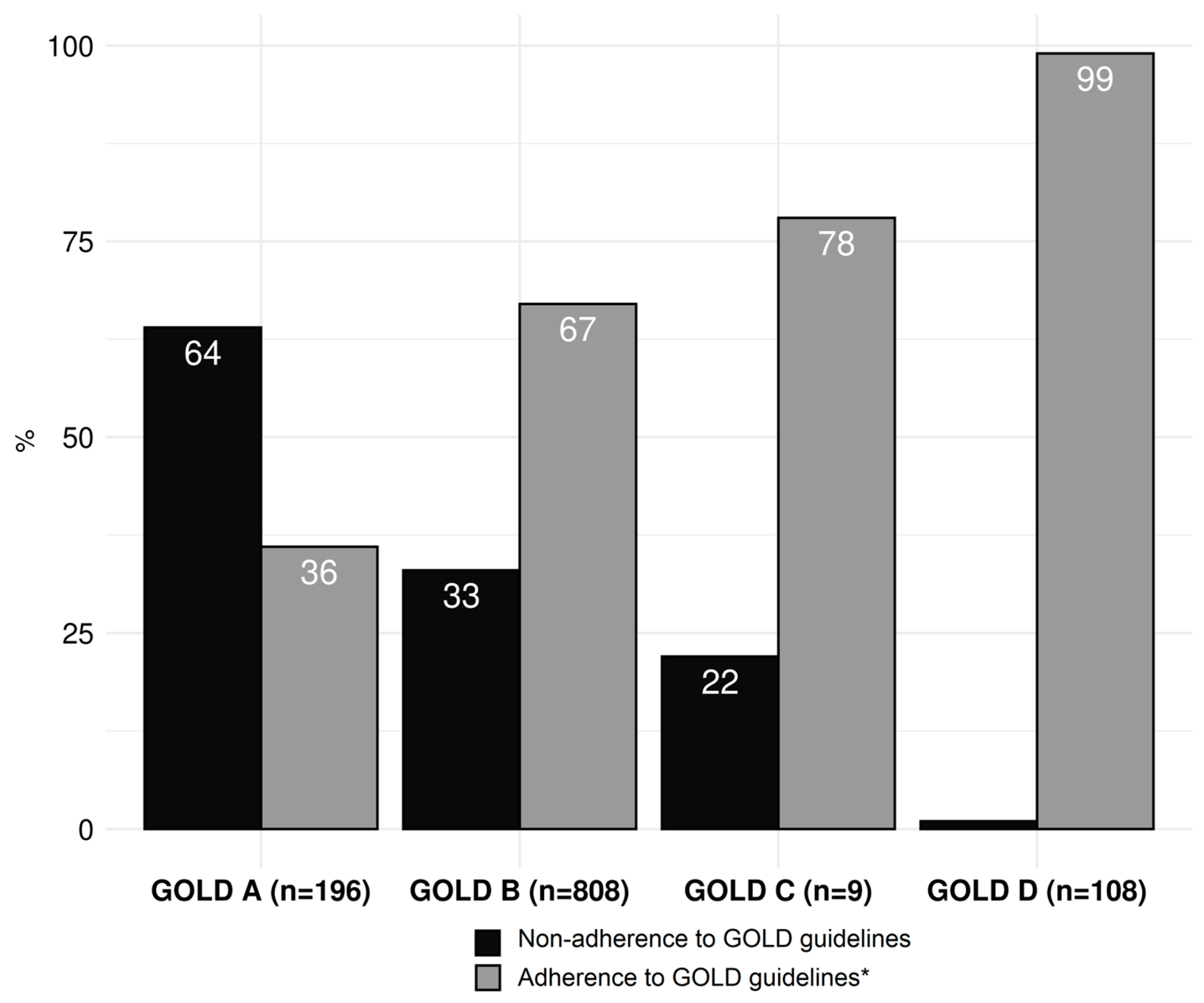
| Conformity, n (%) | Non-Conformity, n (%) | Missing, n (%) | |
|---|---|---|---|
| Overall (n = 1121) | 726 (64.8) | 395 (35.2) | 42 (3.6) |
| GOLD A (n = 195) | 70 (35.9) | 125 (64.1) | |
| no bronchodilator | 20 (10.3) | ||
| two long-acting bronchodilators | 98 (50.3) | ||
| any ICS a | 21 (10.8) | ||
| GOLD B (n = 808) | 541 (67.0) | 267 (33.0) | |
| no long-acting bronchodilator | 71 (8.8) | ||
| any ICS a | 198 (24.5) | ||
| GOLD C (n = 9) | 7 (77.8) | 2 (22.2) | |
| no long-acting bronchodilator | 1 (11.1) | ||
| LABA only | 0 (0.0) | ||
| LAMA plus ICS a | 1 (11.1) | ||
| GOLD D (n = 109) | 108 (99.1) | 1 (0.9) | |
| no long-acting bronchodilator | 1 (0.9) | ||
| LABA only | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangold, V.; Boesing, M.; Berset, C.; Bridevaux, P.-O.; Geiser, T.; Joos Zellweger, L.; Kohler, M.; Lüthi-Corridori, G.; Maier, S.; Miedinger, D.; et al. Adherence to the GOLD Guidelines in Primary Care: Data from the Swiss COPD Cohort. J. Clin. Med. 2023, 12, 6636. https://doi.org/10.3390/jcm12206636
Mangold V, Boesing M, Berset C, Bridevaux P-O, Geiser T, Joos Zellweger L, Kohler M, Lüthi-Corridori G, Maier S, Miedinger D, et al. Adherence to the GOLD Guidelines in Primary Care: Data from the Swiss COPD Cohort. Journal of Clinical Medicine. 2023; 12(20):6636. https://doi.org/10.3390/jcm12206636
Chicago/Turabian StyleMangold, Veronika, Maria Boesing, Camille Berset, Pierre-Olivier Bridevaux, Thomas Geiser, Ladina Joos Zellweger, Malcolm Kohler, Giorgia Lüthi-Corridori, Sabrina Maier, David Miedinger, and et al. 2023. "Adherence to the GOLD Guidelines in Primary Care: Data from the Swiss COPD Cohort" Journal of Clinical Medicine 12, no. 20: 6636. https://doi.org/10.3390/jcm12206636
APA StyleMangold, V., Boesing, M., Berset, C., Bridevaux, P.-O., Geiser, T., Joos Zellweger, L., Kohler, M., Lüthi-Corridori, G., Maier, S., Miedinger, D., Thurnheer, R., von Garnier, C., & Leuppi, J. D. (2023). Adherence to the GOLD Guidelines in Primary Care: Data from the Swiss COPD Cohort. Journal of Clinical Medicine, 12(20), 6636. https://doi.org/10.3390/jcm12206636