Challenges in Coagulation Management in Neurosurgical Diseases: A Scoping Review, Development, and Implementation of Coagulation Management Strategies
Abstract
:1. Introduction
- existing guidelines on coagulation management have several limitations;
- an aging population with increased use of antithrombotic medications;
- a higher incidence of coagulation-associated postoperative complications due to the aging population;
- the availability of prediction models for the development of thromboembolic complications that are seldom incorporated into decision-making processes;
- the potential profound impact of reducing bleeding and thromboembolic complications on the healthcare and socioeconomic systems, and;
- the global objective of the WHO is to implement patient blood management
2. Scoping Review of Literature and Identification of Knowledge Gaps
2.1. Literature Search
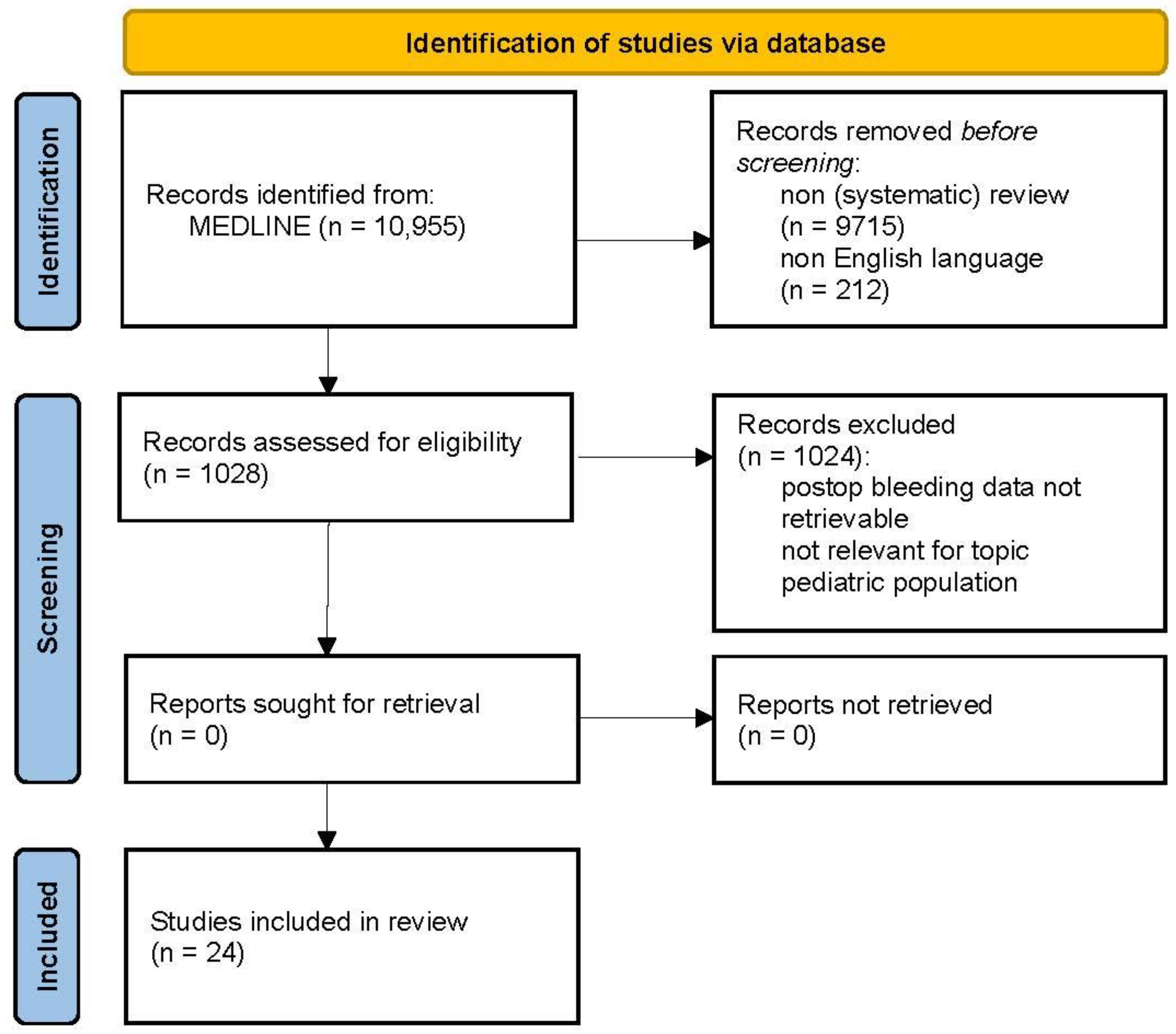
2.2. Bleeding Complications
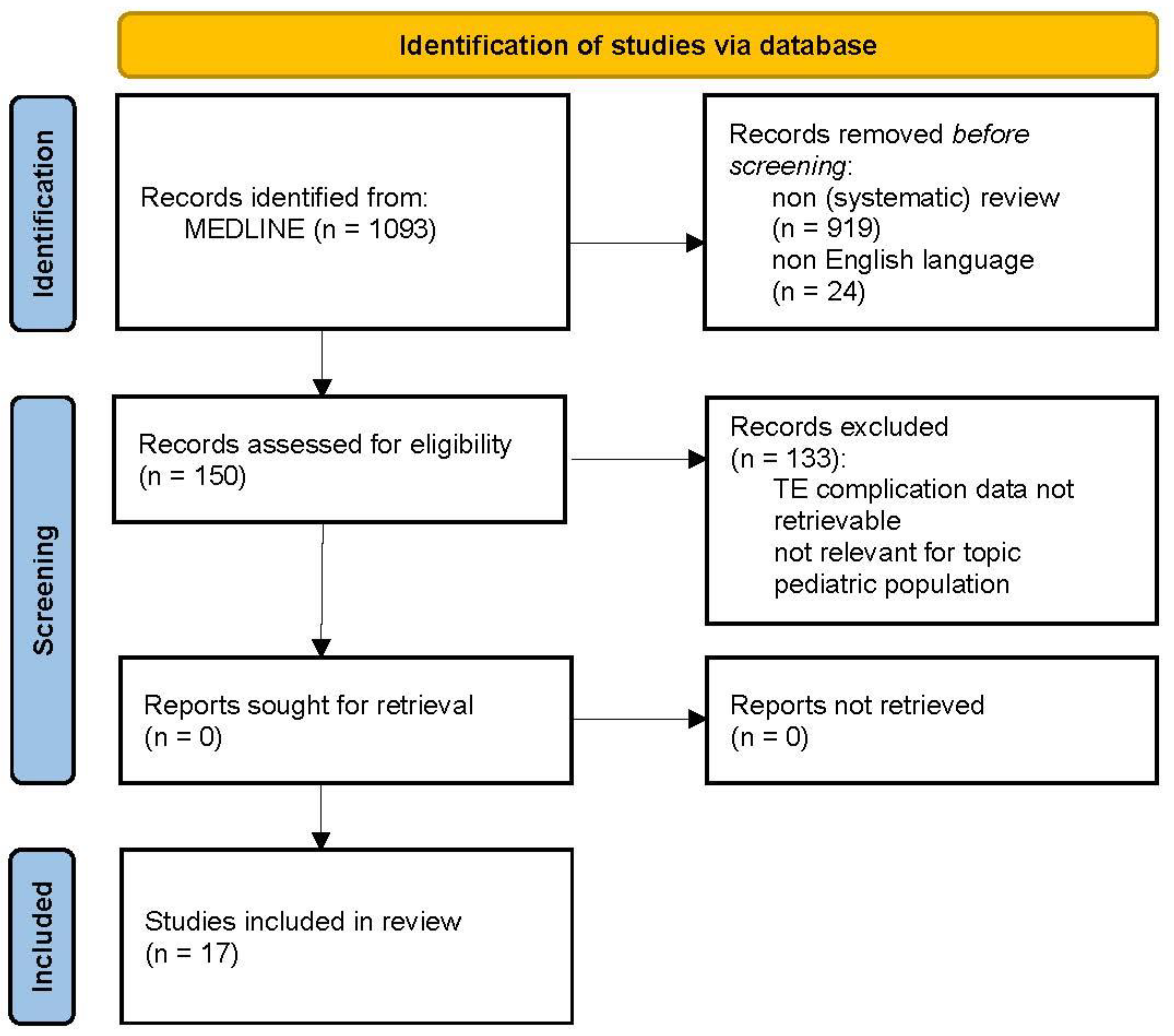
2.3. Thromboembolic Complications
2.4. Available Guidelines
2.5. Knowledge Gaps
2.6. The COMAND Project
- summarize existing evidence on the optimal management of coagulation disorders and prevention of thromboembolic complications for different neurosurgical diseases, and identify knowledge gaps;
- generate new evidence on the optimal management of coagulation disorders and the prevention of thromboembolic complications to cover identified knowledge gaps, and;
- develop recommendations on the sustainable integration and implementation of the evidence-based guideline.
COMAND Project and Medical Research Council Framework
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nittby, H.R.; Maltese, A.; Stahl, N. Early postoperative haematomas in neurosurgery. Acta Neurochir. 2016, 158, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Sparrow, O.C.; Iannotti, F. Postoperative hematoma: A 5-year survey and identification of avoidable risk factors. Neurosurgery 1994, 35, 1061–1064; discussion 1064–1065. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Bertuetti, R.; Rasulo, F.; Bertuccio, A.; Matta, B. Coagulation management in patients undergoing neurosurgical procedures. Curr. Opin. Anaesthesiol. 2017, 30, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Germans, M.; Burkhardt, J.K.; Neidert, M.C.; Fung, C.; Bervini, D.; Zumofen, D.; Rothlisberger, M.; Marbacher, S.; Maduri, R.; et al. Predictors of In-Hospital Death After Aneurysmal Subarachnoid Hemorrhage: Analysis of a Nationwide Database (Swiss SOS [Swiss Study on Aneurysmal Subarachnoid Hemorrhage]). Stroke 2018, 49, 333–340. [Google Scholar] [CrossRef]
- Boluijt, J.; Meijers, J.C.; Rinkel, G.J.; Vergouwen, M.D. Hemostasis and fibrinolysis in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: A systematic review. J. Cereb. Blood Flow Metab. 2015, 35, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.V.; Suggs, J.M.; Diwan, D.; Lee, J.V.; Lipsey, K.; Vellimana, A.K.; Zipfel, G.J. Microvascular platelet aggregation and thrombosis after subarachnoid hemorrhage: A review and synthesis. J. Cereb. Blood Flow Metab. 2020, 40, 1565–1575. [Google Scholar] [CrossRef]
- Fletcher-Sandersjoo, A.; Thelin, E.P.; Maegele, M.; Svensson, M.; Bellander, B.M. Time Course of Hemostatic Disruptions After Traumatic Brain Injury: A Systematic Review of the Literature. Neurocrit. Care 2021, 34, 635–656. [Google Scholar] [CrossRef]
- Broderick, J.P.; Bonomo, J.B.; Kissela, B.M.; Khoury, J.C.; Moomaw, C.J.; Alwell, K.; Woo, D.; Flaherty, M.L.; Khatri, P.; Adeoye, O.; et al. Withdrawal of antithrombotic agents and its impact on ischemic stroke occurrence. Stroke 2011, 42, 2509–2514. [Google Scholar] [CrossRef]
- Collet, J.P.; Montalescot, G.; Blanchet, B.; Tanguy, M.L.; Golmard, J.L.; Choussat, R.; Beygui, F.; Payot, L.; Vignolles, N.; Metzger, J.P.; et al. Impact of prior use or recent withdrawal of oral antiplatelet agents on acute coronary syndromes. Circulation 2004, 110, 2361–2367. [Google Scholar] [CrossRef]
- Senders, J.T.; Goldhaber, N.H.; Cote, D.J.; Muskens, I.S.; Dawood, H.Y.; De Vos, F.; Gormley, W.B.; Smith, T.R.; Broekman, M.L.D. Venous thromboembolism and intracranial hemorrhage after craniotomy for primary malignant brain tumors: A National Surgical Quality Improvement Program analysis. J. Neurooncol. 2018, 136, 135–145. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Cordonnier, C.; Korv, J.; Lal, A.; Ovesen, C.; Purrucker, J.C.; Toni, D.; Steiner, T. European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage. Eur. Stroke J. 2019, 4, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, D.; Comes, R.F.; Geerts, W.; Wiles, M.D.; Force, E.V.G.T. European guidelines on perioperative venous thromboembolism prophylaxis: Neurosurgery. Eur. J. Anaesthesiol. 2018, 35, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Lewin, J.J., 3rd; Rabinstein, A.A.; Aisiku, I.P.; Alexandrov, A.W.; Cook, A.M.; del Zoppo, G.J.; Kumar, M.A.; Peerschke, E.I.; Stiefel, M.F.; et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit. Care 2016, 24, 6–46. [Google Scholar] [CrossRef]
- Gil-Jardine, C.; Payen, J.F.; Bernard, R.; Bobbia, X.; Bouzat, P.; Catoire, P.; Chauvin, A.; Claessens, Y.E.; Douay, B.; Dubucs, X.; et al. Management of Patients Suffering from Mild Traumatic Brain Injury 2023. Anaesth. Crit. Care Pain Med. 2023, 42, 101260. [Google Scholar] [CrossRef]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Hsiang-Yi Chou, S.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hanggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients with Aneurysmal Subarachnoid Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2023, 54, E314–E370. [Google Scholar] [CrossRef]
- Kietaibl, S.; Ahmed, A.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe peri-operative bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second update 2022. Eur. J. Anaesthesiol. 2023, 40, 226–304. [Google Scholar] [CrossRef]
- Ntalouka, M.P.; Brotis, A.G.; Angelis, F.A.; Peroulis, M.; Matsagkas, M.; Fountas, K.N.; Arnaoutoglou, E.M. Appraisal of the Clinical Practice Guidelines for the Use of Antithrombotic Therapy in Elective Spinal Procedures: Do We AGREE (II)? Asian Spine J. 2023, 17, 790–802. [Google Scholar] [CrossRef]
- Schirmer, C.M.; Bulsara, K.R.; Al-Mufti, F.; Haranhalli, N.; Thibault, L.; Hetts, S.W.; Standards, S.; Guidelines, C. Antiplatelets and antithrombotics in neurointerventional procedures: Guideline update. J. Neurointerv. Surg. 2023, 15, 1155–1162. [Google Scholar] [CrossRef]
- Steiner, T.; Juvela, S.; Unterberg, A.; Jung, C.; Forsting, M.; Rinkel, G.; European Stroke, O. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc. Dis. 2013, 35, 93–112. [Google Scholar] [CrossRef]
- Ding, M.; Fratiglioni, L.; Johnell, K.; Fastbom, J.; Ljungdahl, M.; Qiu, C. Atrial fibrillation and use of antithrombotic medications in older people: A population-based study. Int. J. Cardiol. 2017, 249, 173–178. [Google Scholar] [CrossRef] [PubMed]
- EANS Neurotrauma Section. Neurotrauma Section Newsletter. 2023. Available online: https://cdn.ymaws.com/www.eans.org/resource/resmgr/documents/trauma_section/newsletter_April23.pdf (accessed on 15 September 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ye, F.; Long, X.; Li, A.; Xu, H.; Zou, L.; Yang, Y.; You, C. Ultra-Early Treatment for Poor-Grade Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 115, e160–e171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Ding, J.; Chen, H.; Guo, Y.; Wang, G.; Gao, W.W.; Chen, S.W.; Tian, H.L. Predicting progressive hemorrhagic injury after traumatic brain injury: Derivation and validation of a risk score based on admission characteristics. J. Neurotrauma 2012, 29, 2137–2142. [Google Scholar] [CrossRef]
- Caspers, M.; Holle, J.F.; Limper, U.; Frohlich, M.; Bouillon, B. Global Coagulation Testing in Acute Care Medicine: Back to Bedside? Hamostaseologie 2022, 42, 400–408. [Google Scholar] [CrossRef]
- Bonhomme, F.; Boehlen, F.; Clergue, F.; de Moerloose, P. Preoperative hemostatic assessment: A new and simple bleeding questionnaire. Can. J. Anaesth. 2016, 63, 1007–1015. [Google Scholar] [CrossRef]
- Ding, H.; Liu, S.; Quan, X.; Liao, S.; Liu, L. Subperiosteal versus Subdural Drain After Burr Hole Drainage for Chronic Subdural Hematomas: A Systematic Review and Meta-Analysis. World Neurosurg. 2020, 136, 90–100. [Google Scholar] [CrossRef]
- Phan, K.; Moore, J.M.; Griessenauer, C.; Dmytriw, A.A.; Scherman, D.B.; Sheik-Ali, S.; Adeeb, N.; Ogilvy, C.S.; Thomas, A.; Rosenfeld, J.V. Craniotomy Versus Decompressive Craniectomy for Acute Subdural Hematoma: Systematic Review and Meta-Analysis. World Neurosurg. 2017, 101, 677–685.e2. [Google Scholar] [CrossRef]
- Rychen, J.; Saemann, A.; Fingerlin, T.; Guzman, R.; Mariani, L.; Greuter, L.; Soleman, J. Risks and benefits of continuation and discontinuation of aspirin in elective craniotomies: A systematic review and pooled-analysis. Acta Neurochir. 2023, 165, 39–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Schultz, C.; Lanzino, G.; Rabinstein, A.A. Postoperative outcome of cerebral amyloid angiopathy-related lobar intracerebral hemorrhage: Case series and systematic review. Neurosurgery 2012, 70, 125–130; discussion 130. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, X.; Liu, W.; Wong, C.; He, Q.; Chen, Y. Incidence of postoperative symptomatic spinal epidural hematoma requiring surgical evacuation: A systematic review and meta-analysis. Eur. Spine J. 2022, 31, 3274–3285. [Google Scholar] [CrossRef] [PubMed]
- Kao, F.C.; Tsai, T.T.; Chen, L.H.; Lai, P.L.; Fu, T.S.; Niu, C.C.; Ho, N.Y.; Chen, W.J.; Chang, C.J. Symptomatic epidural hematoma after lumbar decompression surgery. Eur. Spine J. 2015, 24, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Goes, R.; Muskens, I.S.; Smith, T.R.; Mekary, R.A.; Broekman, M.L.D.; Moojen, W.A. Risk of aspirin continuation in spinal surgery: A systematic review and meta-analysis. Spine J. 2017, 17, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, D.; Kwee, L.E.; van Putten, E.H.; Kros, J.M.; Dirven, C.M.; Dammers, R. The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma. A retrospective study of a large single center patient cohort. Clin. Neurol. Neurosurg. 2014, 123, 150–154. [Google Scholar] [CrossRef]
- Sweetland, S.; Green, J.; Liu, B.; Berrington de Gonzalez, A.; Canonico, M.; Reeves, G.; Beral, V.; Million Women Study Collaborators. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: Prospective cohort study. BMJ 2009, 339, b4583. [Google Scholar] [CrossRef]
- Algattas, H.; Kimmell, K.T.; Vates, G.E.; Jahromi, B.S. Analysis of Venous Thromboembolism Risk in Patients Undergoing Craniotomy. World Neurosurg. 2015, 84, 1372–1379. [Google Scholar] [CrossRef]
- Hallan, D.R.; Sciscent, B.; Rizk, E. A Retrospective Comparative Cohort Study of Craniotomy and Prophylactic Enoxaparin Timing. Cureus 2022, 14, e23867. [Google Scholar] [CrossRef]
- Rinaldo, L.; Brown, D.A.; Bhargav, A.G.; Rusheen, A.E.; Naylor, R.M.; Gilder, H.E.; Monie, D.D.; Youssef, S.J.; Parney, I.F. Venous thromboembolic events in patients undergoing craniotomy for tumor resection: Incidence, predictors, and review of literature. J. Neurosurg. 2019, 132, 10–21. [Google Scholar] [CrossRef]
- Smith, T.R.; Lall, R.R.; Graham, R.B.; McClendon, J., Jr.; Lall, R.R.; Nanney, A.D.; Adel, J.G.; Zakarija, A.; Chandler, J.P. Venous thromboembolism in high grade glioma among surgical patients: Results from a single center over a 10 year period. J. Neurooncol. 2014, 120, 347–352. [Google Scholar] [CrossRef]
- Kim, K.S.; Brophy, G.M. Symptomatic venous thromboembolism: Incidence and risk factors in patients with spontaneous or traumatic intracranial hemorrhage. Neurocrit. Care 2009, 11, 28–33. [Google Scholar] [CrossRef]
- Liang, C.W.; Su, K.; Liu, J.J.; Dogan, A.; Hinson, H.E. Timing of deep vein thrombosis formation after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2015, 123, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, L. Risk Factors for Venous Thrombosis after Spinal Surgery: A Systematic Review and Meta-analysis. Comput. Math. Methods Med. 2022, 2022, 1621106. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Pritchard, M.W.; Schofield-Robinson, O.J.; Alderson, P.; Smith, A.F. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non-cardiac surgery. Cochrane Database Syst. Rev. 2018, 7, CD012584. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Rothwell, P.M.; Nguyen-Huynh, M.N.; Giles, M.F.; Elkins, J.S.; Bernstein, A.L.; Sidney, S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007, 369, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.K.; Lake, D.E.; DiMarco, J.P.; Ferguson, J.D.; Mangrum, J.M.; Bilchick, K.; Moorman, L.P.; Moorman, J.R. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am. J. Med. 2012, 125, e601–e606. [Google Scholar] [CrossRef]
- Cronin, M.; Dengler, N.; Krauss, E.S.; Segal, A.; Wei, N.; Daly, M.; Mota, F.; Caprini, J.A. Completion of the Updated Caprini Risk Assessment Model (2013 Version). Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619838052. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Akrivou, D.; Perlepe, G.; Kirgou, P.; Gourgoulianis, K.I.; Malli, F. Pathophysiological Aspects of Aging in Venous Thromboembolism: An Update. Medicina 2022, 58, 1078. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wooding, S.; Grant, J. The answer is 17 years, what is the question: Understanding time lags in translational research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, L.J.; Reardon, C.M.; Opra Widerquist, M.A.; Lowery, J. Conceptualizing outcomes for use with the Consolidated Framework for Implementation Research (CFIR): The CFIR Outcomes Addendum. Implement. Sci. 2022, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Flottorp, S.A.; Oxman, A.D.; Krause, J.; Musila, N.R.; Wensing, M.; Godycki-Cwirko, M.; Baker, R.; Eccles, M.P. A checklist for identifying determinants of practice: A systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement. Sci. 2013, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Anderson, C.S.; Song, L.; Jan, S.; Sun, L.; Cheng, G.; Chu, H.; Hu, X.; Ma, L.; Chen, X.; et al. Implementing a Goal-Directed Care Bundle after Acute Intracerebral Haemorrhage: Process Evaluation for the Third INTEnsive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial Study in China. Cerebrovasc. Dis. 2022, 51, 373–383. [Google Scholar] [CrossRef]
- Baatiema, L.; Otim, M.E.; Mnatzaganian, G.; de-Graft Aikins, A.; Coombes, J.; Somerset, S. Health professionals’ views on the barriers and enablers to evidence-based practice for acute stroke care: A systematic review. Implement. Sci. 2017, 12, 74. [Google Scholar] [CrossRef]
| Bleeding Complication | |||
|---|---|---|---|
| Disease Type | Before Treatment | During Surgery | After Treatment |
| Intracranial tumor surgery | intratumoral hemorrhage with extension to: epidural subdural intraparenchymal intraventricular | excessive blood loss (remote) intraparenchymal hemorrhage | intraventricular hemorrhage intracavitary hemorrhage intraparenchymal hemorrhage subdural hemorrhage epidural hemorrhage subcutaneous hemorrhage |
| Traumatic brain injury | intraventricular hemorrhage intraparenchymal hemorrhage subarachnoid hemorrhage subdural hemorrhage epidural hemorrhage subcutaneous hemorrhage | ||
| Intracranial hemorrhage | intraventricular hemorrhage intraparenchymal hemorrhage subarachnoid hemorrhage (aneurysm rebleeding) subdural hemorrhage epidural hemorrhage | ||
| Spine surgery | intramedullary hemorrhage intradural, extramedullary hemorrhage epidural hemorrhage | excessive blood loss injury of paraspinal vasculature | subdural hemorrhage epidural hemorrhage subcutaneous hemorrhage |
| 1. Have any medications affecting clotting been used in the last 14 days? |
| 2. Have you ever consulted a doctor or received treatment for prolonged or unusual bleeding (such as nosebleeds, minor wounds)? |
| 3. Do you experience bruises/hematomas larger than 2 cm without trauma or severe bruising after minor trauma? |
| 4. After a tooth extraction, have you ever experienced prolonged bleeding requiring medical/dental consultation? |
| 5. Have you experienced excessive bleeding during or after surgery? |
| 6. Is there anyone in your family who suffers from a coagulation disease (such as hemophilia, von Willebrand disease, etc.)? |
| For females: |
| 1. Have you ever consulted a doctor or received a treatment for heavy or prolonged menstrual periods (contraceptive pill, iron, etc.)? |
| 2. Did you experience prolonged or excessive bleeding after delivery? |
| Type of TE Complication | Risk (%) | Interval | |
|---|---|---|---|
| Craniotomy [37,38] | VTE | 3.2–7.8 | n.a. |
| MI | 2.5 | n.a. | |
| Stroke | 3.6 | n.a. | |
| Intracranial tumor surgery [39,40] | VTE | 3.0–17.0 | <30 days 1 |
| Malignant brain tumor [10] | VTE | 3.5 | <30 days |
| Intracranial meningioma [35] | VTE | 7.2 | <60 days |
| TBI [41] | VTE | 3.8 | <60 days |
| SAH [41,42] | VTE | 6.7–21 | <60 days 2 |
| Intracerebral hemorrhage [41] | VTE | 2.9 | <60 days |
| Spinal surgery [34,43] | VTE | 5.1 | n.a. |
| MI | 1.3 | n.a. | |
| Stroke | 0.9 | n.a. | |
| Surgery in general [36,44] | IE | 4.4 | <30 days |
| VTE | 3.3 | <84 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germans, M.R.; Rohr, J.; Globas, C.; Schubert, T.; Kaserer, A.; Brandi, G.; Studt, J.-D.; Greutmann, M.; Geiling, K.; Verweij, L.; et al. Challenges in Coagulation Management in Neurosurgical Diseases: A Scoping Review, Development, and Implementation of Coagulation Management Strategies. J. Clin. Med. 2023, 12, 6637. https://doi.org/10.3390/jcm12206637
Germans MR, Rohr J, Globas C, Schubert T, Kaserer A, Brandi G, Studt J-D, Greutmann M, Geiling K, Verweij L, et al. Challenges in Coagulation Management in Neurosurgical Diseases: A Scoping Review, Development, and Implementation of Coagulation Management Strategies. Journal of Clinical Medicine. 2023; 12(20):6637. https://doi.org/10.3390/jcm12206637
Chicago/Turabian StyleGermans, Menno R., Jonas Rohr, Christoph Globas, Tilman Schubert, Alexander Kaserer, Giovanna Brandi, Jan-Dirk Studt, Matthias Greutmann, Katharina Geiling, Lotte Verweij, and et al. 2023. "Challenges in Coagulation Management in Neurosurgical Diseases: A Scoping Review, Development, and Implementation of Coagulation Management Strategies" Journal of Clinical Medicine 12, no. 20: 6637. https://doi.org/10.3390/jcm12206637
APA StyleGermans, M. R., Rohr, J., Globas, C., Schubert, T., Kaserer, A., Brandi, G., Studt, J.-D., Greutmann, M., Geiling, K., Verweij, L., & Regli, L. (2023). Challenges in Coagulation Management in Neurosurgical Diseases: A Scoping Review, Development, and Implementation of Coagulation Management Strategies. Journal of Clinical Medicine, 12(20), 6637. https://doi.org/10.3390/jcm12206637






