Neuroendocrine Carcinoma of the Urinary Bladder: CT Findings and Radiomics Signature
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Series
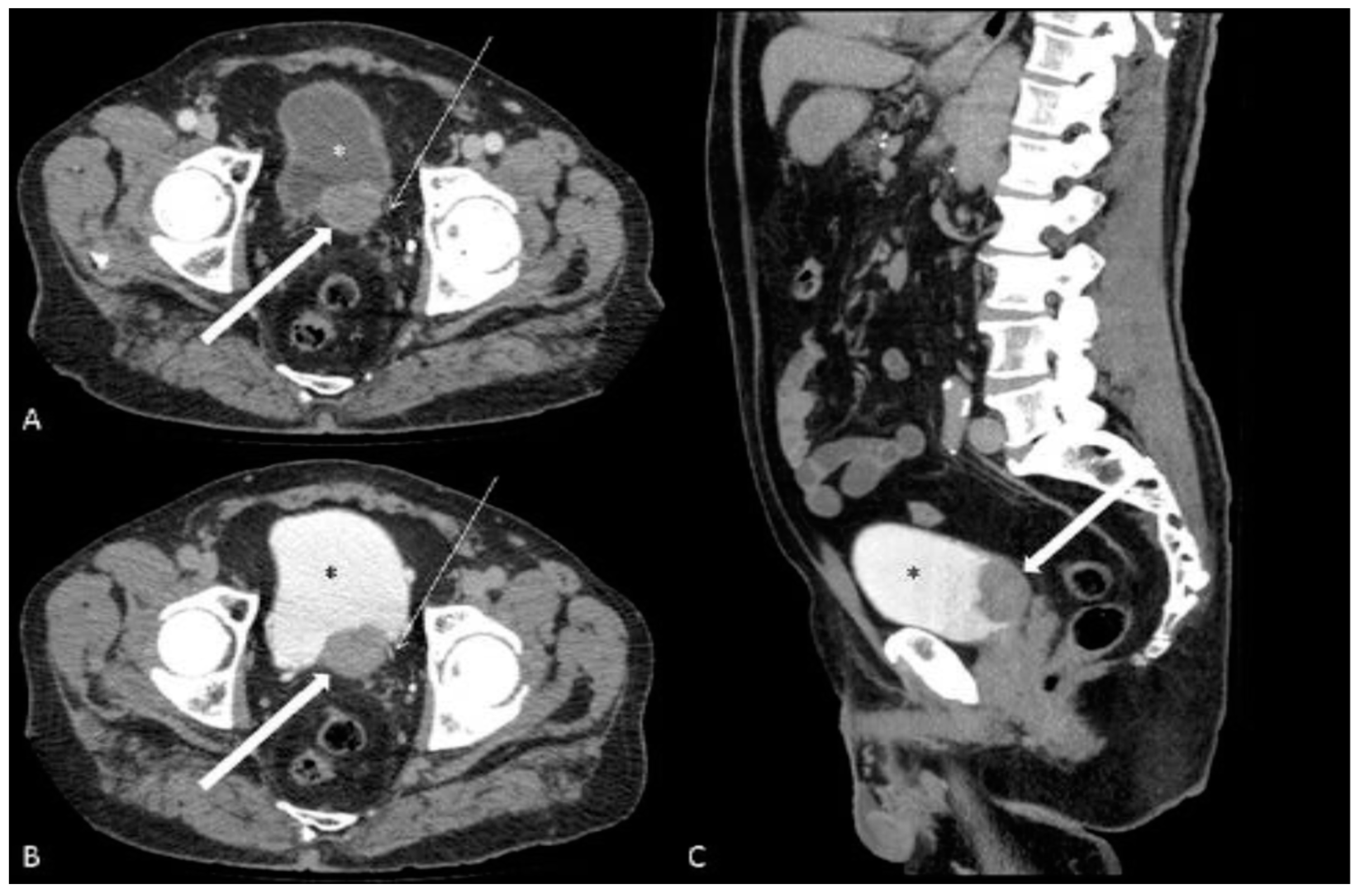
2.2. Image Data Acquisition and Interpretation
2.3. Volume of Interest (VOI) Segmentation
2.4. Radiomics Feature Extraction
2.5. Literature Review
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koay, E.J.; Teh, B.S.; Paulino, A.C.; Butler, E.B. A Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: Epidemiology, prognostic variables, and treatment trends. Cancer 2011, 117, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Pompas-Veganzones, N.; Gonzalez-Peramato, P.; Sanchez-Carbayo, M. The neuroendocrine component in bladder tumors. Curr. Med. Chem. 2014, 21, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.S.; Wein, A.J.; Bing, Z.; Malkowicz, S.B.; Guzzo, T.J. Neuroendocrine tumor of the bladder. Rev. Urol. 2010, 12, e197–e201. [Google Scholar] [PubMed]
- Pósfai, B.; Kuthi, L.; Varga, L.; Laczó, I.; Révész, J.; Kránicz, R.; Maráz, A. The Colorful Palette of Neuroendocrine Neoplasms in the Genitourinary Tract. Anticancer Res. 2018, 38, 3243–3254. [Google Scholar] [CrossRef] [PubMed]
- Lozano, F.; Raventós, C.X.; Carrión, A.; Trilla, E. Optimización del seguimiento de tumores vesicales no músculo invasivo con biomarcadores [Optimization biomarkers in the surveillance of non muscle invasive bladder cancer.]. Arch. Esp. Urol. 2022, 75, 133–143. [Google Scholar]
- Manunta, A.; Vincendeau, S.; Kiriakou, G.; Lobel, B.; Guille, F. Non-transitional cell bladder carcinomas. BJU Int. 2005, 95, 497–502. [Google Scholar] [CrossRef]
- Moretto, P.; Wood, L.; Emmenegger, U.; Blais, N.; Mukherjee, S.D.; Winquist, E.; Belanger, E.C.; MacRae, R.; Balogh, A.; Cagiannos, I.; et al. Management of small cell carcinoma of the bladder: Consensus guidelines from the Canadian Association of Genitourinary Medical Oncologists (CAGMO). Can. Urol. Assoc. J. 2013, 7, E44–E56. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Kamat, A.M.; Grossman, H.B.; Williams, D.L.; Qiao, W.; Thall, P.F.; Dinney, C.P.; Millikan, R.E. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J. Clin. Oncol. 2009, 27, 2592–2597. [Google Scholar] [CrossRef]
- Koenigkam-Santos, M.; Ferreira-Junior, J.R.; Wada, D.T.; Tenório, A.P.M.; Nogueira-Barbosa, M.H.; Azevedo-Marques, P.M.A. Artificial intelligence, machine learning, computer-aided diagnosis, and radiomics: Advances in imaging towards to precision medicine. Radiol. Bras. 2019, 52, 387–396. [Google Scholar] [CrossRef]
- Ge, L.; Chen, Y.; Yan, C.; Zhao, P.; Zhang, P.; ARand Liu, J. Study Progress of Radiomics with Machine Learning for Precision Medicine in Bladder Cancer Management. Front. Oncol. 2019, 9, 1296. [Google Scholar] [CrossRef]
- Kouba, E.; Cheng, L. Neuroendocrine Tumors of the Urinary Bladder According to the 2016 World Health Organization Classification: Molecular and Clinical Characteristics. Endocr. Pathol. 2016, 27, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Kim, K.H.; Jung, S. Small cell carcinoma of the urinary bladder: CT and MR imaging findings. Korean J. Radiol. 2003, 4, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Boyer, A.C.; Jafri, S.Z.; Jafri, S.M.A.; Amin, M.B. Neuroendocrine carcinoma of the urinary bladder: A retrospective study of CT findings. Abdom. Imaging 2013, 38, 870–876. [Google Scholar] [CrossRef]
- Xia, K.; Zhong, W.; Chen, J.; Lai, Y.; Huang, G.; Liu, H.; Dong, W.; He, W.; Lin, T.; Huang, J. Clinical Characteristics, Treatment Strategy, and Outcomes of Primary Large Cell Neuroendocrine Carcinoma of the Bladder: A Case Report and Systematic Review of the Literature. Front. Oncol. 2020, 10, 1291. [Google Scholar] [CrossRef]
- Bote, H.E.; Alaoui, A.E.; Oussama, Z.; El Sayegh, H.; Iken, A.; Benslimane, L.; Nouini, Y. Neuroendocrine carcinoma of the bladder: About 5 cases. Pan. Afr. Med. J. 2017, 26, 92. [Google Scholar] [CrossRef] [PubMed]
- Colarossi, C.; Pino, P.; Giuffrida, D.; Aiello, E.; Costanzo, R.; Martinetti, D.; Memeo, L. Large cell neuroendocrine carcinoma (LCNEC) of the urinary bladder: A case report. Diagn. Pathol. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Rebuzzi, S.E.; Magliocca, F.M.; Speranza, I.; Corongiu, E.; Borgoni, G.; Perugia, G.; Liberti, M.; Bianco, V. Neoadjuvant Chemotherapy in Neuroendocrine Bladder Cancer: A Case Report. Am. J. Case Rep. 2016, 17, 248–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chong, V.; Zwi, J.; Hanning, F.; Lim, R.; Williams, A.; Cadwallader, J. A case of large cell neuroendocrine carcinoma of the bladder with prolonged spontaneous remission. J. Surg. Case Rep. 2017, 2017, rjw179. [Google Scholar] [CrossRef]
- Bertaccini, A.; Marchiori, D.; Cricca, A.; Garofalo, M.; Giovannini, C.; Manferrari, F.; Gerace, T.G.; Pernetti, R.; Martorana, G. Neuroendocrine carcinoma of the urinary bladder: Case report and review of the literature. Anticancer Res. 2008, 28, 1369–1372. [Google Scholar]
- Olivieri, V.; Fortunati, V.; Bellei, L.; Massarelli, M.; Ruggiero, G.; Abate, D.; Serra, N.; Griffa, D.; Forte, F.; Corongiu, E. Primary small-cell neuroendocrine carcinoma of the bladder: Case report and literature review. Arch. Ital. Urol. Androl. 2020, 92. [Google Scholar] [CrossRef]
- Praveen, B.; Varghese, J.; Krishnamoorthy, H.; Pillai, B.S. A rare case of small cell neuroendocrine tumor of the urinary bladder. Indian J. Pathol. Microbiol. 2020, 63, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Chekrine, T.; De Bari, B.; Cassier, P.; Kulisa, M.; Chapet, O.; Mornex, F. Small cell neuroendocrine carcinoma of the bladder: A case report and review of the literature. Cancer Radiother. 2011, 15, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, C.; Busetto, G.M.; Antonini, G.; Giovannone, R.; Di Placido, M.; Soda, G.; De Berardinis, E.; Gentile, V. Primary metastatic neuroendocrine small cell bladder cancer: A case report and literature review. Urol. Int. 2012, 88, 365–369. [Google Scholar] [CrossRef]
- Masood, B.; Iqbal, N.; Iqbal, W.; Masood, Y.; Akbar, M.K.; Mamoon, N. Small-cell neuroendocrine carcinoma of the urinary bladder: A case report. Int. J. Health Sci. 2020, 14, 53–55. [Google Scholar]
- He, B.; Ye, L.; Zeng, H.; Yao, J. Differentiation of urothelial carcinoma and neuroendocrine carcinoma in bladder based on imaging: A CT-based texture analysis. Asian J. Surg. 2023. ahead of print. [Google Scholar] [CrossRef]
- Yang, F.; Wen, Z. Computed tomography manifestations and pathological features of neuroendocrine carcinoma in uncommon sites. Transl. Cancer Res. 2020, 9, 6912–6918. [Google Scholar] [CrossRef]
- Abouelkheir, R.T.; Abdelhamid, A.; Abou El-Ghar, M.; El-Diasty, T. Imaging of Bladder Cancer: Standard Applications and Future Trends. Medicina 2021, 57, 220. [Google Scholar] [CrossRef]
- Browne, R.F.J.; Meehan, C.P.; Colville, J.; Power, R.; Torreggiani, W.C. Transitional cell carcinoma of the upper urinary tract: Spectrum of imaging findings. Radiographics 2005, 25, 1609–1627. [Google Scholar] [CrossRef]
- Ng, C.S. Radiologic diagnosis and staging of renal and bladder cancer. Semin. Roentgenol. 2006, 41, 121–138. [Google Scholar] [CrossRef]
- Bezzi, C.; Mapelli, P.; Presotto, L.; Neri, I.; Scifo, P.; Savi, A.; Bettinardi, V.; Partelli, S.; Gianolli, L.; Falconi, M.; et al. Radiomics in pancreatic neuroendocrine tumors: Methodological issues and clinical significance. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4002–4015. [Google Scholar] [CrossRef]
- Canellas, R.; Burk, K.S.; Parakh, A.; Sahani, D.V. Prediction of Pancreatic Neuroendocrine Tumor Grade Based on CT Features and Texture Analysis. AJR Am. J. Roentgenol. 2018, 210, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhuge, X.; Wang, Z.; Wang, Q.; Sun, K.; Feng, Z.; Chen, X. Textural analysis on contrast-enhanced CT in pancreatic neuroendocrine neoplasms: Association with WHO grade. Abdom. Radiol. 2019, 44, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, C.; Ye, Z. Prediction of Pancreatic Neuroendocrine Tumor Grading Risk Based on Quantitative Radiomic Analysis of MR. Front. Oncol. 2021, 11, 758062. [Google Scholar] [CrossRef] [PubMed]

| Author | No. | Sex | Age | Haematuria | LUTS | Hydronephrosis | RE | Location | Dimensions (cm) | Density (HU) | Margins | Necrosis | Calcifications | Wall Thickness (mm) | DOI (mm) | T3 | T4 | Lymphnodes | Metastasis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study | # 1 | M | 79 | yes | no | yes | yes | PT | 3.1 × 2.3 | 67 | ill | no | no | 11 | 6,5 | yes | yes | no | Liver |
| # 2 | M | 83 | yes | no | yes | yes | ALT | 9.5 × 4.5 | 30 | sharp | no | no | 17 | 32 | yes | no | no | no | |
| # 3 | M | 76 | yes | yes | yes | yes | ART | 6.0 × 3.2 | 51 | sharp | no | no | 6 | 25 | yes | no | no | Liver | |
| # 4 | M | 56 | yes | yes | yes | yes | PRT | 8.4 × 7.8 | 38 | ill | no | no | 19 | 21 | yes | yes | yes | Bone | |
| # 5 | M | 62 | yes | no | yes | no | ALRT | 15.1 × 12.6 | 55 | ill | no | yes | 43 | 17 | yes | yes | yes | no | |
| # 6 | M | 75 | no | yes | yes | no | T | 3.3 × 1.6 | 44 | ill | no | no | 14 | 5 | no | no | no | no | |
| # 7 | M | 84 | yes | no | yes | no | PLART | 12.4 × 12.2 | 31 | sharp | no | no | 30 | 9,5 | yes | yes | yes | no | |
| # 8 | M | 70 | yes | yes | yes | no | PT | 3.6 × 1.7 | 34 | sharp | no | no | 9 | 10 | yes | yes | no | no | |
| # 9 | M | 85 | yes | no | no | yes | A | 4.5 × 2.0 | 84 | ill | no | no | 17 | 5 | yes | no | no | no | |
| # 10 | M | 76 | yes | no | yes | yes | PRT | 3.0 × 3.3 | 59 | ill | yes | no | 15 | 5 | no | no | yes | no | |
| # 11 | M | 83 | yes | no | no | yes | PT | 5.0 × 2.5 | 57 | ill | no | no | 17 | 13 | yes | no | no | no | |
| # 12 | M | 76 | yes | no | yes | no | T | 2.5 × 1.9 | 49 | ill | no | no | 10 | 8 | no | no | no | no | |
| # 13 | M | 83 | yes | yes | no | yes | PR | 3.0 × 1.3 | 68 | ill | no | no | 15 | 5 | yes | no | no | no | |
| # 14 | M | 83 | yes | no | no | yes | PL | 2.5 × 2.5 | 48 | ill | yes | no | 2 | 22 | yes | no | yes | Liver | |
| Kim [12] | # 1 | M | 44 | AT | 3.8 | yes | no | yes | yes | yes | Brain | ||||||||
| # 2 | M | 56 | PLT | 5.5 | yes | no | yes | yes | yes | no | |||||||||
| # 3 | M | 57 | AL | 3 | yes | no | yes | yes | yes | Liver | |||||||||
| # 4 | M | 59 | AR | 4.1 | no | no | yes | yes | no | no | |||||||||
| # 5 | M | 66 | APT | 5.2 | no | no | yes | yes | yes | Liver | |||||||||
| # 6 | F | 59 | PT | 8.2 | no | yes | yes | yes | yes | no | |||||||||
| Author | No./Sex | Age | Haematuria | LUTS | Hydronephrosis | RE | Location | Dimensions (cm) | Density (HU) | Margins | Necrosis | Calcifications | Wall Thickness (mm) | DOI (mm) | T3 | T4 | Lymphnodes | Metastasis | |
| Boyer [13] | 13 M/3 F | 75.5 | 11/16 | 7/16 | 4.9 | 3/16 | 1/16 | 9 (mean) | 9/16 | 6/16 | 5/16 | 8/16 (Liver, bone, lung) | |||||||
| Xia [14] | 31 M/8 F | 61.5 | 20/39 | 4.1 × 1.8 | 1/39 | 38/39 | 7/39 | 6/39 | |||||||||||
| Bote [15] | 4 M/1 F | 63 | 5/5 | 2/5 | T in 4/5 | 3/5 | 2/5 | ||||||||||||
| Colarossi [16] | 1/F | 53 | P | 4 | sharp | yes | yes | yes | |||||||||||
| Prelaj [17] | 1/M | 71 | yes | yes | yes | A | 3.4 × 2.4 | ill | yes | no | no | no | no | no | |||||
| Chong [18] | 1/M | 72 | yes | T | yes | yes | yes | no | |||||||||||
| Bertaccini [19] | 1/M | 37 | yes | P | 2.5 × 2 | ill | yes | no | yes | no | yes | no | |||||||
| Olivieri [20] | 1/M | 78 | yes | yes | no | no | L | 3 | ill | no | no | no | no | no | no | ||||
| Praveen [21] | 1/M | 50 | yes | yes | yes | yes | L | 3 × 3 | no | yes | no | no | no | ||||||
| Chekrine [22] | 1/M | 84 | yes | yes | PR | 2.6 × 5.7 | sharp | yes | no | yes | yes | yes | no | ||||||
| Cerulli [23] | 1/M | 60 | yes | yes | T | 6 | yes | yes | yes | yes | |||||||||
| Masood [24] | 1/M | 60 | yes | yes | L | 3 × 4 | no | no | no | ||||||||||
| He [25] | 10/M | 64.9 | T in 7/10 | 5.2 | 0/10 | 6/10 | 2/10 | 1/10 | 0/10 | ||||||||||
| NECB | Control | p-Value | |
|---|---|---|---|
| Age (years) | 76.5 ± 8.7 | 78.66 ± 6.60 | |
| Male Sex | 14/14—100.0% | 29/42—69.05% | 0.025 |
| Hydronephrosis | 10/14—71.43% | 14/42—33.33% | 0.027 |
| Haematuria | 13/14—92.86% | 7/42—16.67% | |
| LUTS | 5/14—35.71% | 19/42—45.24% | |
| density (HU) | 51.01 ± 15.48 | 76.27 ± 22.26 | <0.001 |
| Product of dimensions (cm2) | 38.1 ± 59.3 | 14.44 ± 12.98 | 0.033 |
| Trigonal region involvement | 11/14—78.57% | 8/42—19.05% | <0.001 |
| Ring enhancement | 9/14—64.29% | 28/42—66.67% | |
| Ill-defined margins | 10/14—71.43% | 29/42—69.05% | |
| Intra-mass necrosis | 2/14—14.29% | 11/42—26.19% | |
| Calcifications | 1/14—7.14% | 2/42—4.76% | |
| cT3 | 3/14—21.43% | 33/42—78.57% | |
| cT4 | 5/14—35.71% | 22/42—52.38% | |
| Lymph-node involvement | 5/14—35.71% | 23/42—54.76% | |
| Distant metastasis | 4/14—28.57% | 10/42—23.81% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, A.; Gatta, T.; Pini, G.M.; Scordi, G.; Fontana, F.; Piacentino, F.; Minici, R.; Laganà, D.; Basile, A.; Dehò, F.; et al. Neuroendocrine Carcinoma of the Urinary Bladder: CT Findings and Radiomics Signature. J. Clin. Med. 2023, 12, 6510. https://doi.org/10.3390/jcm12206510
Coppola A, Gatta T, Pini GM, Scordi G, Fontana F, Piacentino F, Minici R, Laganà D, Basile A, Dehò F, et al. Neuroendocrine Carcinoma of the Urinary Bladder: CT Findings and Radiomics Signature. Journal of Clinical Medicine. 2023; 12(20):6510. https://doi.org/10.3390/jcm12206510
Chicago/Turabian StyleCoppola, Andrea, Tonia Gatta, Giacomo Maria Pini, Giorgia Scordi, Federico Fontana, Filippo Piacentino, Roberto Minici, Domenico Laganà, Antonio Basile, Federico Dehò, and et al. 2023. "Neuroendocrine Carcinoma of the Urinary Bladder: CT Findings and Radiomics Signature" Journal of Clinical Medicine 12, no. 20: 6510. https://doi.org/10.3390/jcm12206510
APA StyleCoppola, A., Gatta, T., Pini, G. M., Scordi, G., Fontana, F., Piacentino, F., Minici, R., Laganà, D., Basile, A., Dehò, F., Carcano, G., Franzi, F., Uccella, S., Sessa, F., & Venturini, M. (2023). Neuroendocrine Carcinoma of the Urinary Bladder: CT Findings and Radiomics Signature. Journal of Clinical Medicine, 12(20), 6510. https://doi.org/10.3390/jcm12206510








