Abstract
This study aimed to determine the effect of long-term exercise on the risk of developing cardiovascular diseases (CVD) and all-cause mortality in patients with diabetic kidney disease (DKD). A single-center, prospective intervention study using propensity score matching was performed over 24 months. The intervention group (n = 67) received six months of individual exercise instruction from a physical therapist, who performed aerobic and muscle-strengthening exercises under unsupervised conditions. New events were defined as the composite endpoint of stroke or CVD requiring hospitalization, initiation of hemodialysis or peritoneal dialysis, or all-cause mortality. The cumulative survival rate without new events at 24 months was significantly higher in the intervention group (0.881, p = 0.016) than in the control group (n = 67, 0.715). Two-way analysis of variance revealed a significant effect of the group factor on high density lipoprotein-cholesterol (HDL-C) which was higher in the intervention group than in the control group (p = 0.004); eGFRcr showed a significant effect of the time factor, which was lower at 24 months than before intervention (p = 0.043). No interactions were observed for all items. In conclusion, aerobic exercises combined with upper and lower limb muscle strengthening for six months reduce the risk of developing CVD and all-cause mortality in patients with DKD.
1. Introduction
The global diabetic population in 2021 was 536.6 million, estimated to increase to 783.2 million by 2045, with the Western Pacific region, including Japan, reported as having the largest diabetic population worldwide [1]. A multicenter, cross-sectional study in Japan found that 54% of patients with type 2 diabetes mellitus had diabetic kidney disease (DKD) [2]. DKD is a significant cause of end-stage renal disease (ESRD) [3,4,5,6]. Diabetes treatment is associated with high medical costs [7]. In addition, DKD is associated with a high mortality rate and high socioeconomic costs; therefore, there is an urgent need to establish effective strategies to slow the progression of DKD [8].
DKD is strongly affected by cardiovascular diseases (CVD) associated with diabetes mellitus. Notably, diabetes mellitus is an independent and definitive risk factor for CVD [9,10]. Furthermore, between 1990 and 2019, the number of patients with CVD almost doubled from 271 million to 523 million; deaths from CVD increased, accounting for about one-third of global deaths [11]. DKD is more strongly associated with excess risk, as patients with DKD and diabetic retinopathy have a significantly higher risk of death from all-cause mortality and cardiovascular complications [12].
Since the risk of death from CVD increases with the development of DKD [13], preventing CVD may effectively alleviate the severity of DKD. Therefore, increasing physical activity through regular exercise is an essential component of the treatment plan for patients with diabetes. In addition, there is strong evidence of an inverse association between the amount of physical activity and the risk of CVD mortality [14]. Furthermore, moderate- to vigorous-intensity aerobic exercise significantly reduces CVD risk and all-cause mortality in patients with type 1 and type 2 diabetes [15]. While the evidence for exercise in diabetic patients is well established, there is still no consensus on the effectiveness of training for DKD [16,17]. Exercise may be equally effective for DKD patients with underlying diabetes mellitus.
Consequently, we hypothesized that increased physical activity through regular aerobic exercise would be an effective therapeutic tool to reduce the incidence of CVD in patients with DKD. Therefore, this study aimed to determine the impact of increased physical activity through a long-term hybrid exercise program (supervised sessions + home-based sessions) under the guidance of physical DKD therapists on the risk of developing CVD and all-cause mortality.
2. Materials and Methods
2.1. Study Population
A total of 850 patients registered in our hospital’s outpatient database of diabetes mellitus were included in this study. Of these, 187 cases were excluded, including patients who were difficult to follow up on due to interruption of hospital care, death, and transfer to another hospital and those for whom DKD severity classification was impossible due to lack of data. Of the final 663 participants included in the study, interventions were provided to 89 with consecutive prescriptions for exercise instruction by a physician. Twenty-two patients dropped out during the intervention, and the remaining 67 were classified as the intervention group. A propensity score matching was performed from the historical cohort group based on the basic information of the intervention group and blood sampling data; 67 cases were selected as the control group for this study (Figure 1). Inclusion criteria for the intervention group were type 2 diabetes, DKD (stage I-V), no maintenance dialysis therapy, physician permission for exercise therapy, and no dementia. DKD severity was classified according to eGFR and urinary albumin levels [18] (Table S1).
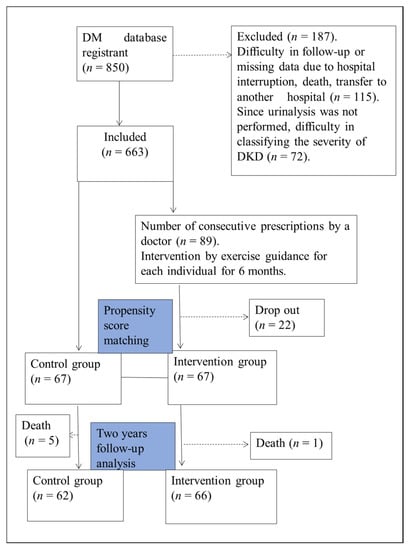
Figure 1.
Study flowchart. This is a single-center, prospective intervention study using propensity score matching. The study population comprised 663 patients who could be followed up. Sixty-seven patients who completed the 6-month intervention were designated as the Intervention group. The 67 patients in the cohort group matched by propensity score matching with the intervention group were designated as the control group and followed up for 24 months.
2.2. Study Design
This is a single-center, prospective interventional study. The observation period was between August 2016 and August 2019; the intervention period was six months. The follow-up period was set as 24 months after the intervention, and the data after 24 months were used for analysis.
2.3. Ethics Approval
The study was conducted per the Declaration of Helsinki guidelines and the ethics guidelines for clinical research from the Ministry of Health, Labor, and Welfare (Tokyo, Japan). Furthermore, the protocol was reviewed and accepted by the ethics committee of Dokkyo Medical University Nikko Medical Center (Nikko 27001). Written informed consent was obtained from all participants in the intervention group. In addition, informed consent was obtained using the opt-out system in the non-intervention group; those who rejected it were excluded.
2.4. Intervention
2.4.1. Educational Programs
The educational session on exercise instruction was designed to help the participants acquire an exercise habit and included diabetes self-management education (DSME) [19]. Because DSME interventions reportedly improve glycemic control [20] and reduce the risk of all-cause mortality [21] with a minimum of 10 h of contact between 6 and 12 months, our educational intervention was conducted following these criteria. In the first month of the intervention, we focused on motivation, including group education, individual education, and counseling; in the second month, the participants were given activity meters and individual exercise instruction and were allowed to exercise unsupervised until the sixth month. During this period of exercise guidance, the patients visited the clinic at least once a month for educational intervention (e.g., benefits and necessity of exercise therapy) and counseling (e.g., listening to factors that inhibit exercise and motivation), confirmation of the content and load of exercise, collection of activity meter data, and measurement of body composition to support the continued exercise.
These educational interventions were conducted by physical therapists certified as cardiac rehabilitation instructors and Certified Diabetes Educators from Japan.
In the control group, the physical therapists did not provide exercise instruction using the DSME. Conversely, diabetic nurses and dietitians provided lifestyle and nutritional guidance to both groups. Therefore, the difference in intervention between the two groups was the physical therapists’ presence or absence of DSME-based exercise instruction.
2.4.2. Exercise Instruction Program
The home-based exercise program consisted of aerobic exercises and eight muscle-strengthening exercises [22,23]. The content of the exercise instruction is as follows;
- (1)
- Walking as a mild-to-moderate aerobic exercise for at least 20 min/day and at least three times/week [24], with a goal of performing this at least 150 min/week [22]. For intensity setting, the target heart rate by the Karvonen method was set according to the stage of each participant (DKD stage I, II: 0.6, III: 0.5, IV: 0.4); if pulse detection was difficult, the Rate of Perceived Exertion by the Borg Scale was set at 12–13 [23].
- (2)
- Resistance exercises were performed using TheraBand® and bodyweight resistance for major muscle groups in the upper and lower extremities at least three days per week, with no more than two consecutive days. Participants performed eight resistance exercises (side raise, biceps curl, triceps pull-down, sitting knee raise, knee extension, hamstring curl, and calf raises and squats), with 8–12 repetitions at an intensity that felt somewhat tight (Borg scale: 13) [23,24]. If they could not keep up with these exercises, they were instructed to perform them at an intensity that would not leave them fatigued the next day. In addition, a pamphlet illustrating the contents of the home exercise program was distributed to all participants so they could check the contents at any time.
2.4.3. Encouragement of Physical Activity
To increase adherence to exercise and establish an exercise habit, we measured and recorded the extent of daily physical activity. Consequently, the participants were lent an activity meter (Activity Style Pro HJA-350IT; Omron Healthcare, Kyoto, Japan) and instructed to wear it from when they woke up to when they went to bed for five months, from the second to the sixth month of the exercise instruction intervention. In addition, a self-management notebook was distributed to the participants; they were instructed to record the number of steps, walking time, resistance exercise, weight, and blood pressure at the end of each day.
2.5. Physical Activity Measurement
In this study, physical activity was assessed in the intervention group using an activity meter and a short-form of the International Physical Activity Questionnaire (IPAQ) [25].
A 3-axis acceleration sensor was built to measure the amount of physical activity; it can be used with commercially available activity meters [26,27]. The activity meter was worn on the waist for five months (size: 80 × 50 × 20 mm; weight: 60 g including batteries); the participant was instructed to wear it throughout the day except when sleeping and bathing. If the participants wore it for > 10 h per day, the data of that day was adopted; if there were more than two good days that they wore it on weekdays and one day at the weekend, the individual’s data was adopted [28]. The participants were asked to visit the clinic once a month to capture the data from the activity meter; the data were analyzed using 10-s epochs. From the obtained individual data, wear time (min/day), walking time (min/day), and activity time by intensity (min/day) was used for analysis. In addition, the ratio of walking and activity time by intensity was calculated from the recruitment data (min/day/wearing time). These values were not included in the data analysis but were used to ensure that the instructed exercises were performed objectively. We defined light physical activity as activity below 3 METs, moderate-to-vigorous physical activity (MVPA) from 3–6 METs, and vigorous physical activity as activity above 6 METs [24,29].
IPAQ estimates were obtained verbally and face-to-face with each participant by trained physical therapists. Physical activity in IPAQ was assessed separately for weekdays and weekends, and these estimates were used to calculate physical activity per week (kcal/week). Physical activity was calculated using the type of activity (indoor, outdoor, etc.), frequency of exercise of 10 min or more (days/week), and duration of exercise (minutes/day) as intensity and type components. Then, METs were calculated and applied to each activity; MET intensity levels used for IPAQ scores were vigorous (8 METs), moderate (4 METs), and walking (3.3 METs; www.ipaq.ki.se (accessed on 10 August 2020)). Using the definition for a MET as the ratio of work metabolic rate to a standard resting metabolic rate of 1.0 (4.184 kJ) ·kg−1·h−1, 1 MET was considered a resting metabolic rate obtained during quiet sitting [29]. The physical-activity quantity (kcal/week) was calculated from the IPAQ data and weight [30].
2.6. Propensity Score Matching
Propensity score matching was used to adjust for differences in background factors between the control and intervention groups [31]. The covariates included in the model were age, gender, body mass index, diabetes history, DKD stage, systolic blood pressure, glycated hemoglobin (HbA1c), hemoglobin (Hb), hi-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Based on these parameters, the authors aimed to generate pairs of control and intervention groups using logistic regression-based propensity scores. A predefined caliper width of 0.20 was used. The area under the receiver operator acting characteristic curve was drawn to assess the accuracy of the logistic regression model used to calculate the propensity score [32].
2.7. Clinical Measurements
The primary endpoint was cumulative survival for the composite endpoint, including new CVD and stroke, hemodialysis induction, and all-cause mortality at 24 months. The definition of CVD was myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, and arrhythmia. All of this information was collected from the electronic medical record.
Secondary endpoints included estimated glomerular filtration rates (eGFR), Urinary Albumin/Creatinine ratio (Urinary Alb/Cre ratio), body weight, blood pressure, Triglyceride (TG), High-density lipoprotein cholesterol (HDL-C), LDL-C, DKD Stage, HbA1c, and Hb. The values of these endpoints were obtained from blood and urine collection data during outpatient visits or hospitalization.
eGFR was calculated using the Japanese eGFR equations based on standardized serum creatinine levels as follows: eGFRcr (mL/min/1.73 m2) = 194 × serum creatinine − 1.094 × Age − 0.287 (× 0.739 if female) [33].
2.8. Statistical Analysis
Data are presented as mean ± standard deviation for continuous variables and as numbers and percentages for categorical variables. Baseline comparisons were conducted using the Wilcoxon rank sum test, Student’s t-test, chi-squared test, and Fisher’s exact test. In addition, groups (control, intervention) and by-time (pre, post) repeated-measures analyses of variance were performed to examine interactions. The log-rank test was used to compare the cumulative survival rate against new events within the observation period. COX regression analysis was performed to calculate hazard ratios for CVD occurrence and all-cause mortality risk. JMP ® Pro, Version 14.0 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Statistical significance was set as a two-tailed p-value of <5%.
In diabetes, the relative risk of the higher physical activity group is reportedly 0.61 (range: 0.52–0.70) compared with that of the lower physical activity group [34]. In this study, the sample size was calculated according to the report by Kodama [34] and Dupont’s formula [35]. With a significance level of 0.05, a power of 80%, and a median event duration of 24 months [21], the required sample size was 128 cases. Because the dropout rate for intervention studies with follow-up longer than 24 months exceeds 19% [36], we set the dropout rate for this study at 25%.
3. Results
The clinical characteristics of the study participants (mean ± SD age, 69 ± 11 years) are shown in Table 1. DKD stage I was represented by 50 cases [37.3%], stage II by 58 cases [43.3%], stage III by 18 cases [13.4%], stage IV by 8 cases [6.0%], and stage V by 0 cases. Ten participants (9.0%) had had a stroke, and 38 (28.4%) had established CVD. When comparing the baseline values of the control and intervention groups, the proportion of users of insulin injections and beta-blockers was significantly higher in the intervention group; however, there were no significant differences in the other items (Table 1, Figure S1).

Table 1.
Baseline characteristics of participants and non-participants in the intervention before and after propensity score matching.
After 24 months of observation, the cumulative survival rate was significantly higher in the intervention group (0.881) than in the control group (0.715). (Figure 2, p = 0.016), with fewer new events. The hazard ratio for combined risk, including all event occurrences, was significantly higher in the control group (HR:2.48, p = 0.03). There were 27 cases of new events during the observation period. In the control group, there was 1 case of stroke, 12 of CVD, 1 of HD, and 5 of all-cause mortality. In contrast, in the intervention group, there was one case of stroke, four of CVD, 2 of HD, 1 of all-cause mortality, and fewer cases of CVD and all-cause mortality (Table 2). Hazard ratios for CVD occurrence and all-cause mortality risk were calculated. These results are shown in Table 3. The hazard ratio for the combined risk of CVD and all-cause mortality was significantly higher in the control group (HR:3.70, p = 0.01).
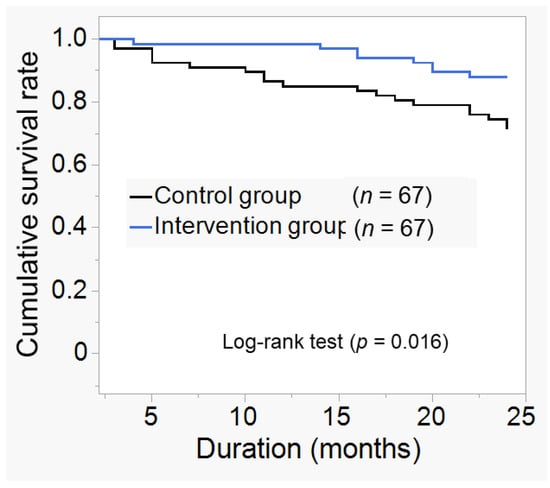
Figure 2.
Kaplan–Meier curve for new event incidence. Cumulative survival rates for the composite endpoint included new cardiovascular disease (CVD) and stroke, hemodialysis (HD) induction, and all-cause mortality at 24 months compared to the two groups. The definition of CVD was myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, and arrhythmia. After 24 months of observation, the cumulative survival rate for new events was 0.881 in the intervention group and 0.715 in the control group, and was significantly higher in the intervention group (p = 0.016).

Table 2.
Breakdown of newly generated events.

Table 3.
Hazard ratio for occurrence of new events for CVD and all-cause mortality combined.
Two-way analysis of variance revealed a significant effect of the group factor on HDL-C, which was higher in the intervention group (Group, F (1, 263) = 8.28, p = 0.004 vs. matched control); eGFRcr showed a significant effect of the time factor, which was lower at 24 months than before intervention (Time, F1, 260 = 4.12, p = 0.043). However, no interaction was observed for all items (Table 4).

Table 4.
Clinical characteristics between the two groups at the start and end of the observation.
3.1. Data from Activity Meter
The activity meter data of the intervention group are shown in Table 5. Forty-two of the 67 participants met the inclusion criteria for the activity meter data. During the intervention period, the mean percentage of MVPA to daily wearing time (%) was 9.5 ± 6.1 in the second month, 9.4 ± 6.3 in the third month, 8.8 ± 5.5 in the fourth month, 8.3 ± 5.8 in the fifth month, and 8.7 ± 5.1 in the sixth month.

Table 5.
Physical activity during the intervention period.
3.2. Data from IPAQ
The amount of physical activity before and during the intervention period is shown in the Supplementary Table S2. Compared to the amount of physical activity before the intervention, the amount of physical activity in the third (p = 0.0052), fourth (p = 0.003), and fifth months (p = 0.0013) was significantly higher.
4. Discussion
To our knowledge, this is the first study in which exercise guided by a trained physical therapist decreases the risk of new cardiovascular and all-cause death in patients with DKD. This study’s results contribute to future planning for adequate care and rehabilitation for DKD.
This study showed that increased physical activity through exercise was associated with reduced risk of CVD and all-cause mortality in patients with DKD. The present promising results could have been obtained through consistent instruction provided by the expert physical therapist regarding diabetes management and cardiac rehabilitation instruction [37]. This suggests the potential to expand further the profession of exercise and health professionals with specialized knowledge (regarding pathology, patient education, or exercise). It has been shown that increased physical activity is associated with a significant reduction in CVD risk and all-cause mortality in patients with type 2 diabetes [14,15]; this effect may be similar in patients with DKD. In the intervention group of this study, the ratio of MVPA to the time spent wearing an activity meter per day was approximately 9% (Table 5). Furthermore, since the average time spent wearing an activity meter per day was about 11 h, the participants performed about 415 min of MVPA per week by a simple calculation. The amount of physical activity obtained from the questionnaire shows that the amount of physical activity increased significantly during the intervention period compared to the pre-intervention period (Table S2). Notably, the participants obtained sufficient physical activity. The fact that the participants in the intervention group achieved sufficient physical activity indicates the usefulness of the DSME-based exercise instruction by a professional physical therapist. In addition, previous studies have shown that patients with diabetes who participated in or continued to participate in more than 10 h of DSME over a 6- to 12-month period had significantly lower mortality and HbA1c than those who spent less time with diabetes care and health education professionals [20,21]. Furthermore, early and tailored progressive rehabilitation interventions have been shown to produce better physical function than usual care [38]. These reports demonstrate the importance of professionals considering the individual’s situation and providing ongoing and tailor-made support. In the present study, HbA1c levels did not change in the intervention group despite adequate physical activity. This may be attributed not only to physical activity, but also to eating habits (quantity, frequency, content, etc.) and medication use. Therefore, these conditions should also be evaluated when implementing rehabilitation.
On the other hand, the 25% dropout rate in this study raises an essential question of how to support these individuals. Notably, there are two main categories of non-participants in the DSME: those who are unable to participate for logistical, medical, or financial reasons (e.g., timing, cost, and existing complications) and those who feel that there is no benefit in participating or who have emotional or cultural concerns (e.g., negative feelings about education that they do not find problematic) [39]. Efforts should be made to identify and address these potential barriers [40]. Therefore, it is an essential future task to analyze the factors that led to dropouts in this study and consider the best approach for those who drop out.
In this study, HDL-C levels were significantly higher in the intervention group at 24 months than in the control. This may be because exercise improved HDL-C levels, which is not easy to obtain with drug treatment, and possibly reduced the risk of CVD and all-cause mortality. Increased physical activity through exercise reportedly prevents atherosclerosis [41] and is a factor in increased blood HDL-C concentration [42]. HDL-C has been reported to increase after ≥120 min of aerobic exercise per week [42]. Since the intervention group in this study could perform approximately 415 min of MVPA per week, we believe that the conditions for increased HDL-C were satisfied. On the other hand, blood glucose and LDL-C levels did not change significantly. This may have been because of the medication. The patients were already well-controlled with statins and hypoglycemic drugs and may have been less affected by exercise.
Carotid artery echo and flow-mediated dilation (FMD) are essential to evaluate blood vessels’ functional and morphological changes due to atherosclerosis. The intima-media thickness obtained by carotid artery echo is closely related to the risk of stroke [43] and CVD [44]; it has been reported that a 1% improvement in FMD reduces the risk of CVD by 13% [45]. Therefore, it is possible that the severity of atherosclerosis also influenced the results of the present study; however, we are not able to address this issue with the present results. To prove this hypothesis, investigating the relationship between vascular assessment using carotid artery echo and FMD and the amount of physical activity would be necessary.
The eGFRcr showed a significant main effect of the time factor and was lower after 24 months in both groups; therefore, we could not show that exercise improved renal function in patients with DKD (Table 4, Figure S2). However, the fact that exercise did not induce decreased renal function or increased urinary Alb/Cre is profound (Table 4, Figure S3). Albuminuria is a useful diagnostic and prognostic indicator of diabetic kidney disease [46]; meta-analysis has shown it to be an independent predictor of CVD and overall mortality [13]. An RCT examining the effects of unsupervised exercise on patients with CKD found that combining aerobic and muscle-strengthening exercises significantly reduced albuminuria better than combining aerobic and balance exercises [47]. The present study’s results suggest that a combined intervention of aerobic and muscle-strengthening exercises does not exacerbate albuminuria in patients with DKD.
An RCT study of exercise and caloric restriction in patients with stage III DKD showed that they reduced inflammation and oxidative stress and delayed renal fibrosis in the intervention group [48]. Low- to moderate-intensity aerobic exercises exert a nephroprotective effect in patients with type 2 diabetes and CKD [49,50]. Conversely, recent advances in treating diabetes and diabetic complications and the aging of patients have increased the number of patients with diabetes who do not follow a typical clinical course. The annual decline in eGFR is 0.7–14.3% in patients with type 2 diabetes, with considerable variability concerning renal function decline [51]. In addition, cystatin C-based eGFR may be more appropriate [52] because eGFR measured with serum creatine is greatly influenced by muscle mass [53]. These findings suggest that the variation in the clinical course of eGFR and changes in muscle mass due to interventions may have influenced eGFR. In the future, it may be necessary to establish an evaluation method that accurately reflects the changes in renal function over time in patients with DKD.
There are some limitations to this study. First, the sample size was limited to a single institution. Therefore, the study can be limited to a small part of the population that can be influenced by different factors. Second, we were not able to assess vascular function. Third, the amount of physical activity in the control group could not be assessed. Finally, given that about 25% of the intervention group dropped out, the study may have been limited to participants with relatively high adherence.
5. Conclusions
Exercise in diabetic patients is an effective treatment to reduce the risk of CVD development and death, which may also be effective in DKD. In this study, in patients with DKD, increasing physical activity through a 6-month exercise instruction intervention reduces the risk of developing CVD and all-cause mortality. Our findings indicate that long-term exercise for DKD is an effective means of reducing CVD incidence and all-cause mortality and may be applied to future DKD treatment.
Supplementary Materials
The following information can be downloaded at https://www.mdpi.com/article/10.3390/jcm12020691/s1, Figure S1. Logit conversion score and distribution of propensity scores before and after matching, Figure S2. Changes in eGFRcr over time. Change in eGFRcr over time in the group that received exercise instruction in addition to usual care (Intervention group, n = 67) and the group that received usual care (Control group, n = 67). Abbreviations: eGFRcr, estimated glomerular filtration rate by creatinine; Figure S3. Changes in urinary Alb/Cre ratio over time. Change in urinary Alb/Cre ratio in the group that received exercise instruction in addition to usual care (Intervention group, n = 67) and the group that received usual care (Control group, n = 67). Abbreviations: urinary Alb/Cre ratio, albumin to creatine ratio in urine. Table S1. DKD severity classification. Table S2. Physical activity of IPAQ in the intervention group during the six months intervention preceding follow-up.
Author Contributions
Conceptualization, H.T. and Y.T.; methodology, H.T., Y.T., Y.N. (Yuki Nakatani) and N.B.; formal analysis, H.T. and Y.T.; investigation, H.T., Y.N. (Yasuko Nagashima), T.T. (Tomoki Tsurumi), M.T., K.O., K.E., T.F., M.H., A.U., T.T. (Takashi Tomoe), A.K., T.S. and N.B.; resources, H.T., M.H., Y.N. (Yuki Nakatani) and N.B.; data curation, H.T. and Y.T.; writing—original draft preparation, H.T.; writing—review and editing, H.T., S.K. and T.Y.; visualization, H.T. and T.Y.; supervision, T.Y.; project administration, T.Y.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to A.U. 19K19840, to H.T. 20H01125), grants for medical staff from the Japan Association for Diabetes Education and Care (to H.T); the APC was funded by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.T. 22K17640), and by the Vehicle Racing Commemorative Foundation (to T.Y.).
Institutional Review Board Statement
This study was performed according to the principles of the Declaration of Helsinki and approved by the institutional ethics committee of Dokkyo Medical University Nikko Medical Center (approval number: Nikko 27001).
Informed Consent Statement
Written informed consents were was obtained from all participants in the intervention group. In addition, informed consent was obtained through an opt-out system in the non-intervention group; those did not wish to participate were excluded.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the staff of the Department of Diabetes and Endocrinology and the Department of Cardiovascular Medicine and Nephrology at Dokkyo Medical University Nikko Medical Center for their cooperation. The authors thank Keiko Yoshizawa for administrative assistance.
Conflicts of Interest
Takanori Yasu reports receiving grant support and lecture fees from AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., and Kowa Co., Ltd.; grant support from MTG Co., Ltd. and Abbott Vascular Japan Co., Ltd.; lecture fees from Actelion Pharmaceuticals Ltd., Novartis Pharma K.K, Sanofi K.K., Sanofi S.A., Daiichi Sankyo Co., Ltd., and Takeda Pharmaceutical Co. Ltd. The remaining authors have no conflict of interest to disclose.
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kashiwabara, K.; Hirakawa, Y.; Tanaka, T.; Noso, S.; Ikegami, H.; Ohsugi, M.; Ueki, K.; Mita, T.; Watada, H.; et al. Conditions, pathogenesis, and progression of diabetic kidney disease and early decliner in Japan. BMJ Open Diabetes Res. Care 2020, 8, e000902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X.; et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; De Boer, I.H. Clinical Manifestations of Kidney Disease among US Adults with Diabetes, 1988–2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Amaral, L.; Souza, C.S.; Lima, H.N.; Soares, T.D.J. Influence of exercise training on diabetic kidney disease: A brief physiological approach. Exp. Biol. Med. 2020, 245, 1142–1154. [Google Scholar] [CrossRef]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Krolewski, A.S.; Rosner, B.; Arky, R.A.; Speizer, F.E.; Hennekens, C.H. A Prospective Study of Maturity-Onset Diabetes Mellitus and Risk of Coronary Heart Disease and Stroke in Women. Arch. Intern. Med. 1991, 151, 1141–1147. [Google Scholar] [CrossRef]
- Eberly, L.E.; Cohen, J.D.; Prineas, R.; Yang, L.; Multiple Risk Factor Intervention Trial Research Group. Impact of Incident Diabetes and Incident Nonfatal Cardiovascular Disease on 18-Year Mortality: The Multiple Risk Factor Intervention Trial experience. Diabetes Care 2003, 26, 848–854. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Chee, M.L.; Banu, R.; Cheng, C.-Y.; Lim, S.C.; Tai, E.S.; Coffman, T.; Wong, T.Y. Association of Diabetic Retinopathy and Diabetic Kidney Disease with All-Cause and Cardiovascular Mortality in a Multiethnic Asian Population. JAMA Netw. Open 2019, 2, e191540. [Google Scholar] [CrossRef] [PubMed]
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; Van Der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.; Buchner, D.M.; Marquez, D.X.; Pate, R.R.; Pescatello, L.S.; Whitt-Glover, M.C. New scientific basis for the 2018 U.S. Physical Activity Guidelines. J. Sport Health Sci. 2019, 8, 197–200. [Google Scholar] [CrossRef]
- Sluik, D.; Buijsse, B.; Muckelbauer, R.; Kaaks, R.; Teucher, B.; Johnsen, N.F.; Tjønneland, A.; Overvad, K.; Østergaard, J.N.; Amiano, P.; et al. Physical Activity and Mortality in Individuals with Diabetes Mellitus: A prospective study and meta-analysis. Arch. Intern. Med. 2012, 172, 1285–1295. [Google Scholar] [CrossRef]
- American Diabetes Association. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S48–S65. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Zoungas, M.S.; Caramori, M.L.; Chan, M.J.C.; Heerspink, H.J.; Hurst, B.C.; Liew, M.A.; Michos, E.D.; Olowu, M.W.A.; Sadusky, M.T.; et al. Diabetes Management in Chronic Kidney Disease: Synopsis of the 2020 KDIGO Clinical Practice Guideline. Ann. Intern. Med. 2021, 174, 385–394. [Google Scholar] [CrossRef]
- Haneda, M.; Utsunomiya, K.; Koya, D.; Babazono, T.; Moriya, T.; Makino, H.; Kimura, K.; Suzuki, Y.; Wada, T.; Ogawa, S.; et al. A new Classification of Diabetic Nephropathy 2014: A report from Joint Committee on Diabetic Nephropathy. J. Diabetes Investig. 2015, 6, 242–246. [Google Scholar] [CrossRef]
- Haas, L.; Maryniuk, M.; Beck, J.; Cox, C.E.; Duker, P.; Edwards, L.; Fisher, E.; Hanson, L.; Kent, D.; Kolb, L.; et al. National Standards for Diabetes Self-Management Education and Support. Diabetes Educ. 2012, 38, 619–629. [Google Scholar] [CrossRef]
- Chrvala, C.A.; Sherr, D.; Lipman, R.D. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Educ. Couns. 2016, 99, 926–943. [Google Scholar] [CrossRef]
- He, X.; Li, J.; Wang, B.; Yao, Q.; Li, L.; Song, R.; Shi, X.; Zhang, J.-A. Diabetes self-management education reduces risk of all-cause mortality in type 2 diabetes patients: A systematic review and meta-analysis. Endocrine 2017, 55, 712–731. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2016. [Google Scholar]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A.; et al. Physical Activity and Public Health: Updated Recommendation for Adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Iwamoto, J.; Saeki, K.; Okamoto, N. Reliability and Validity of the International Physical Activity Questionnaire (IPAQ) in Elderly Adults: The Fujiwara-kyo Study. J. Epidemiol. 2011, 21, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, K.; Oshima, Y.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I.; Tanaka, S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br. J. Nutr. 2011, 105, 1681–1691. [Google Scholar] [CrossRef]
- Oshima, Y.; Kawaguchi, K.; Tanaka, S.; Ohkawara, K.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture 2010, 31, 370–374. [Google Scholar] [CrossRef]
- Mâsse, L.; Fuemmeler, B.; Anderson, C.B.; Matthews, C.; Trost, S.; Catellier, D.J.; Treuth, M. Accelerometer Data Reduction: A Comparison of Four Reduction Algorithms on Select Outcome Variables. Med. Sci. Sports Exerc. 2005, 37, S544–S554. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S516. [Google Scholar] [CrossRef]
- Maddison, R.; Ni Mhurchu, C.; Jiang, Y.; Vander Hoorn, S.; Rodgers, A.; Lawes, C.M.; Rush, E. International Physical Activity Questionnaire (IPAQ) and New Zealand Physical Activity Questionnaire (NZPAQ): A doubly labelled water validation. Int. J. Behav. Nutr. Phys. Act. 2007, 4, 62. [Google Scholar] [CrossRef]
- Cham, H.; West, S.G. Propensity score analysis with missing data. Psychol. Methods 2016, 21, 427–445. [Google Scholar] [CrossRef]
- Stürmer, T.; Joshi, M.; Glynn, R.J.; Avorn, J.; Rothman, K.J.; Schneeweiss, S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J. Clin. Epidemiol. 2006, 59, 437.e1–437.e24. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Tanaka, S.; Heianza, Y.; Fujihara, K.; Horikawa, C.; Shimano, H.; Saito, K.; Yamada, N.; Ohashi, Y.; Sone, H. Association between Physical Activity and Risk of All-Cause Mortality and Cardiovascular Disease in Patients with Diabetes: A meta-analysis. Diabetes Care 2013, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Dupont, W.D.; Plummer, W.D., Jr. Power and sample size calculations: A review and computer program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
- Gagliardino, J.J.; Lapertosa, S.; Pfirter, G.; Villagra, M.; Caporale, J.E.; Gonzalez, C.; Elgart, J.; González, L.; Cernadas, C.; Rucci, E.; et al. Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR). Diabet. Med. 2013, 30, 1102–1111. [Google Scholar] [CrossRef]
- Gillett, P.A.; White, A.T.; Caserta, M.S. Effect of Exercise and/or Fitness Education on Fitness in Older, Sedentary, Obese Women. J. Aging Phys. Act. 1996, 4, 42–55. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Whellan, D.J.; Duncan, P.; Pastva, A.M.; Mentz, R.J.; Reeves, G.R.; Nelson, M.B.; Chen, H.; Upadhya, B.; Reed, S.D.; et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N. Engl. J. Med. 2021, 385, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Horigan, G.; Davies, M.; Findlay-White, F.; Chaney, D.; Coates, V. Reasons why patients referred to diabetes education programmes choose not to attend: A systematic review. Diabet. Med. 2016, 34, 14–26. [Google Scholar] [CrossRef]
- Powers, M.A.; Bardsley, J.K.; Cypress, M.; Funnell, M.M.; Harms, D.; Hess-Fischl, A.; Hooks, B.; Isaacs, D.; Mandel, E.D.; Maryniuk, M.D.; et al. Diabetes Self-management Education and Support in Adults With Type 2 Diabetes: A Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care 2020, 43, 1636–1649. [Google Scholar] [CrossRef]
- Diep, L.; Kwagyan, J.; Kurantsin-Mills, J.; Weir, R.; Jayam-Trouth, A. Association of Physical Activity Level and Stroke Outcomes in Men and Women: A Meta-Analysis. J. Women’s Health 2010, 19, 1815–1822. [Google Scholar] [CrossRef]
- Kodama, S.; Tanaka, S.; Saito, K.; Shu, M.; Sone, Y.; Onitake, F.; Suzuki, E.; Shimano, H.; Yamamoto, S.; Kondo, K.; et al. Effect of Aerobic Exercise Training on Serum Levels of High-Density Lipoprotein Cholesterol: A Meta-analysis. Arch. Intern. Med. 2007, 167, 999–1008. [Google Scholar] [CrossRef]
- Kitamura, A.; Iso, H.; Imano, H.; Ohira, T.; Okada, T.; Sato, S.; Kiyama, M.; Tanigawa, T.; Yamagishi, K.; Shimamoto, T. Carotid Intima-Media Thickness and Plaque Characteristics as a Risk Factor for Stroke in Japanese Elderly Men. Stroke 2004, 35, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B. Carotid-Wall Intima–Media Thickness and Cardiovascular Events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, C.E. Microalbuminuria Predicts Clinical Proteinuria and Early Mortality in Maturity-Onset Diabetes. N. Engl. J. Med. 1984, 310, 356–360. [Google Scholar] [CrossRef]
- Hellberg, M.; Höglund, P.; Svensson, P.; Clyne, N. Randomized Controlled Trial of Exercise in CKD—The RENEXC Study. Kidney Int. Rep. 2019, 4, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, J.; Lian, Y.; Tang, Z.-X.; Zen, Z.; Yu, P.; Li, Y. Long-Term Intensive Lifestyle Intervention Promotes Improvement of Stage III Diabetic Nephropathy. Med. Sci. Monit. 2019, 25, 3061–3068. [Google Scholar] [CrossRef]
- Lazarevic, G.; Antić, S.; Vlahovic, P.; Djordjevic, V.; Zvezdanovic, L.; Stefanovic, V. Effects of Aerobic Exercise on Microalbuminuria and Enzymuria in Type 2 Diabetic Patients. Ren. Fail. 2007, 29, 199–205. [Google Scholar] [CrossRef]
- Pechter, U.; Ots, M.; Mesikepp, S.; Zilmer, K.; Kullissaar, T.; Vihalemm, T.; Zilmer, M.; Maaroos, J. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int. J. Rehabil. Res. 2003, 26, 153–156. [Google Scholar] [CrossRef]
- Jiang, G.; Luk, A.O.Y.; Tam, C.H.T.; Xie, F.; Carstensen, B.; Lau, E.S.H.; Lim, C.K.P.; Lee, H.M.; Ng, A.C.W.; Ng, M.C.Y.; et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with Type 2 diabetes. Kidney Int. 2019, 95, 178–187. [Google Scholar] [CrossRef]
- Nishimoto, M.; Murashima, M.; Kokubu, M.; Matsui, M.; Eriguchi, M.; Samejima, K.-I.; Akai, Y.; Tsuruya, K. Kidney function at 3 months after acute kidney injury is an unreliable indicator of subsequent kidney dysfunction: The NARA-AKI Cohort Study. Nephrol. Dial. Transplant. 2022. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M.; Gunst, J.; Van den Berghe, G. The impact of using estimated GFR versus creatinine clearance on the evaluation of recovery from acute kidney injury in the ICU. Intensiv. Care Med. 2014, 40, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).