Abstract
The aim of this study was to assess Corvis ST biomechanical indices in reference to corneal enantiomorphism. In a prospective observational cohort study, 117 eyes from 63 patients with normal or keratoconus corneas were assessed by three independent observers. In the control group (n = 62), no significant differences were observed between the three observers for all indices. The best reproducibility was obtained with pachymetry and the weakest with CBI. All indices but CBI and arc length featured COV < 10%. All indices except the PD and SSI correlated with pachymetry; all but Rad correlated with IOP. The comparison of the thinnest with the thickest corneas showed no significant differences for any index except pachymetry. In the keratoconus group (n = 55), loss of corneal enantiomorphism was confirmed for all indices except the arc length, velocity, and PD. Significant differences between both groups were found for all indices, even after adjustment for pachymetry and intraocular pressure. The CBI featured the best accuracy (92%), sensitivity (91%), and graphical relevance for keratoconus diagnosis. However, its reproducibility was weak in normal corneas and was strongly dependent on corneal thickness. The SSI was independent of corneal thickness, highly reproducible, and provided the expected enantiomorphism characteristics in both groups, making it a relevant biomarker of biomechanical corneal behavior.
1. Introduction
The cornea exhibits a viscoelastic behavior that is crucial for maintaining its curvature and subsequent refractive power despite changes in intraocular pressure and various forces, such as external shocks or eye-rubbing. Corneal biomechanical properties are closely related to the structure of stroma, which consists of several hundred 1–3 µm-thick stacked lamellae made of collagen fibrils that are aligned and regularly packed. This corneal viscoelastic behavior is explained by the rearrangement and sliding of the stromal lamellae and the stretching of the stromal striae during stress [1].
Keratoconus is a bilateral progressive corneal disease characterized by focal biomechanical weakening, localized conical protrusion, apical thinning, and irregular astigmatism. Areas outside the conus feature normal biomechanical properties. The finite element model of keratoconus shows eccentric thinning and a reduced modulus [2]. Specific changes in the corneal stroma structure are associated with alterations in the viscous and elastic properties of the keratoconus cornea [3]. The reported incidence of keratoconus is much higher among candidates for refractive surgery [4]. Preoperative accurate detection of keratoconus among refractive surgery candidates is crucial because refractive surgery might be contraindicated in these patients.
Corneal biomechanical behavior can be assessed with various technologies, including strip extensometry, eye inflation, Brillouin microscopy, air-puff systems, ultrasound elastography, optical coherence tomography elastography, enzymatic digestion, and laser interferometry [5]. In routine practice, only air-puff systems are currently available for the clinical assessment of corneal biomechanical behavior [6]; they provide a global evaluation of corneal biomechanics. The Ocular Response Analyzer device is the first widely used air-puff device that provides an assessment of corneal hysteresis. Its limitations include its weak reproducibility and the high variability of its measurements [7]. In addition, hysteresis is highly dependent on corneal central thickness, so it cannot be used alone to identify keratoconus suspect corneas [8,9]. The corneal visualization Scheimpflug technology (Corvis ST) device analysis method is based on a corneal dynamic deformation video that is recorded using Scheimpflug technology [10]; it has been reported to provide an analysis of corneal deformation independent of central corneal thickness, corneal volume, or anterior corneal curvature [11]. It provides relevant indicators of biomechanical dysfunction in keratoconus corneas. In a study including 12 normal corneas and 12 keratoconus corneas, a significant difference was found in Deformation Amplitude between normal and keratoconus corneas after adjusting for age, corneal central thickness, and intraocular pressure [12]. In experimental settings, the cross-linking of rabbit corneas results in a significant decrease in the Peak Distance and Deformation Amplitude assessed using Corvis ST [13]. The same findings have recently been reported in a clinical study [14].
The limitation of clinical studies assessing air-puff-derived corneal biomechanical indices is the absence of a reference technology that directly assesses stiffness and viscoelasticity. Enantiomorphism is a major physiological property of the normal cornea; it shows mirror symmetry, with the body median axis as the symmetry axis. This has been verified for many of its physiological parameters including its thickness and curvature [15]. Corneal enantiomorphism in normal eyes is not defined by only the mirror symmetry of the corneal anterior surface astigmatism axis, but also by the symmetry of various corneal features including the keratometry, cylinder, and thickness. Mirror symmetry is also observed when pachymetry maps of fellow eyes are compared. Interestingly, the loss of these corneal features is a hallmark of keratoconus. We hypothesize that corneal biomechanical behavior should also be enantiomorph in normal corneas—as suggested by the mass distribution of preferentially aligned fibrils in the cornea, which appear to exhibit a degree of midline symmetry between the left and right eyes [16]. Conversely, keratoconus corneas feature a loss of corneal enantiomorphism as a major diagnosis indicator [17]. Asymmetry of corneal biomechanics should also characterize keratoconus corneas.
In the present study, we assessed the reproducibility and diagnosis value of Corvis ST indices in normal and keratoconus corneas, considering their ability to distinguish keratoconus from normal corneas and to demonstrate the expected enantiomorphism features of both corneal conditions.
2. Patients and Methods
We retrospectively selected patients with either mild to advanced keratoconus or normal corneas from a prospective observational cohort of patients (CCK-CONE) registered in the Health Data Hub and approved by the Institut National des Données de Santé (#255645). All patients gave informed consent. All procedures followed the tenets of the declaration of Helsinki.
All eyes were assessed with Corvis ST (Oculus Optikgeräte GmbH, Wetzlar, Germany), specular corneal topography (MS39; CSO, Firenze, Italy), and spectral-domain optical coherence tomography (OCT; RTVue; Optovue, Inc., Fremont, CA, USA). The main outcome measures were the 15 Corvis ST indices.
The inclusion criteria for the keratoconus group were the following: a positive keratoconus diagnosis based on slit-lamp examination, specular topography and spectral-domain OCT, the absence of a history of ocular surgery (including collagen cross-linking, intra corneal ring segment implantation, and keratoplasty) or trauma, the absence of associated corneal pathologic features, and the absence of contact lens wear during the 3 weeks preceding the assessment. The clinical features of keratoconus include corneal ectasia, apical thinning, Vogt striae, the Fleischer ring, the Munson sign, the Rizutti sign, or corneal scarring consistent with keratoconus. The diagnosis of keratoconus was facilitated by the use of corneal topographic data: a keratoconus appearance on the topography (skewed asymmetric bowtie, central or inferior steep zone, or claw shape), positive topographic indices (mean keratometry >47 diopters, or inferior–superior value >1.4 diopters in the central 3.0 mm). We did not use the grade of severity to analyze subgroups, but all stages of severity were included except forme fruste keratoconus. The presence of epithelial thinning in the thinnest corneal zone or a doughnut pattern on the SD-OCT epithelial map, and positive keratoconus OCT classification on OCT scans including the conus, were used as SD-OCT indicators of keratoconus [18,19]. The keratoconus group included 55 eyes from 32 patients.
The inclusion criteria for the control group were the following: normal topography, negative topography indices (K ≤ 47 D and I-S ≤ 1.4), SD-OCT indicators negative of keratoconus, normal SD-OCT scans, normal slit-lamp examination, no history of ocular surgery or trauma, no contact lens wear, and no history of eye diseases. Sixty-two eyes from 31 normal subjects were included in the control group.
Each Corvis ST exam consisted of three measurements for each eye. Three exams were performed by three orthoptists for each patient to assess reproducibility.
Inter-observer reproducibility was assessed with the coefficient of variation of the three measurements. Friedman’s analysis of variance was used to assess inter-observer differences. Differences between fellow eyes and between groups were assessed with non-parametric tests (Wilcoxon paired test and Mann–Whitney U test). The Spearman rank correlation coefficient was used to assess the correlations between indices and corneal thickness and intraocular pressure. To compare the Corvis ST indices for their ability to distinguish keratoconus from normal corneas, we calculated their sensitivity (i.e., true positive/(true positive + false negative) and accuracy (i.e., (true positive + true negative)/total number of observations) using a specificity (i.e., true negative/(true negative + false positive) set at 95 ± 1% (i.e., diagnosis thresholds = 5th or 95th percentiles of the indices in the control group). Analyses were performed using a software program (Statistica version 6.1; StatSoft France, Maisons-Alfort, France). As the location of the cone may affect the deformation following the air puff, and therefore the outcomes, the results were stratified according to the location of the thinnest point.
3. Results
One hundred and seventeen eyes were included in the study. Patients were assessed between January 2022 and July 2022. Keratoconus patients featured a mean age of 34.2 ± 14.2 years (mean ± standard deviation). In nine patients, the Corvis ST analysis was unsuccessful in the most-affected eye due to severe corneal ectasia. Normal subjects featured a mean age of 23.2 ± 2.6 years. Keratoconus patients were significantly older than controls (p < 0.00001).
Table 1 shows the Corvis ST indices assessed in both groups.

Table 1.
Corvis indices in normal and keratoconus corneas.
3.1. Control Group
In the control group, Friedman’s analysis of variance showed no significant differences between the three observers for all indices (p > 0.1). The lowest coefficient of variation (COV) of the Corvis indices (i.e., best reproducibility) was obtained with Pachymetry and the highest (weakest reproducibility) with the Corvis Biomechanical Index (CBI; Figure 1). The mean Corvis Biomechanical Index was 28.9%. All indices except the CBI and arc length—Applanation 1 and 2—featured a COV of less than 10%. The COV of the SSI (Stress Strain Index) and PD (Peak Distance) were, respectively, 5.5% and 2.7%.
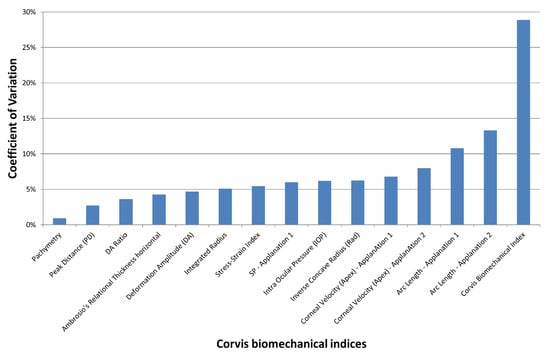
Figure 1.
Mean coefficient of variation (COV) of the Corvis ST indices obtained by 3 different orthoptists in the control group. The inter-observer reproducibility decreases with increased COV.
All the Corvis ST indices except the Peak Distance and Strain Stress index significantly correlated with Pachymetry (Table 1). All the Corvis ST indices except the inverse concave Radius significantly correlated with intraocular pressure (Table 1).
Corneal enantiomorphism was confirmed for all the Corvis ST indices except IOP—which was significantly higher in the right eye compared with the left eye—and the Corvis Biomechanical Index, which was significantly lower in the right eye compared with the left eye (Table 2). The comparison of the thinnest corneas with the thickest showed no significant differences for any of the Corvis ST indices except pachymetry, as expected (Table 2).

Table 2.
Biomechanical enantiomorphism analysis in normal and keratoconus corneas.
3.2. Keratoconus Group
In the keratoconus group, Friedman’s analysis of variance showed no significant differences between the three observers for all indices (p > 0.1). The lowest coefficient of variation of the Corvis ST indices (i.e., best reproducibility) was obtained with Pachymetry and the highest (weakest reproducibility) with the Corneal Velocity (apex)—Applanation 2 (Figure 2). All indices except the Corneal Velocity (Apex)—Applanation 2; the Stiffness Parameter—Applanation 1; the Arc Length—Applanation 1 and 2; and Ambrosio’s Relational Thickness horizontal featured a COV of less than 10%. The COV of the SSI was 5.9%.
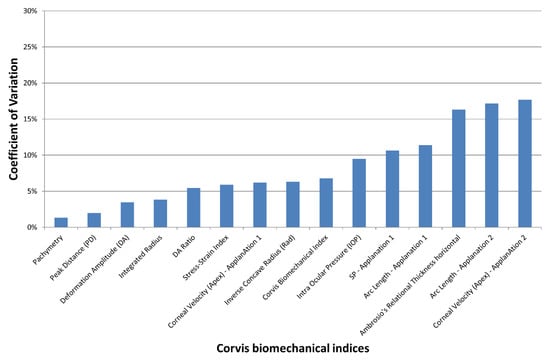
Figure 2.
Mean coefficient of variation (COV) of the Corvis ST indices obtained by 3 different orthoptists in the keratoconus group. The inter-observer reproducibility decreases with increased COV.
All the Corvis ST indices except the Peak Distance significantly correlated with Pachymetry (Table 1). All the Corvis ST indices except the Arc Length—Applanation 1 and 2 and Corneal Velocity (Apex)—Applanation 1 significantly correlated with intraocular pressure (Table 1).
For all keratoconus patients, the most-affected cornea (i.e., the steepest cornea on the specular corneal topography) was the thinnest one, as assessed with Corvis ST. The loss of corneal enantiomorphism along with more altered biomechanical indices in the thinnest cornea was confirmed for all the Corvis ST indices except the Arc Length—Applanation 1 and 2; the Corneal Velocity (Apex)—Applanation 1 and 2; and the Peak Distance.
Among the indices independent of corneal thickness in the control group, the SSI featured unique properties (high reproducibility, expected enantiomorphism characteristics in both groups), making it a relevant biomarker of corneal biomechanical behavior.
3.3. Comparison of Keratoconus with Control Corneas
Significant differences between both groups, with more altered biomechanical indices in the keratoconus group, were found for all the Corvis ST indices (Table 1). A posteriori power analysis showed that this figure was at least 80% (i.e., β < 20%) for all indices and at least 90% for 11 out of 15 indices. As most parameters were dependent on Pachymetry and intraocular pressure, we assessed differences after the indices were divided by pachymetry or intraocular pressure. The differences were still present after adjustment for pachymetry and intraocular pressure for all indices except the Arc Length—Applanation 1. Figure 3 shows the scatterplots of the most relevant indices as a function of Pachymetry in both groups. Graphically, the Corvis Biomechanical Index featured the best ability to distinguish keratoconus from normal corneas independently of Pachymetry.
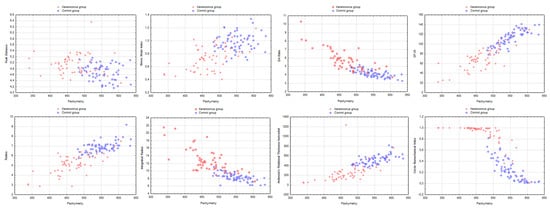
Figure 3.
Scatterplots of the most relevant Corvis ST indices as a function of Pachymetry in both groups.
To compare the Corvis ST indices for their ability to distinguish keratoconus from normal corneas, we calculated their sensitivity and accuracy using either the 5th or 95th percentiles of the indices in the control group as diagnosis thresholds (i.e., specificity set at 95 ± 1% for all indices; Table 3). The best accuracy and sensitivity were obtained with the Corvis Biomechanical Index (accuracy, 92%; sensitivity, 91%). The weakest accuracy and sensitivity were obtained with the Peak Distance (accuracy, 66%; sensitivity, 33%).

Table 3.
Assessment of Corvis indices for the diagnosis of keratoconus.
3.4. Stratified Analysis
Thirty-one keratoconi featured a thinnest point that was less than 1 mm from the corneal apex on the vertical axis, as measured with SD-OCT (central keratoconus), and 24 featured a thinnest point that was more than 1 mm from the corneal apex (peripheral keratoconus). No significant differences between central and peripheral keratoconus were observed for any of the Corvis ST indices (Table 4).

Table 4.
Analysis stratified according to the location of the thinnest point.
Keratoconus corneas with their thinnest point at less than 1 mm from the corneal apex on the vertical meridian were classified as central. Keratoconus corneas with their thinnest point farther than 1 mm from the corneal apex on the vertical meridian were classified as peripheral.
4. Discussion
In the present study, most standard Corvis ST indices and indices provided by the Vinciguerra screening report featured good repeatability, as previously demonstrated for standard and recent indices [20,21] The exam could be performed in all normal corneas, but 9 out of 55 keratoconus corneas could not be assessed due to advanced keratoconus.
Among the 15 indices provided by the Corvis ST device, we found the Corvis Biomechanical Index (CBI) to be the most accurate in distinguishing keratoconi from normal corneas. The CBI featured high reproducibility in keratoconus corneas, as previously reported [22]. We did not find studies specifically addressing the influence of tear film on the reproducibility of Corvis data; however, the use of an ultra-high-speed Scheimpflug camera should allow this device to not be dependent on tear film quality [11]—it can still differentiate keratoconi from normal corneas after adjusting for pachymetry and IOP. Although linked to pachymetry, its performance appears to be highly independent of pachymetry, as assessed graphically. This result was expected, as the CBI was developed for improving keratoconus diagnosis by combining several indices including the DA, velocity, ARTh, and a stiffness parameter [23,24] Whereas standard indices of the Corvis ST device cannot readily be used for the diagnosis of keratoconus or to demonstrate the effect of CXL in vivo, indices developed in the Vinciguerra screening report—including CBI—allow for a more accurate keratoconus diagnosis compared with standard indices [11,25]. Moreover, the diagnostic ability of the CBI is comparable to that of the Pentacam indices for differentiating normal eyes and eyes with forme fruste keratoconus [26]. Recently, a modified linear term of the Corvis Biomechanical Index (i.e., Corvis Biomechanical Factor) has been developed for improving the ABCD keratoconus classification [27]. The CBF correlates with keratoconus severity [28].
Whereas the CBI is a strong biomarker of keratoconus, it may be a weaker indicator of corneal biomechanical behavior. Its reproducibility in the control group was weak, with a 28.9% coefficient of variation between the three different observers, which makes it a poor indicator of changes in biomechanics in a given cornea over time. It was also strongly correlated (rs = −0.86) to pachymetry in the control group. Corneal stiffness increases with thickness; the biomechanical behavior of any tissue is influenced by its thickness. However, assessing corneal biomechanical properties independently of corneal thickness is an unmet need which, at least conceptually, laser interferometry should significantly help with. For instance, two corneas featuring the same central corneal thickness may have different stiffnesses and different risks of developing ectasia after refractive surgery. The effect of a given photoablation procedure on these corneas and the resulting change in refractive power may be different. Lastly, significant differences in the CBI between the left and right eyes were observed in the control group, which is a weakness of this index.
Among the Corvis ST indices, the stress-strain index (SSI) presented several interesting features, making it a valuable index for assessing corneal stiffness. The SSI is a stiffness parameter, derived from an algorithm, that showed no significant correlation with either central corneal thickness or intraocular pressure but was significantly correlated with age in the dataset used for its production [29]. First, its reproducibility was good, with a coefficient of variation between three different observers of less than 6%. This means that a 10% change in the SSI should be considered significant. Second, it was not significantly correlated with pachymetry in the control group, showing that it could assess corneal stiffness independently of corneal thickness. Lastly, the SSI featured the expected enantiomorphism properties, i.e., normal corneas were enantiomorph and keratoconus corneas were not. Compared with the CBI, the SSI’s accuracy for diagnosing keratoconus (77%) was lower. However, keratoconus is a localized corneal disorder featuring decreased stiffness in the conus area, with normal stiffness outside the conus. As Corvis ST provides an assessment of the whole cornea’s biomechanical behavior, it may be less accurate for diagnosing keratoconus than a method that provides a localized assessment, such as Brillouin microscopy or OCT-elastography [30,31,32]. Global biomechanical assessments may be more accurate when combined with structural and topographical parameters. Pentacam HR and Corvis ST parameters have been combined and analyzed using a random forest method to improve corneal ectasia detection, with very high sensitivity/specificity—even in forme fruste keratoconus corneas (91% sensitivity, 96% specificity) [33,34,35]. Back propagation neural networks have been used to differentiate form fruste keratoconus from normal thin corneas with a 91% accuracy [36]. A recent study has demonstrated a progressive decrease in the SSI with more advanced keratoconus stages, while the SSI was relatively independent of IOP and corneal central thickness but positively correlated with age [37]. A recent study showed that in eyes with ocular hypertension, the SSI decreases after initiation of treatment with topical antiglaucoma medications [38]. Whether this decrease is related to decreased intraocular pressure or drug-related ocular surface damage is still to be determined.
We found the intraocular pressure to not be enantiomorph in the control group, which was unexpected. However, as the Corvis ST exam was performed in the right eye first, we can hypothesize that the first eye exam influenced the fellow-eye exam.
The study’s limitations include the absence of a successful Corvis ST examination in 9 out of 55 keratoconus corneas, the absence of a reference measure of corneal stiffness, the absence of a keratoconus subgroup analysis, and the difference in age between normal and keratoconus corneas. As corneal stiffness increases with age, comparing older keratoconus patients with younger controls should result in weaker differences between both groups. Despite this age difference, all indices were significantly impaired in the keratoconus group. Whereas we lack a reference technology to directly measure corneal stiffness in vivo—as can be done in an experimental setting with, for instance, extensometry or OCT-elastography—we have used corneal enantiomorphism in addition to repeatability and the ability to distinguish keratoconus from normal corneas as clinical indicators. This led us to select the SSI as the most relevant index for assessing corneal stiffness and the CBI as the most efficient index for diagnosing keratoconus, using a dataset different from those used to develop these indices. These findings are in good agreement with previous studies [19,22,25,33]. As our objective was not to determine the best threshold values for the indices but to compare them with each other, we did not use receiver operating characteristic (ROC) curves, but the 5th and 95th percentiles measured in the control group as threshold values to set the specificity at 95 ± 1%. The absence of a successful Corvis ST examination in severe keratoconus is probably a limitation of the technology itself. However, assessing corneal biomechanics in advanced keratoconus may not be mandatory, as the diagnosis is clinically evident, the corneal condition well-known, and the indication for transplantation is evident.
In conclusions: The CBI was the best index for keratoconus diagnosis amid the Corvis ST indices. As a consequence, it could be considered a relevant supplementary biomarker for screening candidates for refractive surgery if combined with corneal topography and optical coherence tomography. Conversely, relying on this Corvis biomarker alone is not advisable, as the performance would be less than that of the other two technologies. In addition, CBI reproducibility was weak in normal corneas and was strongly dependent on corneal thickness. Conversely, the SSI was independent of corneal thickness, highly reproducible, and provided the expected enantiomorphism characteristics in normal and keratoconus corneas—making it a very acceptable biomarker of corneal biomechanical behavior. The SSI may be an interesting index for assessing changes in corneal stiffness over time or changes induced by corneal refractive surgery or cross-linking.
Author Contributions
Conceptualization, V.B.; Data curation, J.B., R.C., C.G., B.M. and O.S.; Formal analysis, V.B. and O.S.; Funding acquisition, V.B.; Investigation, J.B., R.C., C.G. and B.M.; Methodology, V.B., C.G. and O.S.; Project administration, V.B.; Supervision, V.B.; Validation, J.B. and O.S.; Visualization, J.B. and R.C.; Writing—original draft, V.B.; Writing—review and editing, V.B., J.B., R.C., C.G., B.M. and O.S. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Agence Nationale de la Recherche (grant #ANR-21-CE19-0010-02, CorMecha project). The sponsor or funding organization had no role in the design or conduct of this research. The authors have no financial or proprietary interest in any material or method mentioned in the paper.
Institutional Review Board Statement
The study was approved by the Institut National des Données de Santé (#255645).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Latour, G.; Nahas, A.; Plamann, K.; Crotti, C.; Bocheux, R.; Borderie, M.; Nguyen, T.-M.; et al. Stromal striae: A new insight into corneal physiology and mechanics. Sci. Rep. 2017, 7, 13194–13196. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J.; Dupps, W.J., Jr. Biomechanics of corneal ectasia and biomechanical treatments. J. Cataract. Refract. Surg. 2014, 40, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Vellara, H.R.; Patel, D.V. Biomechanical properties of the keratoconic cornea: A review. Clin. Exp. Optom. 2015, 98, 31–38. [Google Scholar] [CrossRef]
- Randleman, J.; Russell, B.; A Ward, M.; Thompson, K.P.; Stulting, R. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology 2003, 110, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kling, S.; Hafezi, F. Corneal biomechanics—A review. Ophthalmic Physiol. Opt. 2017, 37, 240–252. [Google Scholar] [CrossRef]
- Piñero, D.P.; Alcón, N. In vivo characterization of corneal biomechanics. J. Cataract. Refract. Surg. 2014, 40, 870–887. [Google Scholar] [CrossRef]
- Kopito, R.; Gaujoux, T.; Montard, R.; Touzeau, O.; Allouch, C.; Borderie, V.; Laroche, L. Reproducibility of viscoelastic property and intraocular pressure measurements obtained with the Ocular Response Analyzer. Acta Ophthalmol. 2010, 89, e225–e230. [Google Scholar] [CrossRef]
- Schweitzer, C.; Roberts, C.J.; Mahmoud, A.M.; Colin, J.; Maurice-Tison, S.; Kerautret, J. Screening of Forme Fruste Keratoconus with the Ocular Response Analyzer. Investig. Opthalmol. Vis. Sci. 2010, 51, 2403–2410. [Google Scholar] [CrossRef]
- Saad, A.; Lteif, Y.; Azan, E.; Gatinel, D. Biomechanical Properties of Keratoconus Suspect Eyes. Investig. Opthalmol. Vis. Sci. 2010, 51, 2912–2916. [Google Scholar] [CrossRef]
- Tian-Jie, L.; Ko, M.; Wang, L.-K.; Zhang, J.-Y.; Li, T.-J.; Huang, Y.-F.; Zheng, Y.-P. Assessment of Ocular Biomechanics Using Dynamic Ultra High-Speed Scheimpflug Imaging in Keratoconic and Normal Eyes. J. Refract. Surg. 2014, 30, 785–791. [Google Scholar] [CrossRef]
- Lanza, M.; Cennamo, M.; Iaccarino, S.; Romano, V.; Bifani, M.; Irregolare, C.; Lanza, A. Evaluation of corneal deformation analyzed with a Scheimpflug based device. Contact Lens Anterior Eye 2015, 38, 89–93. [Google Scholar] [CrossRef]
- Ye, C.; Yu, M.; Lai, G.; Jhanji, V. Variability of Corneal Deformation Response in Normal and Keratoconic Eyes. Optom. Vis. Sci. 2015, 92, e149–e153. [Google Scholar] [CrossRef]
- Bekesi, N.; Kochevar, I.E.; Marcos, S. Corneal Biomechanical Response Following Collagen Cross-Linking With Rose Bengal-Green Light and Riboflavin-UVA. Investig. Ophthalmol. Vis. Sci. 2016, 57, 992–1001. [Google Scholar] [CrossRef]
- Jabbarvand, M.; Moravvej, Z.; Shahraki, K.; Hashemian, H.; Ghasemi, H.; Berijani, S.; Amiri, Z.; Jamali, A. Corneal biomechanical outcome of collagen cross-linking in keratoconic patients evaluated by Corvis ST. Eur. J. Ophthalmol. 2020, 31, 1577–1583. [Google Scholar] [CrossRef]
- Touzeau, O.; Gaujoux, T.; Bullet, J.; Allouch, C.; Borderie, V.; Laroche, L. Relationships between refractive parameters: Sphere, cylinder and axis. J. Fr. Ophtalmol. 2012, 35, 587–598. [Google Scholar] [CrossRef]
- Boote, C.; Hayes, S.; Abahussin, M.; Meek, K. Mapping Collagen Organization in the Human Cornea: Left and Right Eyes Are Structurally Distinct. Investig. Opthalmol. Vis. Sci. 2006, 47, 901–908. [Google Scholar] [CrossRef]
- Saad, A.; Guilbert, E.; Gatinel, D. Corneal Enantiomorphism in Normal and Keratoconic Eyes. J. Refract. Surg. 2014, 30, 542–547. [Google Scholar] [CrossRef]
- Temstet, C.; Sandali, O.; Bouheraoua, N.; Hamiche, T.; Galan, A.; El Sanharawi, M.; Basli, E.; Laroche, L.; Borderie, V. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J. Cataract. Refract. Surg. 2015, 41, 812–820. [Google Scholar] [CrossRef]
- Sandali, O.; El Sanharawi, M.; Temset, C.; Hamiche, T.; Galan, A.; Ghouali, W.; Goemaere, I.; Basli, E.; Borderie, V.; Laroche, L. Fourier-Domain Optical coherence tomography imaging in keratoconus: A corneal structural classification. Ophthalmology 2013, 120, 2403–2412. [Google Scholar] [CrossRef]
- Lopes, B.T.; Roberts, C.J.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P.; Reisdorf, S.; Berger, S.; Koprowski, R.; Ambrósio, R. Repeatability and Reproducibility of Intraocular Pressure and Dynamic Corneal Response Parameters Assessed by the Corvis ST. J. Ophthalmol. 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Yang, K.; Xu, L.; Fan, Q.; Zhao, D.; Ren, S. Repeatability and comparison of new Corvis ST parameters in normal and keratoconus eyes. Sci. Rep. 2019, 9, 15379. [Google Scholar] [CrossRef] [PubMed]
- Herber, R.; Vinciguerra, R.; Lopes, B.; Raiskup, F.; E Pillunat, L.; Vinciguerra, P.; Ambrósio, R. Repeatability and reproducibility of corneal deformation response parameters of dynamic ultra-high-speed Scheimpflug imaging in keratoconus. J. Cataract. Refract. Surg. 2020, 46, 86–94. [Google Scholar] [PubMed]
- Vinciguerra, R.; Ambrosio RJr Elsheikh, A.; Roberts, C.J.; Lopes, B.; Morenghi, E.; Azzolini, C.; Vinciguerra, P. Detection of Keratoconus with a New Biomechanical Index. J. Refract. Surg. 2016, 32, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, R.; Ambrósio, R.; Elsheikh, A.; Hafezi, F.; Kang, D.S.Y.; Kermani, O.; Koh, S.; Lu, N.; Padmanabhan, P.; Roberts, C.J.; et al. Detection of postlaser vision correction ectasia with a new combined biomechanical index. J. Cataract. Refract. Surg. 2021, 47, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Bak-Nielsen, S.; Pedersen, I.B.; Ivarsen, A.; Hjortdal, J. Dynamic Scheimpflug-based Assessment of Keratoconus and the Effects of Corneal Cross-linking. J. Refract. Surg. 2014, 30, 408–414. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, T.; Yu, M.; Jhanji, V. Comparison of Corneal Dynamic and Tomographic Analysis in Normal, Forme Fruste Keratoconic, and Keratoconic Eyes. J. Refract. Surg. 2017, 33, 632–638. [Google Scholar] [CrossRef]
- Flockerzi, E.; Vinciguerra, R.; Belin, M.W.; Vinciguerra, P.; Ambrosio, R., Jr.; Seitz, B. Combined biomechanical and tomographic keratoconus staging: Adding a biomechanical parameter to the ABCD keratoconus staging system. Acta Ophthalmol. 2021, 30, 2022. [Google Scholar] [CrossRef]
- Flockerzi, E.; Vinciguerra, R.; Belin, M.W.; Vinciguerra, P.; Ambrosio, R., Jr.; Seitz, B. Correlation of the Corvis Biomechanical Factor with tomographic parameters in keratoconus. J. Cataract. Refract. Surg. 2022, 48, 215–221. [Google Scholar] [CrossRef]
- Eliasy, A.; Chen, K.J.; Vinciguerra, R.; Lopes, B.T.; Abass, A.; Vinciguerra, P.; Ambrosio, R., Jr.; Roberts, C.J.; Elsheikh, A. Determination of Corneal Biomechanical Behavior in-vivo for Healthy Eyes Using CorVis ST Tonometry: Stress-Strain Index. Front Bioeng Biotechnol. 2019, 7, 105. [Google Scholar] [CrossRef]
- Scarcelli, G.; Pineda, R.; Yun, S.H. Brillouin Optical Microscopy for Corneal Biomechanics. Investig. Opthalmology Vis. Sci. 2012, 53, 185–190. [Google Scholar] [CrossRef]
- Lopes, B.T.; Elsheikh, A. In Vivo Corneal Stiffness Mapping by the Stress-Strain Index Maps and Brillouin Microscopy. Curr. Eye Res. 2022, 30, 1–7. [Google Scholar] [CrossRef]
- Ghoubay, D.; Borderie, M.; Grieve, K.; Martos, R.; Bocheux, R.; Nguyen, T.-M.; Callard, P.; Chédotal, A.; Borderie, V.M. Corneal stromal stem cells restore transparency after N2 injury in mice. Stem Cells Transl. Med. 2020, 9, 917–935. [Google Scholar] [CrossRef]
- Salomao, M.Q.; Hofling-Lima, A.L.; Gomes Esporcatte, L.P.; Lopes, B.; Vinciguerra, R.; Vinciguerra, P.; Bühren, J.; Sena, N., Jr.; Luz Hilgert, G.S.; Ambrosio, R., Jr. The Role of Corneal Biomechanics for the Evaluation of Ectasia Patients. Int. J. Environ. Res. Public Health 2020, 17, 2113. [Google Scholar] [CrossRef]
- Ambrósio, R., Jr.; Lopes, B.T.; Faria-Correia, F.; Salomão, M.Q.; Bühren, J.; Roberts, C.J.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P. Integration of Scheimpflug-Based Corneal Tomography and Biomechanical Assessments for Enhancing Ectasia Detection. J. Refract. Surg. 2017, 33, 434–443. [Google Scholar] [CrossRef]
- Pérez-Rueda, A.; Jiménez-Rodríguez, D.; Castro-Luna, G. Diagnosis of Subclinical Keratoconus with a Combined Model of Biomechanical and Topographic Parameters. J. Clin. Med. 2021, 10, 2746. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, D.; Guo, L.; Qin, X.; Zhang, H.; Zhang, H.; Jie, Y.; Li, L. Comparisons of corneal biomechanical and tomographic parameters among thin normal cornea, forme fruste keratoconus, and mild keratoconus. Eye Vis. 2021, 8, 44. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Lopes, B.T.; Eliasy, A.M.; Abass, A.; Vinciguerra, R.; Vinciguerra, P.; Ambrósio, R.J.; Elsheikh, A. Evaluation of corneal biomechanical behavior in vivo for healthy and keratoconic eyes using the stress–strain index. J. Cataract. Refract. Surg. 2022, 48, 1162–1167. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, C.; Liu, C.; Cheng, W.; Ming, S.; Du, W.; Feng, X. Correlations among Corneal Biomechanical Parameters, Stiffness, and Thickness Measured Using Corvis ST and Pentacam in Patients with Ocular Hypertension. J. Ophthalmol. 2022, 3, 7387581. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).