Efficacy and Safety Ablation Index-Guided High-Energy Linear Ablation for Persistent Atrial Fibrillation: PVI Plus Linear Ablation of Mitral Isthmus and Posterior Box Isolation
Abstract
1. Introduction
2. Methods
- Electrical cardioversion: If AF has not been terminated after the completion of PVI, the electrical cardioversion (bi-phase 150–200 J) would be performed.
- 3.
- PVI: PVI was performed and verified as previously described.
- 4.
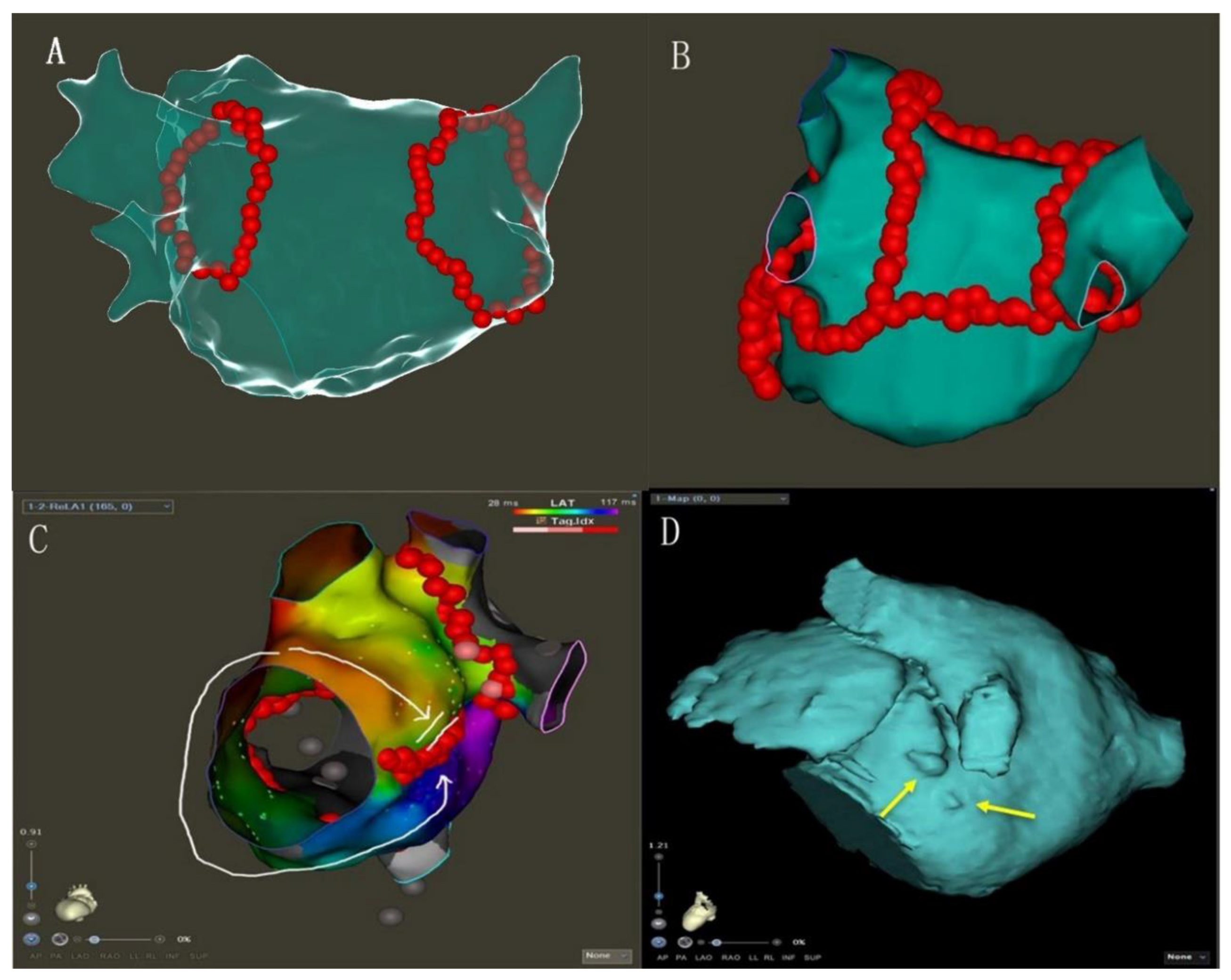
- MI linear ablation: linear ablation was performed across the MI between the left inferior PV and mitral annulus. AI-guided high-power ablation was used with the ablation energy of 45 W and the target AI of 450 (Figure 1B, Supplementary Figure S1).
- 5.
- PWI: LA roof linear ablation was performed across the LA roof joining the two superior isolated PV. LA posterior inferior linear joined the two inferior isolated PV. AI-guided high-power ablation was used with the ablation energy of 45 W and the target AI of 400.
- 6.
- Electrical cardioversion: If AF has not been terminated after the completion of PVI and linear ablation, the electrical cardioversion (bi-phase 150–200 J) would be performed to stop AF.
- 7.
- If AF was converted to AFL (atrial flutter) during ablation, reentrant circus was determined by high-density activation mapping, then linear ablation of the key isthmus was performed, including the tricuspid isthmus. Whenever AF terminated to one or more atrial tachycardias (ATs), these were targeted for ablation until sinus rhythm (SR)was achieved.
- 8.
- 9.
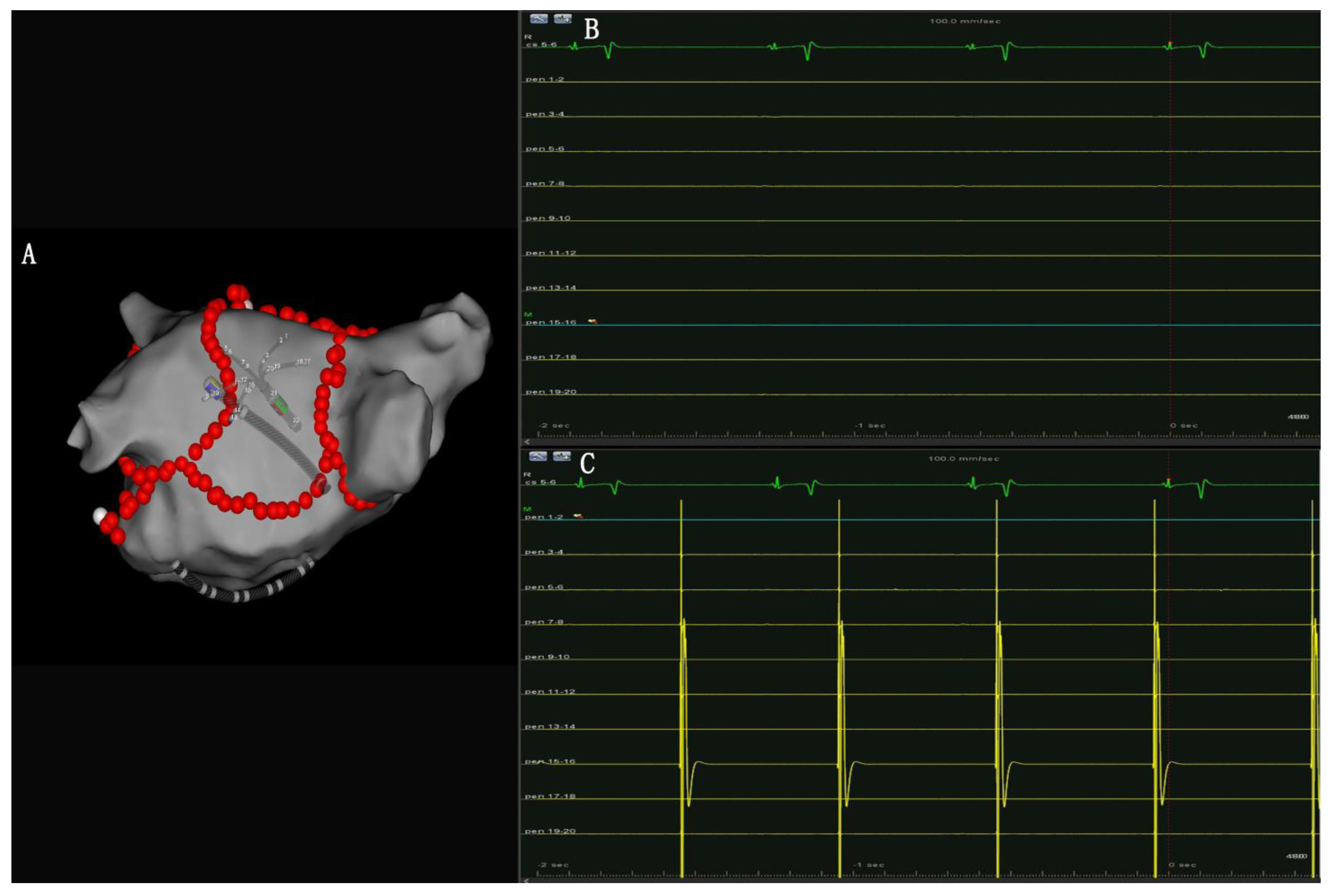
- PW conduction block: After the roof and posterior inferior ablation lines had been completed, the PWI was confirmed with either the electrical silence of the LA posterior wall (Figure 3B), or a conduction block when pacing the LA post wall. (Figure 3C). If the LA posterior wall was not isolated, additional ablations were performed until PWI was achieved.
3. Results
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oral, H.; Knight, B.P.; Tada, H.; Özaydın, M.; Chugh, A.; Hassan, S.; Scharf, C.; Lai, S.W.K.; Greenstein, R.; Morady, F.; et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002, 105, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.A.; Crijns, H.J.G.; Damiano, R.J., Jr.; Davies, D.W.; DiMarco, J.; et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012, 14, 528–606. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Klemm, H.; Rostock, T.; Brandstrup, B.; Ventura, R.; Steven, D.; Risius, T.; Lutomsky, B.; Meinertz, T. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: A prospective randomized comparison. Eur. Heart J. 2006, 27, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Sang, C.H.; Yu, R.H.; Long, D.Y.; Tang, R.B.; Jiang, C.X.; Ning, M.; Liu, N.; Liu, X.-P.; Du, X.; et al. Prospective randomized comparison between a fixed ‘2C3L’ approach vs. stepwise approach for catheter ablation of persistent atrial fibrillation. Europace 2015, 17, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Vogler, J.; Willems, S.; Sultan, A.; Schreiber, D.; Lüker, J.; Servatius, H.; Schäffer, B.; Moser, J.; Hoffmann, B.A.; Steven, D. Pulmonary Vein Isolation Versus Defragmentation: The CHASE-AF Clinical Trial. J. Am. Coll. Cardiol. 2015, 66, 2743–2752. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Schlüter, M.; Heeger, C.H.; Lemes, C.; Maurer, T.; Reissmann, B.; Riedl, J.; Rottner, L.; Santoro, F.; Schmidt, B.; et al. Stand-Alone Pulmonary Vein Isolation Versus Pulmonary Vein Isolation with Additional Substrate Modification as Index Ablation Procedures in Patients With Persistent and Long-Standing Persistent Atrial Fibrillation: The Randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation). Circ. Arrhythmia Electrophysiol. 2017, 10, e005114. [Google Scholar]
- Yao, Y.; Hu, F.; Du, Z.; He, J.; Shi, H.; Zhang, J.; Cai, H.; Jia, Y.; Tang, M.; Niu, G.; et al. The value of extensive catheter linear ablation on persistent atrial fibrillation (the CLEAR-AF Study). Int. J. Cardiol. 2020, 316, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Ho, S.Y.; Sánchez-Quintana, D.; Becker, A.E. A review of the coronary venous system: A road less travelled. Heart Rhythm 2004, 1, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, M.; Shah, D.C.; Haïssaguerre, M.; Marcellin, L.; Brechenmacher, C. The anatomic basis of connections between the coronary sinus musculature and the left atrium in humans. Circulation 2000, 101, 647–652. [Google Scholar] [CrossRef]
- Inoue, K.; Hikoso, S.; Masuda, M.; Furukawa, Y.; Hirata, A.; Egami, Y.; Watanabe, T.; Minamiguchi, H.; Miyoshi, M.; Tanaka, N.; et al. Pulmonary vein isolation alone vs. more extensive ablation with defragmentation and linear ablation of persistent atrial fibrillation: The EARNEST-PVI trial. EP Eur. 2021, 23, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Tilz, R.R.; Rillig, A.; Thum, A.M.; Arya, A.; Wohlmuth, P.; Metzner, A.; Mathew, S.; Yoshiga, Y.; Wissner, E.; Kuck, K.-H.; et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J. Am. Coll. Cardiol. 2012, 60, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Pak, H.N.; Oh, Y.S.; Lim, H.E.; Kim, Y.H.; Hwang, C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm 2011, 8, 199–206. [Google Scholar] [CrossRef]
- Sawhney, N.; Anousheh, R.; Chen, W.; Feld, G.K. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2010, 3, 243–248. [Google Scholar] [CrossRef]
- Solimene, F.; Schillaci, V.; Shopova, G.; Urraro, F.; Arestia, A.; Iuliano, A.; Maresca, F.; Agresta, A.; La Rocca, V.; De Simone, A.; et al. Safety and efficacy of atrial fibrillation ablation guided by Ablation Index module. J. Interv. Card. Electrophysiol. 2019, 54, 9–15. [Google Scholar] [CrossRef]
- Hussein, A.; Das, M.; Riva, S.; Morgan, M.; Ronayne, C.; Sahni, A.; Shaw, M.; Todd, D.; Hall, M.; Modi, S.; et al. Use of Ablation Index-Guided Ablation Results in High Rates of Durable Pulmonary Vein Isolation and Freedom from Arrhythmia in Persistent Atrial Fibrillation Patients: The PRAISE Study Results. Circ. Arrhythmia Electrophysiol. 2018, 11, e006576. [Google Scholar] [CrossRef]
- Ravi, V.; Poudyal, A.; Abid, Q.U.A.; Larsen, T.; Krishnan, K.; Sharma, P.S.; Trohman, R.G.; Huang, H.D. High-power short duration vs. conventional radiofrequency ablation of atrial fibrillation: A systematic review and meta-analysis. EP Eur. 2021, 23, 710–721. [Google Scholar] [CrossRef]
- Winkle, R.A.; Mohanty, S.; Patrawala, R.A.; Mead, R.H.; Kong, M.H.; Engel, G.; Salcedo, J.; Trivedi, C.G.; Gianni, C.; Jais, P.; et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm 2019, 16, 165–169. [Google Scholar] [CrossRef]
- Wu, S.H.; Jiang, W.F.; Gu, J.; Zhao, L.; Wang, Y.L.; Liu, Y.G.; Zhou, L.; Gu, J.N.; Xu, K.; Liu, X. Benefits and risks of additional ablation of complex fractionated atrial electrograms for patients with atrial fibrillation: A systematic review and meta-analysis. Int. J. Cardiol. 2013, 169, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jaïs, P.; Hocini, M.; Hsu, L.F.; Sanders, P.; Scavee, C.; Weerasooriya, R.; Macle, L.; Raybaud, F.; Garrigue, S.; Shah, D.C.; et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004, 110, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.J.; Tsao, H.M.; Wu, M.H.; Tai, C.T.; Chang, S.L.; Wongcharoen, W.; Lin, Y.J.; Lo, L.W.; Chen, Y.J.; Sheu, M.H.; et al. Anatomic characteristics of the left atrial isthmus in patients with atrial fibrillation: Lessons from computed tomographic images. J. Cardiovasc. Electrophysiol. 2006, 17, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Yamane, T.; Date, T.; Hioki, M.; Narui, R.; Ito, K.; Tanigawa, S.I.; Nakane, T.; Yamashita, S.; Tokuda, M.; et al. Completion of mitral isthmus ablation using a steerable sheath: Prospective randomized comparison with a nonsteerable sheath. J. Cardiovasc. Electrophysiol. 2011, 22, 1331–1338. [Google Scholar] [CrossRef]
- Pambrun, T.; Derval, N.; Duchateau, J.; Denis, A.; Chauvel, R.; Tixier, R.; Welte, N.; André, C.; Nakashima, T.; Nakatani, Y.; et al. Epicardial course of the musculature related to the great cardiac vein: Anatomical considerations and clinical implications for mitral isthmus block after vein of Marshall ethanol infusion. Heart Rhythm 2021, 18, 1951–1958. [Google Scholar] [CrossRef]
- Lin, W.S.; Tai, C.T.; Hsieh, M.H.; Tsai, C.F.; Lin, Y.K.; Tsao, H.M.; Huang, J.L.; Yu, W.C.; Yang, S.P.; Ding, Y.A.; et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003, 107, 3176–3183. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, S.Y.; Na, J.O.; Choi, C.U.; Kim, S.H.; Kim, J.W.; Kim, E.J.; Rha, S.W.; Park, C.G.; Seo, H.S.; et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation?: A prospective randomized clinical trial. Int. J. Cardiol. 2015, 181, 277–283. [Google Scholar] [CrossRef]
- Markman, T.M.; Hyman, M.C.; Kumareswaran, R.; Arkles, J.S.; Santangeli, P.; Schaller, R.D.; Supple, G.E.; Frankel, D.S.; Riley, M.P.; Lin, D.; et al. Durability of posterior wall isolation after catheter ablation among patients with recurrent atrial fibrillation. Heart Rhythm 2020, 17, 1740–1744. [Google Scholar] [CrossRef]
- Pambrun, T.; Duchateau, J.; Delgove, A.; Denis, A.; Constantin, M.; Ramirez, F.D.; Chauvel, R.; Tixier, R.; Welte, N.; André, C.; et al. Epicardial course of the septopulmonary bundle: Anatomical considerations and clinical implications for roof line completion. Heart Rhythm 2021, 18, 349–357. [Google Scholar] [CrossRef]
- Park, Y.M.; Choi, J.I.; Lim, H.E.; Park, S.W.; KIM, Y.H. Is pursuit of termination of atrial fibrillation during catheter ablation of great value in patients with longstanding persistent atrial fibrillation? J. Cardiovasc. Electrophysiol. 2012, 23, 1051–1058. [Google Scholar] [CrossRef]
- Watanabe, R.; Nagashima, K.; Wakamatsu, Y.; Otsuka, N.; Yokoyama, K.; Matsumoto, N.; Otsuka, T.; Suzuki, S.; Hirata, A.; Murakami, M.; et al. Different Determinants of the Recurrence of Atrial Fibrillation and Adverse Clinical Events in the Mid-Term Period After Atrial Fibrillation Ablation. Circ. J. 2022, 86, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Berruezo, A.; Tamborero, D.; Mont, L.; Benito, B.; Tolosana, J.M.; Sitges, M.; Vidal, B.; Arriagada, G.; Méndez, F.; Matiello, M.; et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 2007, 28, 836–841. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | PVI-Plus Group | PVI-Alone Group | p Value |
|---|---|---|---|
| Age (years) | 58 ± 10 | 59 ± 8 | 0.222 |
| Male | 146 (73%) | 110 (68%) | 0.389 |
| Duration of persistent AF (months) | 28 ± 25 | 30 ± 24 | 0.556 |
| Hypertension | 92 (46%) | 83 (51%) | 0.323 |
| Diabetes mellitus | 21 (10.5%) | 24 (15%) | 0.162 |
| Coronary artery disease | 40 (20%) | 39 (24%) | 0.352 |
| Prior stroke/TIA | 69 (34.5%) | 65 (40%) | 0.359 |
| CHA2DS2-VASc score | 2.0 ± 1.7 | 2.2 ± 1.6 | 0.368 |
| OAC | 196 (98%) | 160 (98%) | 0.568 |
| AADs therapy | 136 (68%) | 107 (66%) | 0.437 |
| Echocardiography parameters | |||
| LVEF (%) | 54 ± 6 | 52 ± 5 | 0.218 |
| LAd (mm) | 45 ± 3.8 | 45 ± 3.7 | 0.736 |
| PVI-Plus Group (n = 200) | PVI-Alone Group (n = 162) | p Value | |
|---|---|---|---|
| Total procedure time, min | 154 ± 21 | 83 ± 10 | <0.001 |
| Fluoroscopy time, min | 19 ± 4 | 10 ± 2 | <0.001 |
| Successful PVI | 200 (100%) | 162 (100%) | - |
| MI ablation | 200 (100%) | - | - |
| Successful MI block achieved | 179 (89.5%) | - | - |
| CS vein ablation | 152 (76%) | - | - |
| Posterior wall isolation | 192 (96%) | - | - |
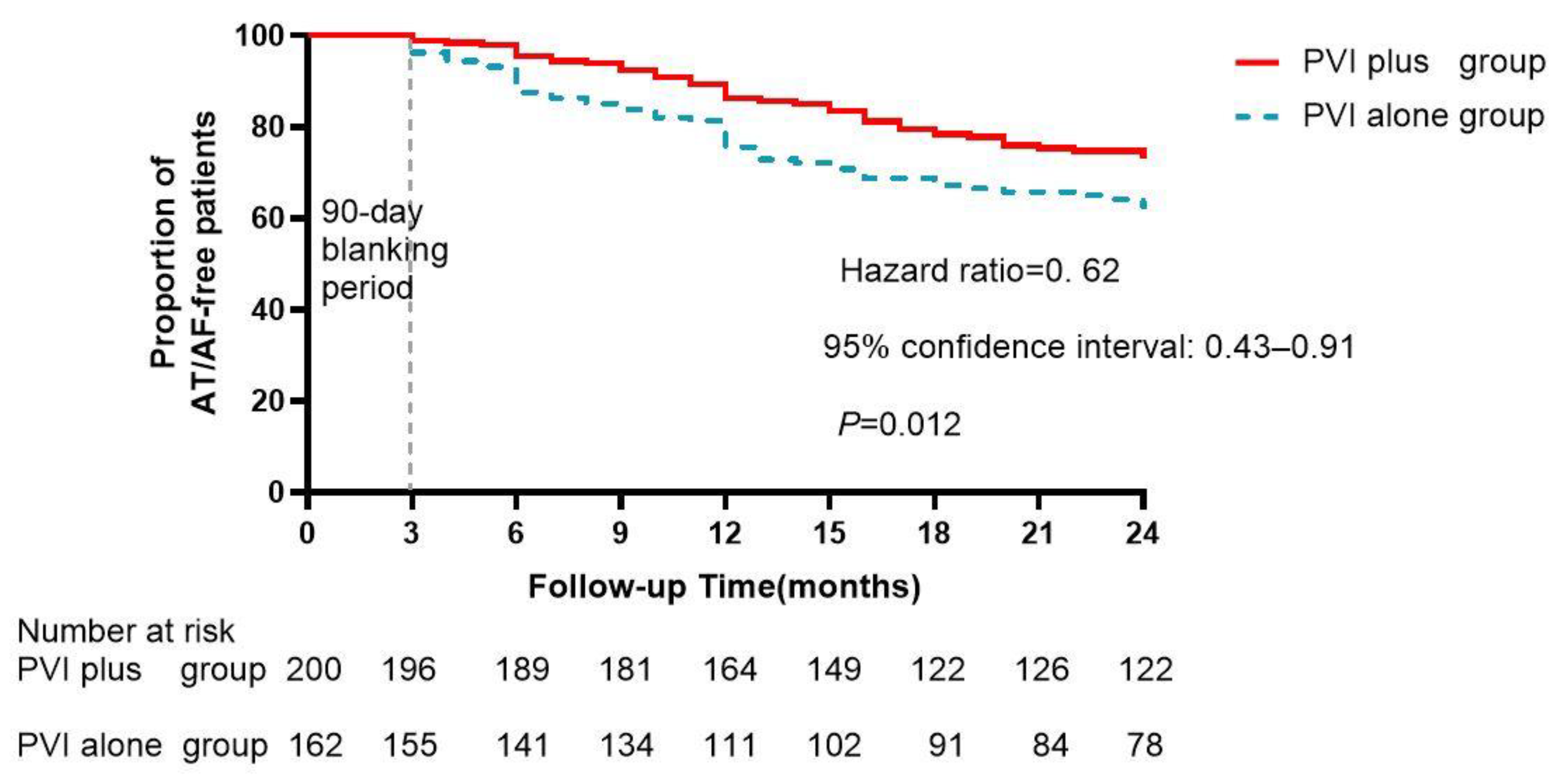
| Conversion to sinus rhythm | 56 (28%) | 13 (8%) | <0.001 |
| Adverse Event | PVI-Plus Group (n = 200) | PVI-Only Group (n = 162) | p Value |
|---|---|---|---|
| Patients with adverse events n (%) | 5 (2.5) | 3 (1.9) | 0.808 |
| Death, n (%) | 0 | 0 | - |
| Symptomatic stroke, n (%) | 1 | 0 | 0.648 |
| Cardiac tamponade, n (%) | 0 | 0 | - |
| Phrenic nerve paralysis, n (%) | 0 | 0 | - |
| Femoral arteriovenous fistula | 0 | 0 | - |
| Femoral pseudoaneurysm | 4 | 3 | 0.924 |
| Atrioesophageal fistula, n (%) | 0 | 0 | - |
| Infection, n (%) | 0 | 0 | - |
| Heart failure, n (%) | 0 | 0 | - |
| Pulmonary vein stenosis | 0 | 0 | - |
| Univariate | Multivariable | |
|---|---|---|
| Variable | HR (95% CL) p | HR (95% CL) p |
| Age(years) | 1.01 (0.98–1.04) 0.580 | - |
| Female sex | 0.91 (0.68–1.22) 0.518 | - |
| AF duration | 1.02 (1.01–1.02) 0.001 | 1.01 (1.00–1.02) 0.012 |
| Hypertension | 0.94 (0.54–1.64) 0.832 | - |
| Diabetes mellitus | 0.91 (0.36–2.29) 0.841 | - |
| Coronary artery disease | 0.87 (0.43–1.79) 0.711 | - |
| Cardiac insufficiency | 1.33 (0.42–4.28) 0.630 | - |
| LA diameter | 3.19 (1.53–6.67) 0.002 | 2.52(1.22–5.20) 0.012 |
| CHA2DS2VASC score | 1.04 (0.89–1.22) 0.637 | - |
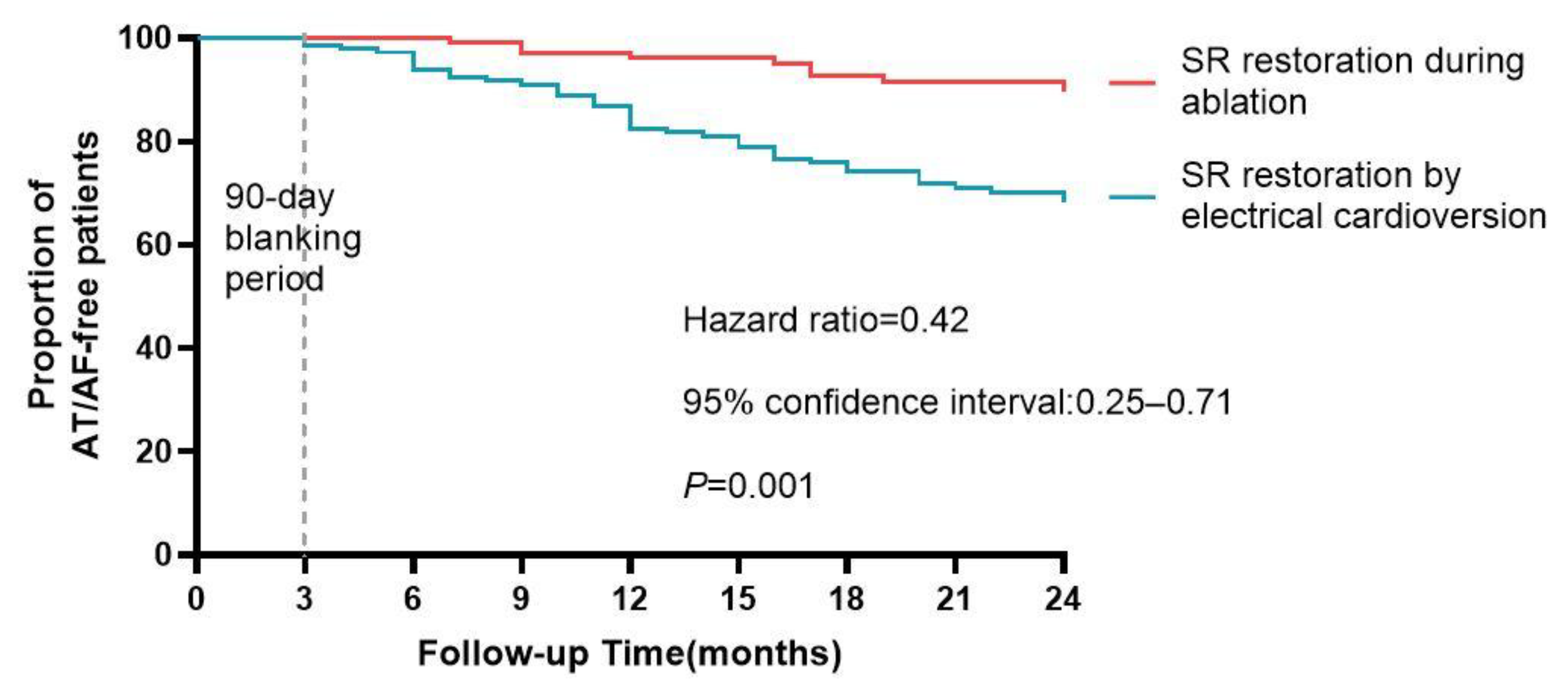
| No conversion to SR | 2.61 (1.17–5.79) 0.019 | 2.45(1.10–5.44) 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, T.; Cui, B.; Zhang, J.; Chen, Y.; Wu, G. Efficacy and Safety Ablation Index-Guided High-Energy Linear Ablation for Persistent Atrial Fibrillation: PVI Plus Linear Ablation of Mitral Isthmus and Posterior Box Isolation. J. Clin. Med. 2023, 12, 619. https://doi.org/10.3390/jcm12020619
Li X, Liu T, Cui B, Zhang J, Chen Y, Wu G. Efficacy and Safety Ablation Index-Guided High-Energy Linear Ablation for Persistent Atrial Fibrillation: PVI Plus Linear Ablation of Mitral Isthmus and Posterior Box Isolation. Journal of Clinical Medicine. 2023; 12(2):619. https://doi.org/10.3390/jcm12020619
Chicago/Turabian StyleLi, Xi, Tao Liu, Bo Cui, Jinlin Zhang, Yanhong Chen, and Gang Wu. 2023. "Efficacy and Safety Ablation Index-Guided High-Energy Linear Ablation for Persistent Atrial Fibrillation: PVI Plus Linear Ablation of Mitral Isthmus and Posterior Box Isolation" Journal of Clinical Medicine 12, no. 2: 619. https://doi.org/10.3390/jcm12020619
APA StyleLi, X., Liu, T., Cui, B., Zhang, J., Chen, Y., & Wu, G. (2023). Efficacy and Safety Ablation Index-Guided High-Energy Linear Ablation for Persistent Atrial Fibrillation: PVI Plus Linear Ablation of Mitral Isthmus and Posterior Box Isolation. Journal of Clinical Medicine, 12(2), 619. https://doi.org/10.3390/jcm12020619




