Safety and Feasibility of Catheter Ablation Procedures in Patients with Bleeding Disorders
Abstract
1. Introduction
2. Materials and Methods
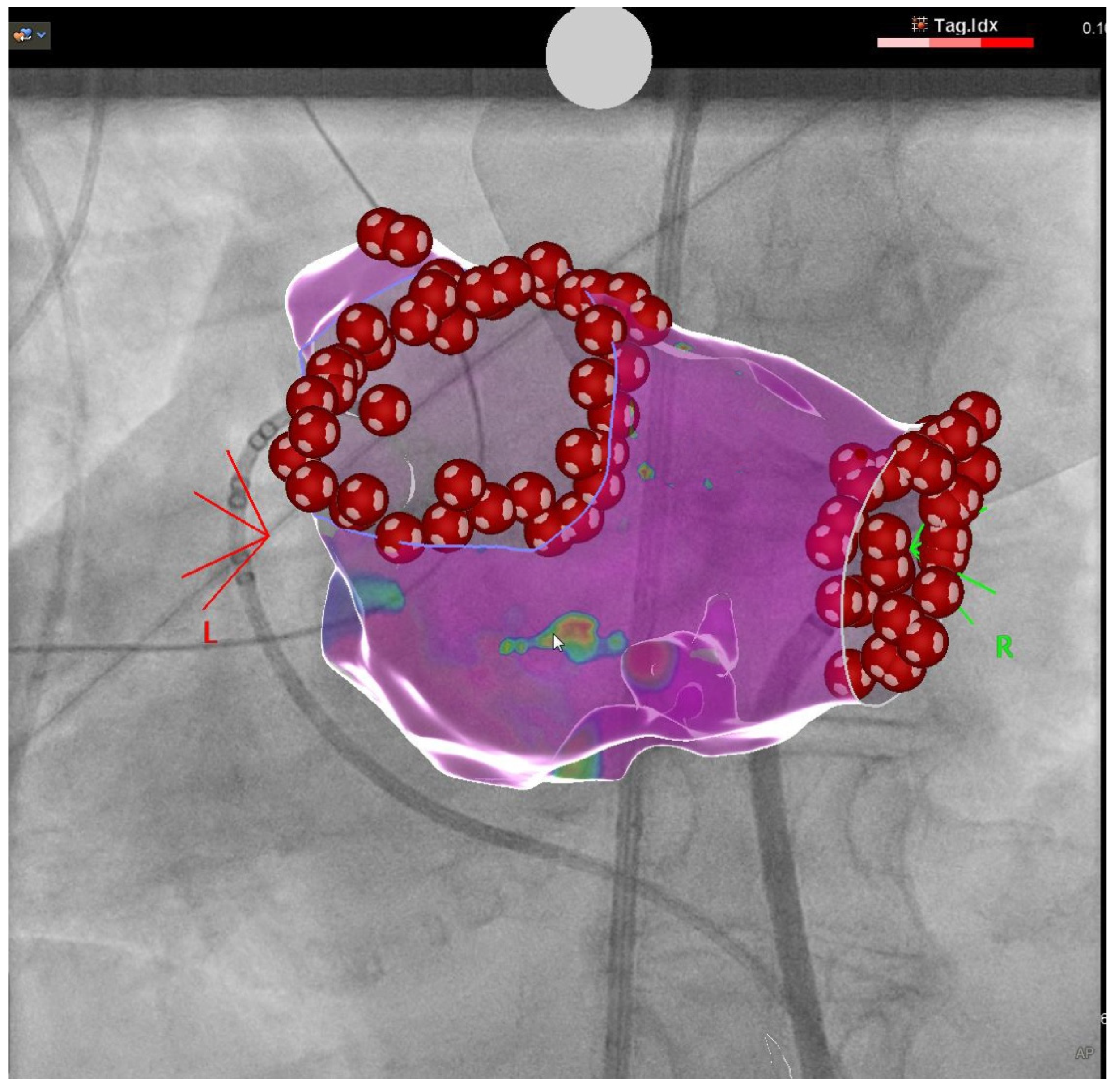
2.1. Pulmonary Vein Isolation
2.2. PVC Ablation
2.3. AVNRT Ablation
2.4. Coagulation Management
2.4.1. Pre- and Intraprocedural Coagulation Management
2.4.2. Postprocedural Management
2.5. Endpoints
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowman, M.; Hopman, W.M.; Rapson, D.; Lillicrap, D.; James, P. The prevalence of symptomatic von Willebrand disease in primary care practice. J. Thromb. Haemost. 2009, 8, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Leebeek, F.W.; Eikenboom, J.C. Von Willebrand’s Disease. N. Engl. J. Med. 2016, 375, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Berntorp, E.; Fischer, K.; Hart, D.P.; Mancuso, M.E.; Stephensen, D.; Shapiro, A.D.; Blanchette, V. Haemophilia. Nat. Rev. Dis. Prim. 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Stonebraker, J.S.; Chambost, H.; Makris, M.; Coffin, D.; Herr, C.; Germini, F.; for the Data and Demographics Committee of the World Federation of Hemophilia. Establishing the Prevalence and Prevalence at Birth of Hemophilia in Males. Ann. Intern. Med. 2019, 171, 540. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, A.A. Molecular genetics of hemophilia A: Clinical perspectives. Egypt. J. Med. Hum. Genet. 2010, 11, 105–114. [Google Scholar] [CrossRef]
- Schutgens, R.E.G.; Klamroth, R.; Pabinger, I.; Malerba, M.; Dolan, G.; the ADVANCE working group. Atrial fibrillation in patients with haemophilia: A cross-sectional evaluation in Europe. Haemophilia 2014, 20, 682–686. [Google Scholar] [CrossRef]
- Schutgens, R.E.G.; Klamroth, R.; Pabinger, I.; Dolan, G.; the ADVANCE working group. Management of atrial fibrillation in people with haemophilia—A consensus view by the ADVANCE Working Group. Haemophilia 2014, 20, e417–e420. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Van der Valk, P.R.; Mauser-Bunschoten, E.P.; van der Heijden, J.F.; Schutgens, R.E.G. Catheter Ablation for Atrial Fibrillation in Patients with Hemophilia or von Willebrand Disease. TH Open 2019, 03, e335–e339. [Google Scholar] [CrossRef]
- Sobolev, M.; Shiloh, A.L.; Di Biase, L.; Slovut, D.P. Ultrasound-guided cannulation of the femoral vein in electrophysiological procedures: A systematic review and meta-analysis. Europace 2017, 19, 850–855. [Google Scholar] [CrossRef]
- Cardoso, R.; Knijnik, L.; Bhonsale, A.; Miller, J.; Nasi, G.; Rivera, M.; Blumer, V.; Calkins, H. An updated meta-analysis of novel oral anticoagulants versus vitamin K antagonists for uninterrupted anticoagulation in atrial fibrillation catheter ablation. Hear. Rhythm 2018, 15, 107–115. [Google Scholar] [CrossRef]
- Gressenberger, P. Reversal strategies in patients treated with direct oral anticoagulants. Vasa 2019, 48, 389–392. [Google Scholar] [CrossRef]
- Schutgens, R.E.G.; van der Heijden, J.F.; Mauser-Bunschoten, E.P.; Mannucci, P.M. New concepts for anticoagulant therapy in persons with hemophilia. Blood 2016, 128, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Badescu, M.C.; Badulescu, O.V.; Butnariu, L.I.; Floria, M.; Ciocoiu, M.; Costache, I.-I.; Popescu, D.; Bratoiu, I.; Buliga-Finis, O.N.; Rezus, C. Current Therapeutic Approach to Atrial Fibrillation in Patients with Congenital Hemophilia. J. Pers. Med. 2022, 12, 519. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.J.; You, C.W. Clinical Analysis of Hospitalized Patients with Hemophilia A: Single-hemophilia Treatment Center Experience in Korea over 10 years. Blood Res. 2021, 56, 141–149. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. 6), 1–158. [Google Scholar] [CrossRef]
- Kramer, A.D.; Korsholm, K.; Kristensen, A.; Poulsen, L.H.; Nielsen-Kudsk, J.E. Left atrial appendage occlusion in haemophilia patients with atrial fibrillation. J. Interv. Card. Electrophysiol. 2021, 64, 95–102. [Google Scholar] [CrossRef]
- Toselli, M.; Bosi, D.; Benatti, G.; Solinas, E.; Cattabiani, M.A.; Vignali, L. Left atrial appendage closure: A balanced management of the thromboembolic risk in patients with hemophilia and atrial fibrillation. J. Thromb. Thrombolysis 2020, 50, 668–673. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Fu, C.; Hou, M.; Yan, L.; Shang, J.; Wang, Z.; Yin, J.; Yu, Z.; Wu, D. Bortezomib provides favorable efficacy in type 3 acquired von willebrand syndrome related to lymphoplasmacytic lymphoma/Waldenstrom’s macroglobulinemia. Leuk. Lymphoma 2021, 63, 491–494. [Google Scholar] [CrossRef]
- Ojeda-Uribe, M.; Caron, C.; Itzhar-Baikian, N.; Debliquis, A. Bortezomib effectiveness in one patient with acquired von Willebrand syndrome associated to monoclonal gammopathy of undetermined significance. Am. J. Hematol. 2010, 85, 396. [Google Scholar] [CrossRef]
- Guillet, B.; Cayla, G.; Lebreton, A.; Trillot, N.; Wibaut, B.; Falaise, C.; Castet, S.; Gautier, P.; Claeyssens, S.; Schved, J.-F. Long-Term Antithrombotic Treatments Prescribed for Cardiovascular Diseases in Patients with Hemophilia: Results from the French Registry. Thromb. Haemost. 2020, 121, 287–296. [Google Scholar] [CrossRef]
- Eghbali, A.; Melikof, L.; Taherahmadi, H.; Bagheri, B. Efficacy of tranexamic acid for the prevention of bleeding in patients with von Willebrand disease and Glanzmann thrombasthenia: A controlled, before and after trial. Haemophilia 2016, 22, e423–e426. [Google Scholar] [CrossRef]
- Wardrop, D.; Estcourt, L.J.; Brunskill, S.J.; Doree, C.; Trivella, M.; Stanworth, S.; Murphy, M.F. Antifibrinolytics (lysine analogues) for the prevention of bleeding in patients with haematological disorders. Cochrane Database Syst. Rev. 2013, CD009733. [Google Scholar] [CrossRef]
| Pt | Age | BMI | Bleeding Disorder | Clotting Levels [%] | Arrhythmia | CHA2DS2-VASc Score (HAS-BLED) Score | OAC Baseline | LV-EF |
|---|---|---|---|---|---|---|---|---|
| [%] | ||||||||
| 1 | 57 | 22.6 | VWD I | FVIII: 72 vWFAc: 64 Ac/Ag: 0.82 * | PersAF | 3 (2) | None | 55 |
| 2 | 56 | 25.7 | HA | FVIII: 15 | PAF | 1 (1) | None | 55 |
| 3 | 23 | 21.5 | VWD I | FVIII: 73 vWFAc: 53 Ac/Ag: 0.92 | PVC | 0 (1) | None | 55 |
| 4 | 76 | 31.6 | VWD IIA | FVIII: 64 vWFAc: 37 Ac: 37% | PersAF | 4 (4) | Dabigatran | 40–45 |
| 5 | 27 | 28.4 | HA | FVIII: 22 | PVC | 1 (1) | None | 55 |
| 6 | 45 | 22.2 | VWD I | FVIII: 58 vWFAc: 37 Ag/Ac: 0.79 | AVNRT | 0 (0) | None | 55 |
| 7 | 72 | 20.7 | HA | FVIII: 19 | PVC | 1 (2) | None | 63% |
| Pt | Procedure | Procedure Time | Preprocedural Management | Periprocedural Management | Postprocedural Management | Complications |
|---|---|---|---|---|---|---|
| 1 | Re-PVI | 125 min | 2000 IE Wilate | 10,000 IE Heparin | Tranexam acid 3 × 1 g/3 d Rivaroxaban | - |
| Re-re-PVI | 95 min | 2000 IE Wilate | 12,000 IE Heparin | Apixaban | - | |
| CTI + LAAI | 145 min | 2000 IE Wilate | 12,000 IE Heparin | Apixaban | ||
| 2 | Laser-PVI | 50 min | 2000 IE Haemoctin | 14,000 IE Heparin | Warfarin | - |
| 3 | RF PVC | 120 min | None (FVIII > 60%) | 8000 IE Heparin | - | - |
| 4 | Cryo-PVI | 70 min | 3000 IE Wilate | 18,000 IE Heparin | Dabigatran | - |
| RF-PVI | 104 min | 3000 IE Wilate | 21,000 IE Heparin | Dabigatran | - | |
| 5 | RF PVC | 70 min | 2000 IE Haemoctin | 14,000 IE Heparin | 1000 IE Haemoctin/3 d 4 week ASA | - |
| 6 | RF AVNRT | 91 min | 2000 IE Wilate | 9000 IE Heparin * | Tranexam acid 3 × 1 g/5 d 1000 IE Wilate/2 d | - |
| 7 | RF PVC | 100 min | 3000 IE NovoEight | 3000 IE Heparin | 1500 IE NovoEight 12 h and 24 h after the procedure 1500 IE NovoEight 3×/wk until ASA withdrawal; 4 week ASA | Minor hematoma, no Hb drop |
| Median PVI: 98.2 (IQR 70–125) Median other: 95.5 (IQR 80–110) | Median Wilate: 2300 (IQR 2–3) Median Factor VIII: 2000 (IQR 2–3) | Median Heparin: 14,500 (IQR 12,000–18,000) Median Heparin 8000 (IQR 3000–14,000) |
| Pt | Procedure | DOP | Anticoagulation | OAC/APT Withdrawal | FFA | Complications | Further Interventions |
|---|---|---|---|---|---|---|---|
| 1 | Redo-PVI | 08/2017 | Rivaroxaban | - | - | - | |
| Redo-PVI | 05/2018 | Apixaban | - | - | - | ||
| CTI + LAAI | 02/2019 | Apixaban | 05/2019 | 02/2019 | - | LAAC 04/2019 after LAAI | |
| 2 | Laser-PVI | 05/2018 | Warfarin | 08/2018 | 05/2018 | - | |
| 3 | RF PVC | 12/2019 | - | - | 12/2019 | - | |
| 4 | Cryo-PVI | 10/2020 | Dabigatran | - | - | GI-Bleeding | |
| RF-PVI | 02/2021 | Dabigatran | 05/2021 | 12/2021 | - | LAAC 04/2021 after GI-Bleeding | |
| 5 | RF PVC | 03/2021 | ASA | 04/2021 | 03/2021 | - | |
| 6 | RF AVNRT | 08/2019 | - | - | 08/2019 | - | |
| 7 | RF PVC | 08/2021 | ASA | 09/2021 | 08/2021 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feher, M.; Saguner, A.M.; Kirstein, B.; Vogler, J.; Eitel, C.; Phan, H.-L.; Keelani, A.; Cimen, T.; Hatahet, S.; Trajanoski, D.; et al. Safety and Feasibility of Catheter Ablation Procedures in Patients with Bleeding Disorders. J. Clin. Med. 2022, 11, 6956. https://doi.org/10.3390/jcm11236956
Feher M, Saguner AM, Kirstein B, Vogler J, Eitel C, Phan H-L, Keelani A, Cimen T, Hatahet S, Trajanoski D, et al. Safety and Feasibility of Catheter Ablation Procedures in Patients with Bleeding Disorders. Journal of Clinical Medicine. 2022; 11(23):6956. https://doi.org/10.3390/jcm11236956
Chicago/Turabian StyleFeher, Marcel, Ardan M. Saguner, Bettina Kirstein, Julia Vogler, Charlotte Eitel, Huong-Lan Phan, Ahmad Keelani, Tolga Cimen, Sascha Hatahet, Darko Trajanoski, and et al. 2022. "Safety and Feasibility of Catheter Ablation Procedures in Patients with Bleeding Disorders" Journal of Clinical Medicine 11, no. 23: 6956. https://doi.org/10.3390/jcm11236956
APA StyleFeher, M., Saguner, A. M., Kirstein, B., Vogler, J., Eitel, C., Phan, H.-L., Keelani, A., Cimen, T., Hatahet, S., Trajanoski, D., Samara, O., Kuck, K.-H., Tilz, R. R., & Heeger, C.-H. (2022). Safety and Feasibility of Catheter Ablation Procedures in Patients with Bleeding Disorders. Journal of Clinical Medicine, 11(23), 6956. https://doi.org/10.3390/jcm11236956







