Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population and Variables
2.3. Statistical Analyses
3. Results
3.1. Participant Clinical Characteristics
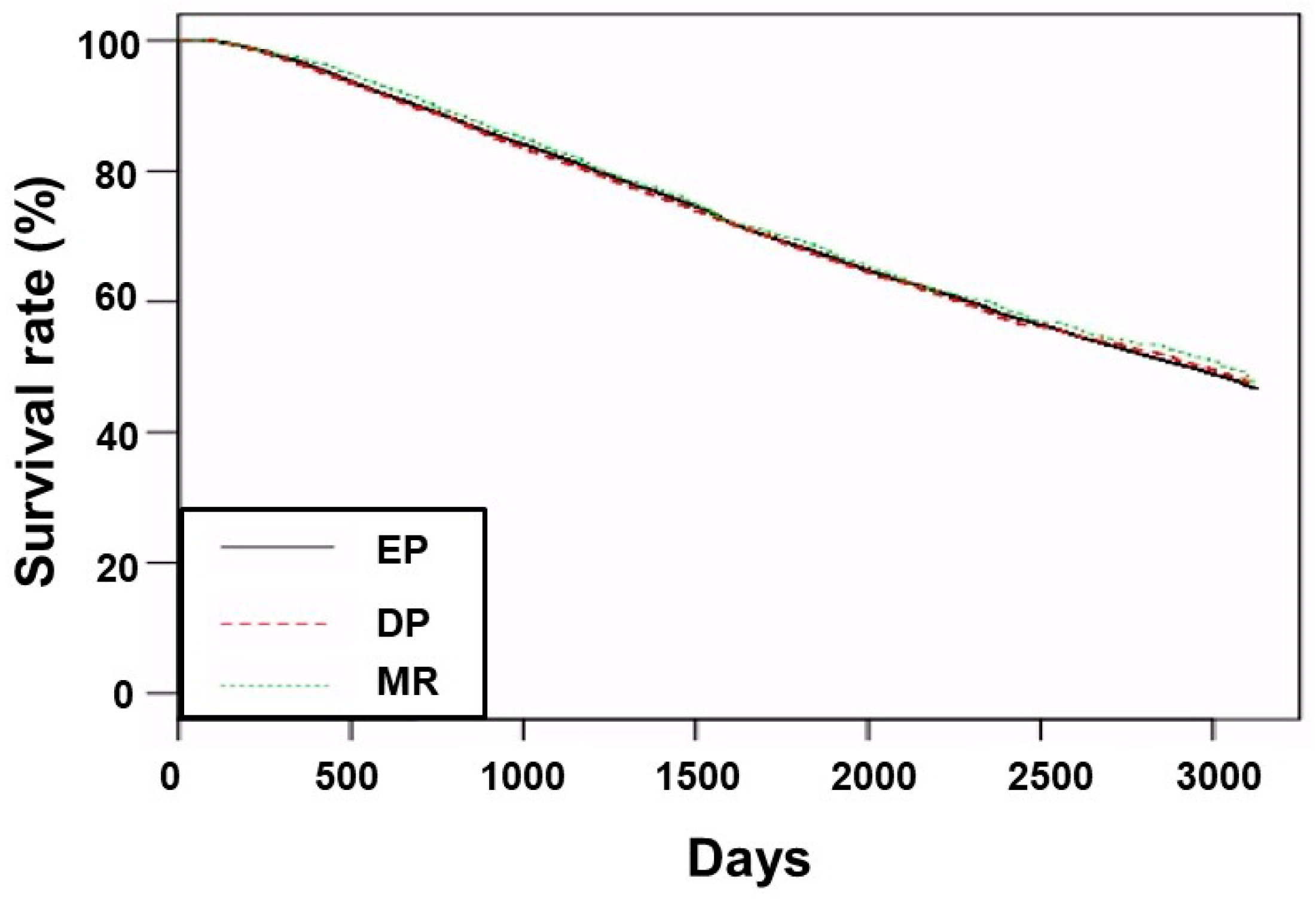
3.2. Survival Analyses
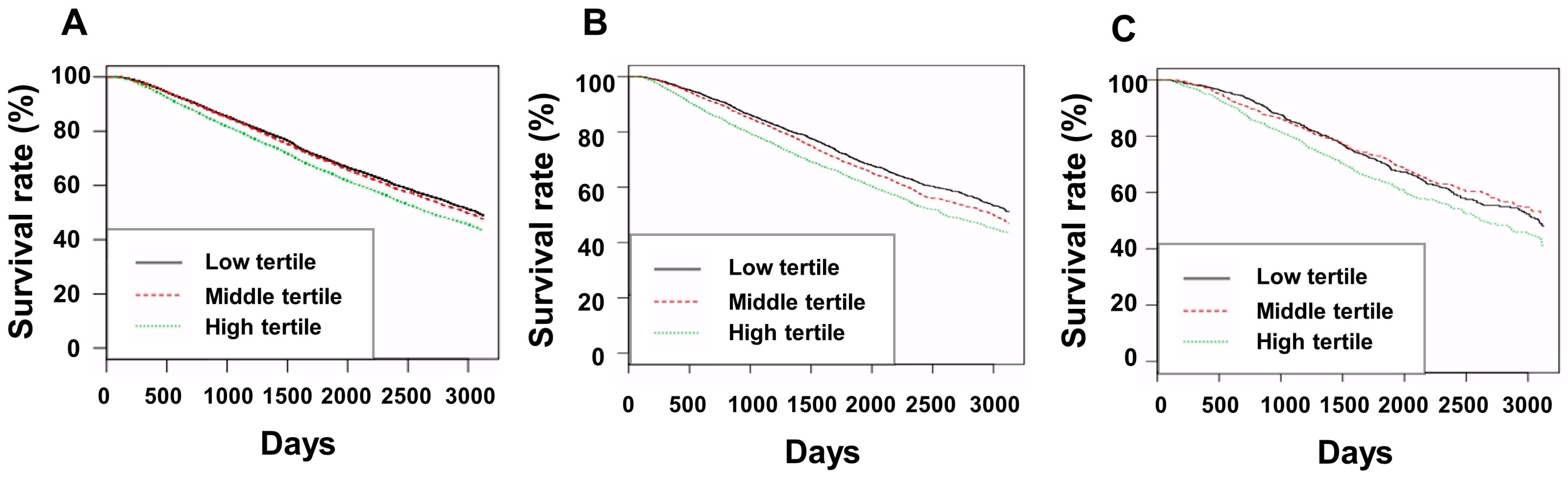
3.3. Subgroup Analyses
3.4. Factors Associated with ERI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ESRD Registry Committee: Korean Society of Nephrology. Factsheet: CKD in Korea. Available online: https://ksn.or.kr/bbs/index.php?page=2&code=Factsheet (accessed on 15 December 2022).
- US Renal Data System, USRDS 2020 Annual Data Report: Atlas of Chronic Kidney Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2020. Available online: https://adr.usrds.org/2020 (accessed on 15 December 2022).
- McMurray, J.; Parfrey, P.S.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- ESRD Registry Committee: Korean Society of Nephrology. Current Renal Replacement Therapy in Korea. 2021. Available online: https://ksn.or.kr/bbs/index.php?code=report (accessed on 15 December 2022).
- Saglimbene, V.M.; Palmer, S.C.; Ruospo, M.; Natale, P.; Craig, J.C.; Strippoli, G.F. Continuous erythropoiesis receptor activator (CERA) for the anaemia of chronic kidney disease. Cochrane Database Syst. Rev. 2017, 8, CD009904. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Wada, A.; Masakane, I. Types of Erythropoietin-Stimulating Agents and Mortality among Patients Undergoing Hemodialysis. J. Am. Soc. Nephrol. 2019, 30, 1037–1048. [Google Scholar] [CrossRef]
- Locatelli, F.; Hannedouche, T.; Fishbane, S.; Morgan, Z.; Oguey, D.; White, W.B. Cardiovascular Safety and All-Cause Mortality of Methoxy Polyethylene Glycol-Epoetin Beta and Other Erythropoiesis-Stimulating Agents in Anemia of CKD: A Randomized Noninferiority Trial. Clin. J. Am. Soc. Nephrol. 2019, 14, 1701–1710. [Google Scholar] [CrossRef]
- Minutolo, R.; Garofalo, C.; Chiodini, P.; Aucella, F.; Del Vecchio, L.; Locatelli, F.; Scaglione, F.; De Nicola, L. Types of erythropoiesis-stimulating agents and risk of end-stage kidney disease and death in patients with non-dialysis chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Jhee, J.H.; Joo, Y.S.; Yang, K.H.; Jung, J.J.; Shin, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Clinical significance of hemodialysis quality of care indicators in very elderly patients with end stage kidney disease. J. Nephrol. 2022, 35, 2351–2361. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service. 6th Hemodialysis Quality Assessment Program. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none (accessed on 15 December 2022).
- Vega, A.; Abad, S.; Verdalles, U.; Aragoncillo, I.; Velazquez, K.; Quiroga, B.; Escudero, V.; López-Gómez, J.M. Dose equivalence between continuous erythropoietin receptor activator (CERA), Darbepoetin and Epoetin in patients with advanced chronic kidney disease. Hippokratia 2014, 18, 315–318. [Google Scholar]
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef]
- Fishbane, S.; Berns, J.S. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 2005, 68, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Pham, E.; Macdougall, I.C. Erythropoietins: A common mechanism of action. Exp. Hematol. 2008, 36, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Rucker, D.; Thadhani, R.; Tonelli, M. Trace element status in hemodialysis patients. Semin. Dial. 2010, 23, 389–395. [Google Scholar] [CrossRef]
- Yasukawa, M.; Arai, S.; Nagura, M.; Kido, R.; Asakawa, S.; Hirohama, D.; Yamazaki, O.; Tamura, Y.; Fujimaki, M.; Kobayashi, S.; et al. Selenium Associates with Response to Erythropoiesis-Stimulating Agents in Hemodialysis Patients. Kidney Int. Rep. 2022, 7, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Anadón Ruiz, A.; Martín Jiménez, E.; Bermejo-Barrera, P.; Lozano, R.; Seijas, V.M. Selenium and All-cause Mortality in End-Stage Renal Disease. Retrospective Observational Cohort Study. J. Ren. Nutr. 2020, 30, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef]
- Garagarza, C.; Valente, A.; Caetano, C.; Ramos, I.; Sebastião, J.; Pinto, M.; Oliveira, T.; Ferreira, A.; Sousa Guerreiro, C. Zinc Deficient Intake in Hemodialysis Patients: A Path to a High Mortality Risk. J. Ren. Nutr. 2022, 32, 87–93. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yang, C.C.; Hung, K.C.; Jiang, M.Y.; Huang, Y.T.; Hwang, J.C.; Hsieh, C.C.; Chuang, M.H.; Chen, J.Y. Association between hypomagnesemia and mortality among dialysis patients: A systematic review and meta-analysis. PeerJ 2022, 10, e14203. [Google Scholar] [CrossRef]
- Yu, L.; Song, J.; Lu, X.; Zu, Y.; Li, H.; Wang, S. Association between Serum Magnesium and Erythropoietin Responsiveness in Hemodialysis Patients: A Cross-Sectional Study. Kidney Blood Press. Res. 2019, 44, 354–361. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, J.H.; Kim, S.J.; Ryu, D.R.; Kang, D.H.; Choi, K.B. The Relationship between Magnesium and Endothelial Function in End-Stage Renal Disease Patients on Hemodialysis. Yonsei Med. J. 2016, 57, 1446–1453. [Google Scholar] [CrossRef]
- Moctezuma-Velázquez, C.; Gómez-Sámano, M.Á.; Cajas-Sánchez, M.B.; Reyes-Molina, D.L.; Galindo-Guzmán, M.; Meza-Arana, C.E.; Cuevas-Ramos, D.; Gómez-Pérez, F.J.; Gulias-Herrero, A. High Dietary Magnesium Intake is Significantly and Independently Associated with Higher Insulin Sensitivity in a Mexican-Mestizo Population: A Brief Cross-Sectional Report. Rev. Investig. Clin. 2017, 69, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toprak, O.; Kurt, H.; Sarı, Y.; Şarkış, C.; Us, H.; Kırık, A. Magnesium Replacement Improves the Metabolic Profile in Obese and Pre-Diabetic Patients with Mild-to-Moderate Chronic Kidney Disease: A 3-Month, Randomised, Double-Blind, Placebo-Controlled Study. Kidney Blood Press. Res. 2017, 42, 33–42. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, A.; Correa-Rotter, R.; Vázquez-Rangel, A.; Vega-Vega, O.; Espinosa-Cuevas, Á. Relationship between protein-energy wasting in adults with chronic hemodialysis and the response to treatment with erythropoietin. BMC Nephrol. 2019, 20, 316. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Yajima, K.; Takahashi, H. Association of the erythropoiesis-stimulating agent resistance index and the geriatric nutritional risk index with cardiovascular mortality in maintenance hemodialysis patients. PLoS ONE 2021, 16, e0245625. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Suh, S.W.; Hwang, J.H.; Shin, J. Responsiveness to an erythropoiesis-stimulating agent is correlated with body composition in patients undergoing chronic hemodialysis. Front. Nutr. 2022, 9, 1044895. [Google Scholar] [CrossRef]
- Mallick, S.; Rafiroiu, A.; Kanthety, R.; Iqbal, S.; Malik, R.; Rahman, M. Factors predicting erythropoietin resistance among maintenance hemodialysis patients. Blood Purif. 2012, 33, 238–244. [Google Scholar] [CrossRef]

| EP (n = 38,043) | DP (n = 10,054) | MR (n = 2253) | p | |
|---|---|---|---|---|
| Age (years) | 60.8 ± 13.0 | 61.4 ± 13.1 * | 62.1 ± 12.6 *,† | <0.001 |
| Sex (male, %) | 22,544 (59.3%) | 5683 (56.5%) | 1325 (58.8%) | <0.001 |
| Hemodialysis vintage (days) | 1468 ± 1618 | 1383 ± 1530 * | 1380 ± 1548 * | <0.001 |
| CCI score | 7.7 ± 2.9 | 7.7 ± 2.8 | 8.1 ± 2.9 *,† | <0.001 |
| Body mass index (kg/m2) | 22.4 ± 3.5 | 22.5 ± 3.5 * | 22.8 ± 3.4 *,† | <0.001 |
| Underlying etiology of ESRD | <0.001 | |||
| Diabetes mellitus | 17,031 (44.8%) | 4681 (46.6%) | 1068 (47.4%) | |
| Hypertension | 10,069 (26.5%) | 2474 (24.6%) | 577 (25.6%) | |
| Glomerulonephritis | 3846 (10.1%) | 1058 (10.5%) | 220 (9.8%) | |
| Other | 2951 (7.8%) | 976 (9.7%) | 175 (7.8%) | |
| Unknown | 4146 (10.9%) | 865 (8.6%) | 213 (9.5%) | |
| Follow-up duration (days) | 1814 ± 847 | 1763 ± 838 * | 1826 ± 830 † | <0.001 |
| Type of vascular access | <0.001 | |||
| Arteriovenous fistula | 31,631 (83.1%) | 8228 (81.8%) | 1867 (82.9%) | |
| Arteriovenous graft | 5502 (14.5%) | 1514 (15.1%) | 345 (15.3%) | |
| Catheter | 910 (2.4%) | 312 (3.1%) | 41 (1.8%) | |
| Kt/Vurea | 1.52 ± 0.27 | 1.56 ± 0.29 * | 1.56 ± 0.27 * | <0.001 |
| Ultrafiltration volume (L/session) | 2.26 ± 0.95 | 2.19 ± 0.97 * | 2.16 ± 0.99 * | <0.001 |
| Hemoglobin (g/dL) | 10.6 ± 0.7 | 10.7 ± 0.7 * | 10.7 ± 0.8 * | <0.001 |
| Serum albumin (g/dL) | 4.00 ± 0.34 | 3.92 ± 0.35 * | 3.93 ± 0.33 * | <0.001 |
| Serum phosphorus (mg/dL) | 5.0 ± 1.4 | 4.8 ± 1.3 * | 4.9 ± 1.4 † | <0.001 |
| Serum calcium (mg/dL) | 8.9 ± 0.8 | 8.8 ± 0.7 * | 8.8 ± 0.8 * | <0.001 |
| Serum creatinine (mg/dL) | 9.4 ± 2.7 | 9.3 ± 2.7 * | 9.0 ± 2.6 *,† | <0.001 |
| Systolic blood pressure (mmHg) | 142 ± 15 | 140 ± 16 * | 143 ± 16 † | <0.001 |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 76 ± 10 * | 76 ± 10 * | <0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Ref: EP group | ||||
| DP group | 1.01 (0.98–1.05) | 0.527 | 1.01 (0.97–1.05) | 0.740 |
| MR group | 0.97 (0.91–1.04) | 0.433 | 0.89 (0.83–0.97) | 0.006 |
| Ref: DP group | ||||
| MR group | 0.96 (0.89–1.04) | 0.302 | 0.89 (0.82–0.97) | 0.006 |
| Age (per 1 year increase) | 1.07 (1.06–1.07) | <0.001 | 1.06 (1.06–1.07) | <0.001 |
| Sex (ref: male) | 0.85 (0.83–0.88) | <0.001 | 0.75 (0.72–0.78) | <0.001 |
| CCI score (per 1 score increase) | 1.14 (1.13–1.14) | <0.001 | 1.06 (1.06–1.07) | <0.001 |
| Body mass index (per 1 kg/m2 increase) | 0.97 (0.97–0.98) | <0.001 | 0.98 (0.97–0.98) | <0.001 |
| Underlying etiology of ESRD (ref: DM) | ||||
| Glomerulonephritis | 0.34 (0.32–0.37) | <0.001 | 0.53 (0.48–0.57) | <0.001 |
| Hypertension | 0.66 (0.64–0.69) | <0.001 | 0.67 (0.64–0.70) | <0.001 |
| Other | 0.53 (0.50–0.56) | <0.001 | 0.69 (0.64–0.74) | <0.001 |
| Unknown | 0.60 (0.57–0.64) | <0.001 | 0.70 (0.65–0.74) | <0.001 |
| Hemoglobin (per 1 g/dL increase) | 0.85 (0.83–0.87) | <0.001 | 0.94 (0.91–0.96) | <0.001 |
| Serum creatinine (per 1 mg/dL increase) | 0.86 (0.86–0.87) | <0.001 | 0.94 (0.94–0.95) | <0.001 |
| SBP (per 1 mmHg increase) | 1.01 (1.01–1.01) | <0.001 | 1.00 (1.00–1.01) | <0.001 |
| DBP (per 1 mmHg increase) | 0.98 (0.98–0.98) | <0.001 | 1.00 (1.00–1.01) | 0.011 |
| Serum calcium (per 1 mg/dL increase) | 0.94 (0.92–0.95) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| Serum phosphorus (per 1 mg/dL increase) | 0.84 (0.83–0.85) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| Kt/Vurea (per 1 unit increase) | 0.91 (0.86–0.97) | 0.002 | 0.74 (0.68–0.80) | <0.001 |
| Serum albumin (per 1 g/dL increase) | 0.36 (0.35–0.38) | <0.001 | 0.63 (0.60–0.67) | <0.001 |
| HD vintage (per 1 day increase) | 1.00 (1.00–1.00) | 0.004 | 1.00 (1.00–1.00) | <0.001 |
| Ultrafiltration volume (per 1 L increase) | 0.92 (0.90–0.93) | <0.001 | 1.06 (1.04–1.08) | <0.001 |
| ERI (per 1 unit increase) | 1.02 (1.02–1.02) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| ESA dose (per 1 unit/week increase) | 1.00 (1.00–1.00) | <0.001 | 1.00 (1.00–1.00) | <0.001 |
| Limitations | |
|---|---|
| Design | Retrospective observational design |
| Data set | No data on iron supplementation or iron status |
| No data on micronutrients | |
| No data on the route of injection of ESAs | |
| No data on patient inflammatory status during the treatment period | |
| No data on safety issues related to ESA treatment | |
| Definitions of types or doses of ESA using claims data | |
| Definitions of comorbidities using claims data | |
| No consideration of the factors related to the centers or physicians | |
| Use of two or more ESAs in a small proportion of patients | |
| Statistical analysis | Imbalance in sample size and baseline characteristics among the three groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.H.; Kim, B.Y.; Son, E.J.; Kim, G.O.; Do, J.Y. Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients. J. Clin. Med. 2023, 12, 625. https://doi.org/10.3390/jcm12020625
Kang SH, Kim BY, Son EJ, Kim GO, Do JY. Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients. Journal of Clinical Medicine. 2023; 12(2):625. https://doi.org/10.3390/jcm12020625
Chicago/Turabian StyleKang, Seok Hui, Bo Yeon Kim, Eun Jung Son, Gui Ok Kim, and Jun Young Do. 2023. "Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients" Journal of Clinical Medicine 12, no. 2: 625. https://doi.org/10.3390/jcm12020625
APA StyleKang, S. H., Kim, B. Y., Son, E. J., Kim, G. O., & Do, J. Y. (2023). Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients. Journal of Clinical Medicine, 12(2), 625. https://doi.org/10.3390/jcm12020625






