Eating Disorders and Dental Erosion: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Extraction
- for PubMed: (eating disorders OR anorexia OR bulimia) AND ((tooth OR dental) AND erosion)
- for Scopus: TITLE-ABS-KEY((“eating disorders” OR anorexia OR bulimia) AND ((tooth OR dental) AND erosion))
- for Web of Science: TS = ((eating disorders OR anorexia OR bulimia) AND ((tooth OR dental) AND erosion)).
2.2. Quality Assessment and Critical Appraisal for the Systematic Review of Included Studies
3. Results
3.1. Search Strategy
3.2. Characteristics of Included Studies
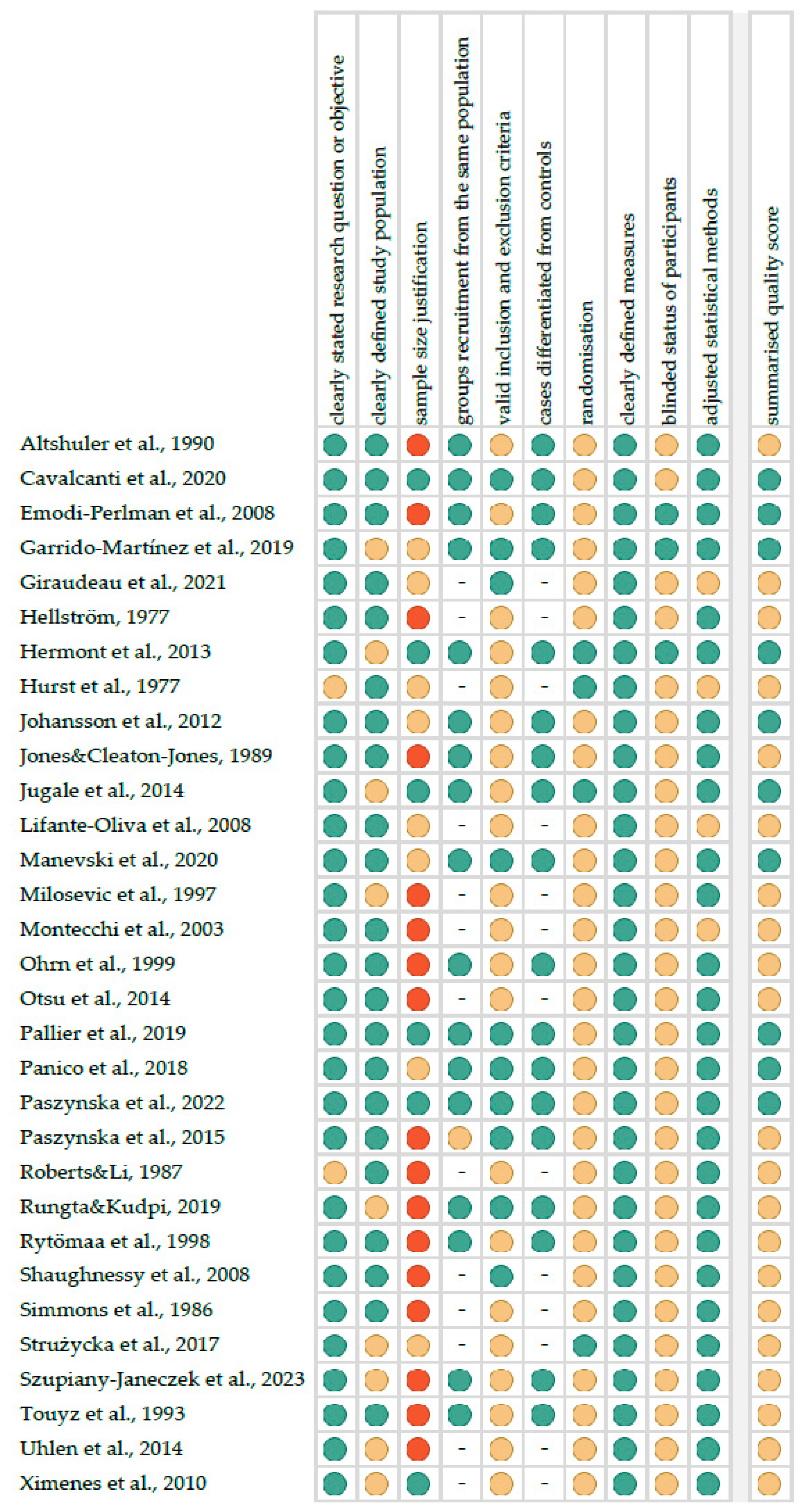
3.3. Quality Assessment of Included Studies
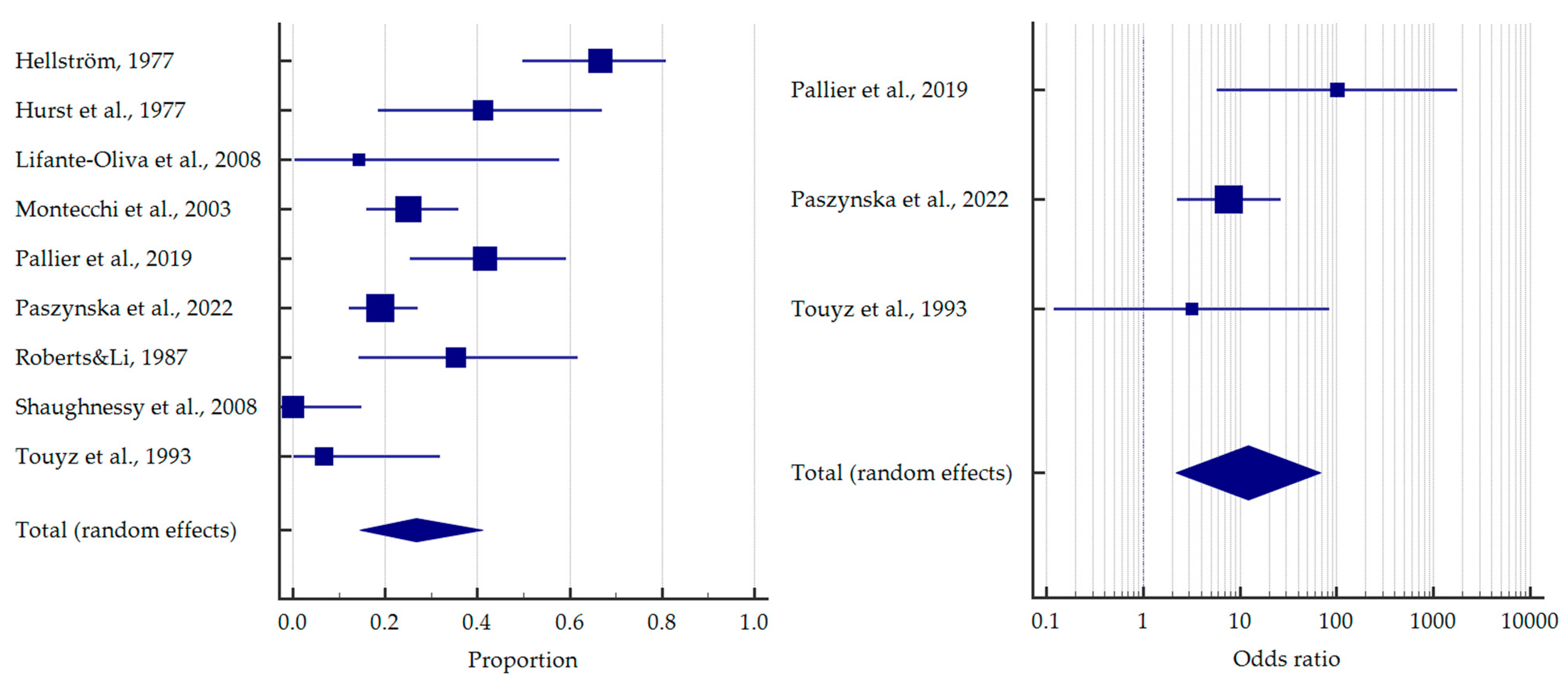
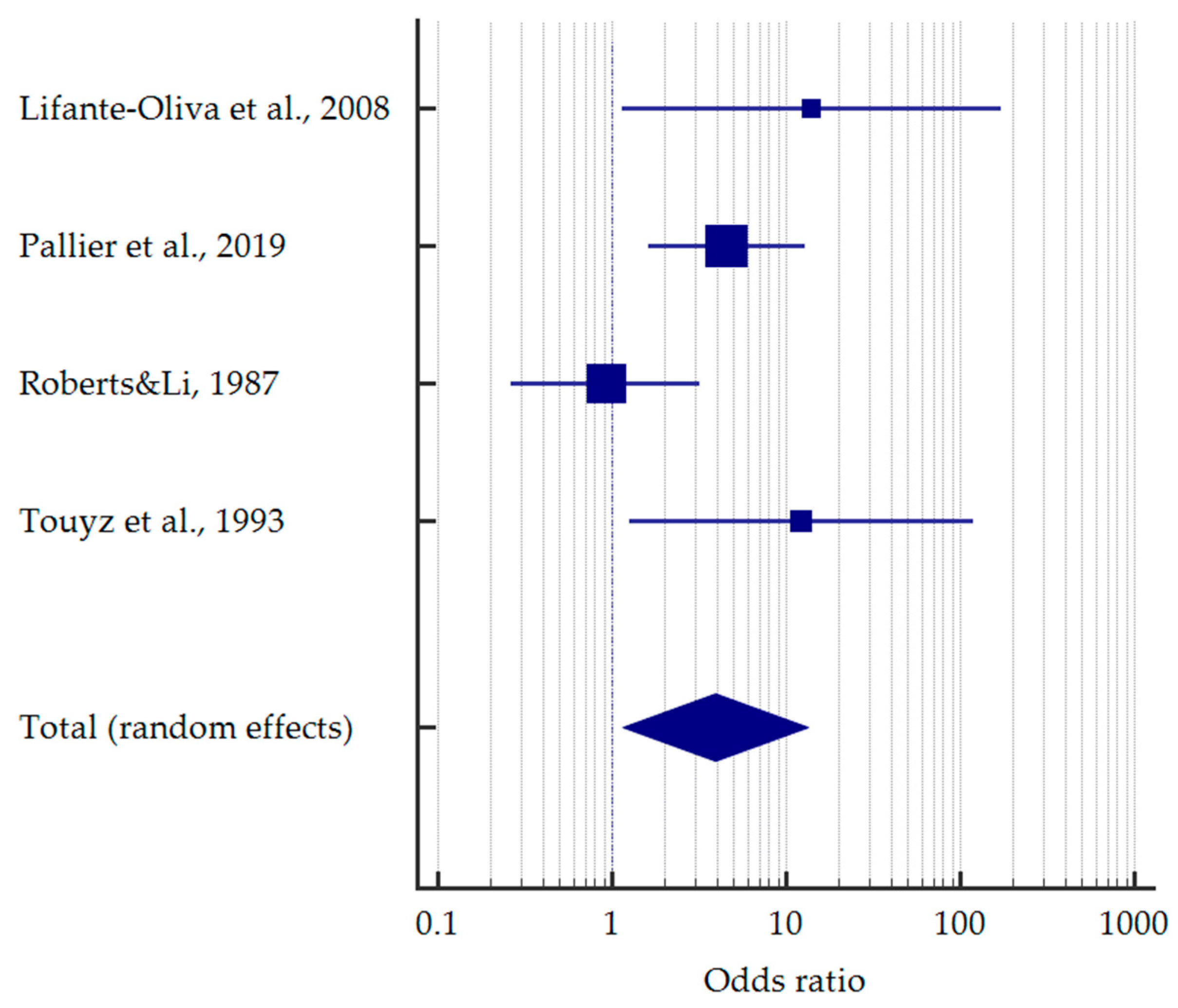
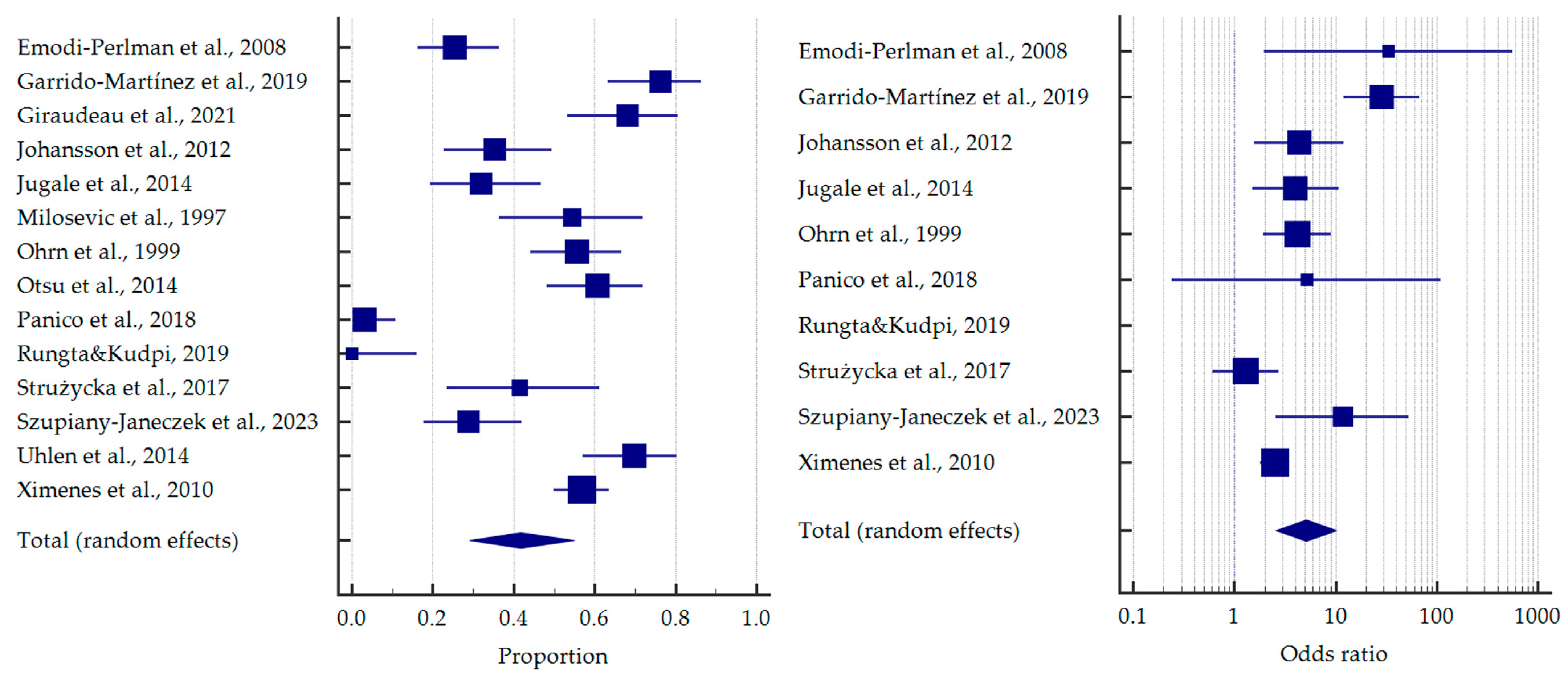
3.4. Results of Meta-Analysis
4. Discussion
4.1. Dental Erosion in Anorexia Nervosa
4.2. Dental Erosion in Bulimia Nervosa
4.3. Dental Erosion in Non-Distinguished Eating Disorders
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating Disorders. Lancet Lond. Engl. 2020, 395, 899–911. [Google Scholar] [CrossRef]
- Stice, E.; Marti, C.N.; Rohde, P. Prevalence, Incidence, Impairment, and Course of the Proposed DSM-5 Eating Disorder Diagnoses in an 8-Year Prospective Community Study of Young Women. J. Abnorm. Psychol. 2013, 122, 445–457. [Google Scholar] [CrossRef]
- Striegel-Moore, R.H.; Rosselli, F.; Perrin, N.; DeBar, L.; Wilson, G.T.; May, A.; Kraemer, H.C. Gender Difference in the Prevalence of Eating Disorder Symptoms. Int. J. Eat. Disord. 2009, 42, 471–474. [Google Scholar] [CrossRef]
- Rikani, A.A.; Choudhry, Z.; Choudhry, A.M.; Ikram, H.; Asghar, M.W.; Kajal, D.; Waheed, A.; Mobassarah, N.J. A Critique of the Literature on Etiology of Eating Disorders. Ann. Neurosci. 2013, 20, 157–161. [Google Scholar] [CrossRef]
- Sharan, P.; Sundar, A.S. Eating Disorders in Women. Indian J. Psychiatry 2015, 57, S286–S295. [Google Scholar] [CrossRef]
- Porras-Garcia, B.; Ferrer-Garcia, M.; Serrano-Troncoso, E.; Carulla-Roig, M.; Soto-Usera, P.; Miquel-Nabau, H.; Fernández-Del castillo Olivares, L.; Marnet-Fiol, R.; de la Montaña Santos-Carrasco, I.; Borszewski, B.; et al. AN-VR-BE. A Randomized Controlled Trial for Reducing Fear of Gaining Weight and Other Eating Disorder Symptoms in Anorexia Nervosa through Virtual Reality-Based Body Exposure. J. Clin. Med. 2021, 10, 682. [Google Scholar] [CrossRef]
- Gaudio, S.; Brooks, S.J.; Riva, G. Nonvisual Multisensory Impairment of Body Perception in Anorexia Nervosa: A Systematic Review of Neuropsychological Studies. PLoS ONE 2014, 9, e110087. [Google Scholar] [CrossRef]
- Nitsch, A.; Dlugosz, H.; Gibson, D.; Mehler, P.S. Medical Complications of Bulimia Nervosa. Cleve. Clin. J. Med. 2021, 88, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; An, Z.; Kim, K.-H.; Kim, D.-M.; Hwang, B.-I.; Kim, M. Factors Associated with Underweight, Overweight, and Eating Disorders in Young Korean Women: A Population-Based Study. Nutrients 2022, 14, 1315. [Google Scholar] [CrossRef] [PubMed]
- Lo Muzio, L.; Lo Russo, L.; Massaccesi, C.; Rappelli, G.; Panzarella, V.; Di Fede, O.; Kerr, A.R.; Campisi, G. Eating Disorders: A Threat for Women’s Health. Oral Manifestations in a Comprehensive Overview. Minerva Stomatol. 2007, 56, 281–292. [Google Scholar] [PubMed]
- Monda, M.; Costacurta, M.; Maffei, L.; Docimo, R. Oral Manifestations of Eating Disorders in Adolescent Patients. A Review. Eur. J. Paediatr. Dent. 2021, 22, 155–158. [Google Scholar] [CrossRef]
- Garbin, C.A.S.; Martins, R.J.; de Melo Belila, N.; Garbin, A.J.Í. Oral Manifestations in Patients with Anorexia and Bulimia Nervosa: A Systematic Review. J. Public Health 2020, 28, 765–771. [Google Scholar] [CrossRef]
- Lo Russo, L.; Campisi, G.; Di Fede, O.; Di Liberto, C.; Panzarella, V.; Lo Muzio, L. Oral Manifestations of Eating Disorders: A Critical Review. Oral Dis. 2008, 14, 479–484. [Google Scholar] [CrossRef]
- Presskreischer, R.; Prado, M.A.; Kuraner, S.E.; Arusilor, I.-M.; Pike, K. Eating Disorders and Oral Health: A Scoping Review. J. Eat. Disord. 2023, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.; Ganss, C.; Lussi, A. Basic Erosive Wear Examination (BEWE): A New Scoring System for Scientific and Clinical Needs. Clin. Oral Investig. 2008, 12 (Suppl. 1), S65–S68. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.K.; Hegde, A.; Thomas, A.; Virdi, M.S. Acidogenic Potential of Packaged Fruit Juices and Its Effect on Plaque and Salivary pH. Int. J. Clin. Pediatr. Dent. 2019, 12, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Valena, V.; Young, W.G. Dental Erosion Patterns from Intrinsic Acid Regurgitation and Vomiting. Aust. Dent. J. 2002, 47, 106–115. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Scardina, G.A.; Pezzullo, S.; Scribante, A. Home Oral Care Domiciliary Protocol for the Management of Dental Erosion in Rugby Players: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 4893. [Google Scholar] [CrossRef]
- Nijakowski, K.; Walerczyk-Sas, A.; Surdacka, A. Regular Physical Activity as a Potential Risk Factor for Erosive Lesions in Adolescents. Int. J. Environ. Res. Public. Health 2020, 17, 3002. [Google Scholar] [CrossRef]
- Nijakowski, K.; Zdrojewski, J.; Nowak, M.; Podgórski, F.; Surdacka, A. Regular Physical Activity and Dental Erosion: A Systematic Review. Appl. Sci. 2022, 12, 1099. [Google Scholar] [CrossRef]
- Johansson, A.-K.; Omar, R.; Carlsson, G.E.; Johansson, A. Dental Erosion and Its Growing Importance in Clinical Practice: From Past to Present. Int. J. Dent. 2012, 2012, 632907. [Google Scholar] [CrossRef] [PubMed]
- Skalsky Jarkander, M.; Grindefjord, M.; Carlstedt, K. Dental Erosion, Prevalence and Risk Factors among a Group of Adolescents in Stockholm County. Eur. Arch. Paediatr. Dent. 2018, 19, 23–31. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Hannas, A.R.; Kato, M.T. Saliva and Dental Erosion. J. Appl. Oral Sci. 2012, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Tran, T.T.K.; Hsu, Y.H.; Liu, S.Y.S.; Kroon, J. A Systematic Review of Dietary Acids and Habits on Dental Erosion in Adolescents. Int. J. Paediatr. Dent. 2020, 30, 713–733. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Magalhães, A.C.; Rios, D. Prevention of Erosive Tooth Wear: Targeting Nutritional and Patient-Related Risks Factors. Br. Dent. J. 2018, 224, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ludovichetti, F.S.; Signoriello, A.G.; Colussi, N.; Zuccon, A.; Stellini, E.; Mazzoleni, S. Soft Drinks and Dental Erosion during Pediatric Age: A Clinical Investigation. Minerva Dent. Oral Sci. 2022, 71, 262–269. [Google Scholar] [CrossRef]
- Ludovichetti, F.S.; Zambon, G.; Cimolai, M.; Gallo, M.; Signoriello, A.G.; Pezzato, L.; Bertolini, R.; Mazzoleni, S. Efficacy of Two Toothpaste in Preventing Tooth Erosive Lesions Associated with Gastroesophageal Reflux Disease. Appl. Sci. 2022, 12, 1023. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2020, 88, 105906. [Google Scholar] [CrossRef]
- NHLBI, NIH. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 22 August 2020).
- OCEBM. Levels of Evidence. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ (accessed on 22 August 2020).
- Altshuler, B.D.; Dechow, P.C.; Waller, D.A.; Hardy, B.W. An Investigation of the Oral Pathologies Occurring in Bulimia Nervosa. Int. J. Eat. Disord. 1990, 9, 191–199. [Google Scholar] [CrossRef]
- Cavalcanti, A.L.; Andrade, N.M.; Brandt, L.M.T.; Fernandes, L.H.F.; Toscano, R.L.; Auad, S.M.; Buldur, B.; Cavalcanti, A.F.C. Risk Behaviors for Eating Disorders among Brazilian Female Adolescents. Open Dent. J. 2020, 14, 7–12. [Google Scholar] [CrossRef]
- Emodi-Perlman, A.; Yoffe, T.; Rosenberg, N.; Eli, I.; Alter, Z.; Winocur, E. Prevalence of Psychologic, Dental, and Temporomandibular Signs and Symptoms among Chronic Eating Disorders Patients: A Comparative Control Study. J. Orofac. Pain 2008, 22, 201–208. [Google Scholar] [PubMed]
- Garrido-Martínez, P.; Domínguez-Gordillo, A.; Cerero-Lapiedra, R.; Burgueño-García, M.; Martínez-Ramírez, M.-J.; Gómez-Candela, C.; Cebrián-Carretero, J.-L.; Esparza-Gómez, G. Oral and Dental Health Status in Patients with Eating Disorders in Madrid, Spain. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e595–e602. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, N.; Camman, P.; Pourreyron, L.; Inquimbert, C.; Lefebvre, P. The Contribution of Teledentistry in Detecting Tooth Erosion in Patients with Eating Disorders. Digit. Health 2021, 7, 20552076211019250. [Google Scholar] [CrossRef] [PubMed]
- Hellström, I. Oral Complications in Anorexia Nervosa. Scand. J. Dent. Res. 1977, 85, 71–86. [Google Scholar] [CrossRef]
- Hermont, A.P.; Pordeus, I.A.; Paiva, S.M.; Abreu, M.H.N.G.; Auad, S.M. Eating Disorder Risk Behavior and Dental Implications among Adolescents. Int. J. Eat. Disord. 2013, 46, 677–683. [Google Scholar] [CrossRef]
- Hurst, P.S.; Lacey, L.H.; Crisp, A.H. Teeth, Vomiting and Diet: A Study of the Dental Characteristics of Seventeen Anorexia Nervosa Patients. Postgrad. Med. J. 1977, 53, 298–305. [Google Scholar] [CrossRef][Green Version]
- Johansson, A.-K.; Norring, C.; Unell, L.; Johansson, A. Eating Disorders and Oral Health: A Matched Case-Control Study. Eur. J. Oral Sci. 2012, 120, 61–68. [Google Scholar] [CrossRef]
- Jones, R.R.; Cleaton-Jones, P. Depth and Area of Dental Erosions, and Dental Caries, in Bulimic Women. J. Dent. Res. 1989, 68, 1275–1278. [Google Scholar] [CrossRef]
- Jugale, P.V.; Pramila, M.; Murthy, A.K.; Rangath, S. Oral Manifestations of Suspected Eating Disorders among Women of 20–25 Years in Bangalore City, India. J. Health Popul. Nutr. 2014, 32, 46–50. [Google Scholar]
- Lifante-Oliva, C.; López-Jornet, P.; Camacho-Alonso, F.; Esteve-Salinas, J. Study of Oral Changes in Patients with Eating Disorders. Int. J. Dent. Hyg. 2008, 6, 119–122. [Google Scholar] [CrossRef]
- Manevski, J.; Stojšin, I.; Vukoje, K.; Janković, O. Dental Aspects of Purging Bulimia. Vojnosanit. Pregl. 2020, 77, 300–307. [Google Scholar] [CrossRef]
- Milosevic, A.; Brodie, D.A.; Slade, P.D. Dental Erosion, Oral Hygiene, and Nutrition in Eating Disorders. Int. J. Eat. Disord. 1997, 21, 195–199. [Google Scholar] [CrossRef]
- Montecchi, P.P.; Custureri, V.; Polimeni, A.; Cordaro, M.; Costa, L.; Marinucci, S.; Montecchi, F. Oral Manifestations in a Group of Young Patients with Anorexia Nervosa. Eat. Weight Disord. EWD 2003, 8, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ohrn, R.; Enzell, K.; Angmar-Månsson, B. Oral Status of 81 Subjects with Eating Disorders. Eur. J. Oral Sci. 1999, 107, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Otsu, M.; Hamura, A.; Ishikawa, Y.; Karibe, H.; Ichijyo, T.; Yoshinaga, Y. Factors Affecting the Dental Erosion Severity of Patients with Eating Disorders. Biopsychosoc. Med. 2014, 8, 25. [Google Scholar] [CrossRef]
- Pallier, A.; Karimova, A.; Boillot, A.; Colon, P.; Ringuenet, D.; Bouchard, P.; Rangé, H. Dental and Periodontal Health in Adults with Eating Disorders: A Case-Control Study. J. Dent. 2019, 84, 55–59. [Google Scholar] [CrossRef]
- Panico, R.; Piemonte, E.; Lazos, J.; Gilligan, G.; Zampini, A.; Lanfranchi, H. Oral Mucosal Lesions in Anorexia Nervosa, Bulimia Nervosa and EDNOS. J. Psychiatr. Res. 2018, 96, 178–182. [Google Scholar] [CrossRef]
- Paszynska, E.; Hernik, A.; Slopien, A.; Roszak, M.; Jowik, K.; Dmitrzak-Weglarz, M.; Tyszkiewicz-Nwafor, M. Risk of Dental Caries and Erosive Tooth Wear in 117 Children and Adolescents’ Anorexia Nervosa Population-A Case-Control Study. Front. Psychiatry 2022, 13, 874263. [Google Scholar] [CrossRef]
- Paszyńska, E.; Słopień, A.; Węglarz, M.; Linden, R.W.A. Parotid Salivary Parameters in Bulimic Patients—A Controlled Clinical Trial. Psychiatr. Pol. 2015, 49, 709–720. [Google Scholar] [CrossRef]
- Roberts, M.W.; Li, S.H. Oral Findings in Anorexia Nervosa and Bulimia Nervosa: A Study of 47 Cases. J. Am. Dent. Assoc. 1939 1987, 115, 407–410. [Google Scholar] [CrossRef]
- Rungta, N.; Kudpi, R. Evaluation of Eating Disorders Using “scoff Questionnaire” among Young Female Cohorts and Its Dental Implications-an Exploratory Study. J. Orofac. Sci. 2019, 11, 27–31. [Google Scholar] [CrossRef]
- Rytömaa, I.; Järvinen, V.; Kanerva, R.; Heinonen, O.P. Bulimia and Tooth Erosion. Acta Odontol. Scand. 1998, 56, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, B.F.; Feldman, H.A.; Cleveland, R.; Sonis, A.; Brown, J.N.; Gordon, C.M. Oral Health and Bone Density in Adolescents and Young Women with Anorexia Nervosa. J. Clin. Pediatr. Dent. 2008, 33, 87–92. [Google Scholar] [CrossRef][Green Version]
- Simmons, M.S.; Grayden, S.K.; Mitchell, J.E. The Need for Psychiatric-Dental Liaison in the Treatment of Bulimia. Am. J. Psychiatry 1986, 143, 783–784. [Google Scholar] [CrossRef] [PubMed]
- Strużycka, I.; Lussi, A.; Bogusławska-Kapała, A.; Rusyan, E. Prevalence of Erosive Lesions with Respect to Risk Factors in a Young Adult Population in Poland-a Cross-Sectional Study. Clin. Oral Investig. 2017, 21, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Szupiany-Janeczek, T.; Rutkowski, K.; Pytko-Polończyk, J. Oral Cavity Clinical Evaluation in Psychiatric Patients with Eating Disorders: A Case-Control Study. Int. J. Environ. Res. Public. Health 2023, 20, 4792. [Google Scholar] [CrossRef] [PubMed]
- Touyz, S.W.; Liew, V.P.; Tseng, P.; Frisken, K.; Williams, H.; Beumont, P.J. Oral and Dental Complications in Dieting Disorders. Int. J. Eat. Disord. 1993, 14, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.-M.; Tveit, A.B.; Stenhagen, K.R.; Mulic, A. Self-Induced Vomiting and Dental Erosion--a Clinical Study. BMC Oral Health 2014, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, R.; Couto, G.; Sougey, E. Eating Disorders in Adolescents and Their Repercussions in Oral Health. Int. J. Eat. Disord. 2010, 43, 59–64. [Google Scholar] [CrossRef]
- Mehler, P.S.; Rylander, M. Bulimia Nervosa—Medical Complications. J. Eat. Disord. 2015, 3, 12. [Google Scholar] [CrossRef]




| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Patients aged from 0 to 99 years, both genders | |
| Intervention/Exposure | eating disorders (e.g., bulimia nervosa, anorexia nervosa) | other diseases (e.g., gastrointestinal reflux) |
| Comparison | not applicable | |
| Outcomes | prevalence of dental erosion | only severity of dental erosion or only other dental indices |
| Study design | case-control, cohort and cross-sectional studies | literature reviews, case reports, expert opinion, letters to the editor, conference reports |
| published until 27 August 2023 | not published in English |
| Author | Setting | Study Group (F/M; Age) | Control Group (F/M; Age) | ED Diagnoses | Clinical Criteria for Dental Erosion |
|---|---|---|---|---|---|
| Altshuler et al., 1990 [32] | USA | 40 (40/0), 23.8 ± 5.5 | 40 (40/0), 24.9 ± 6.1 | BN | loss of enamel with exposure of dentine and/or alteration of morphology |
| Cavalcanti et al., 2020 [33] | Brazil | 100 (100/0), mean 16.1 | 100 (100/0), mean 16.1 | BN | based on the O’Sullivan index |
| Emodi-Perlman et al., 2008 [34] | Israel | 79 (79/0), 23.5 ± 3.5 | 48 (48/0), 24.6 ± 3.0 | chronic EDs: BN (n = 29), AN (n = 24), EDNOS (n = 16), mixed-diagnosis (n = 10) | 4-graded scoring system (0-no, 1-enamel, 2-dentine, 3-pulp) |
| Garrido-Martínez et al., 2019 [35] | Spain | 59 (59/0), range 19–44 | 120 (120/0), range 19–44 | EDs: ARFID (n = 22), AN (n = 16), BN (n = 6), EDNOS (n = 15) | the degree measured according to Johansson et al. (1996) |
| Giraudeau et al., 2021 [36] | France | 50 (48/2), mean 26.8 | n/a | EDs: BN (n = 26), AN (n = 24) | BEWE scoring system using asynchronous telemedicine |
| Hellström, 1977 [37] | Sweden | 39 (38/1), range 14–42 | n/a | AN | diagnostic criteria given by Pindborg (1970) and Eccles and Jenkins (1974) |
| Hermont et al., 2013 [38] | Brazil | 20 (20/0), range 15–18 | 80 (80/0), range 15–18 | BN | based on the O’Sullivan index |
| Hurst et al., 1977 [39] | UK | 17 (14/3), range 13–33 | n/a | AN | different patterns: palatal, labial or generalised |
| Johansson et al., 2012 [40] | Sweden | 54 (50/4), mean 21.5 | 54 (50/4), mean 21.5 | EDs: AN (n = 14), BN (n = 8), EDNOS (n = 32) | the degree measured according to Johansson et al. (1996) |
| Jones and Cleaton-Jones, 1989 [41] | RSA | 11 (11/0), 29.8 ± 8.4 | 22 (22/0), 28.9 ± 9.0 | BN | 4-graded scoring system (0-no, 1-enamel, 2-dentine, 3-pulp) |
| Jugale et al., 2014 [42] | India | 50 (50/0), range 20–25 | 67 (67/0), range 20–25 | EDs | perimolysis |
| Lifante-Oliva et al., 2008 [43] | Spain | 17 (17/0), 20.1 ± 5.6 | n/a | BN (n = 10), AN (n = 7) | 4-graded scoring system (0-no, 1-enamel, 2-dentine, 3-pulp) |
| Manevski et al., 2020 [44] | Serbia | 30 (28/2), 24.6 ± 4.4 | 30 (28/2), 24.7 ± 5.8 | BN | BEWE scoring system |
| Milosevic et al., 1997 [45] | UK | 33 (NR), mean 27.1 | n/a | EDs: BN (n = 28), AN (n = 5) | TWI |
| Montecchi et al., 2003 [46] | Italy | 80 (76/4), mean 15 | n/a | AN | NR |
| Ohrn et al., 1999 [47] | Sweden | 81 (79/2), median 25 | 52 (48/4), median 24 | EDs: BN (n = 46), AN (n = 3), EDNOS (n = 25), mixed-diagnosis (n = 7) | classified according to a modification by Lussi et al. (1991) of the system proposed by Eccles (1979) |
| Otsu et al., 2014 [48] | Japan | 71 (71/0), mean 31.1 | n/a | EDs: AN (n = 35), BN (n = 27), EDNOS (n = 3), unclear diagnosis (n = 6) | diagnostic criteria given by Japanese Society for Oral Health (1985) |
| Pallier et al., 2019 [49] | France | 70 (70/0), 32.1 ± 9.1 | 70 (70/0), 30.2 ± 4.7 | AN (n = 36), BN (n = 34) | BEWE scoring system |
| Panico et al., 2018 [50] | Argentina | 65 (65/0), mean 21.6 | 65 (65/0), mean 23.2 | EDs: BN (n = 46), AN (n = 6), EDNOS (n = 13) | NR |
| Paszynska et al., 2022 [51] | Poland | 117 (117/0), 14.9 ± 1.8 | 103 (103/0), 15.0 ± 1.8 | AN | BEWE scoring system |
| Paszynska et al., 2015 [52] | Poland | 25 (25/0), 21.2 ± 3.2 | 44 (44/0), 25.5 ± 4.6 | BN | TWI |
| Roberts and Li, 1987 [53] | USA | 47 (47/0), mean 25 | n/a | AN (n = 17), BN (n = 30) | erosion of maxillary palatal surfaces |
| Rungta and Kudpi, 2019 [54] | India | 21 (21/0), range 15–17 | 179 (179/0), range 15–17 | EDs | NR |
| Rytömaa et al., 1998 [55] | Finland | 35 (35/0), 25.3 ± 6.8 | 105 (105/0), 25.7 ± 7.0 | BN | diagnostic criteria given by Eccles and Jenkins (1974) |
| Shaughnessy et al., 2008 [56] | USA | 23 (23/0), 18.5 ± 2.9 | n/a | AN | clinically detectable change in enamel smooth surface without evidence of dental caries |
| Simmons et al., 1986 [57] | USA | 66 (66/0), median 26 | n/a | BN | clinically observable features |
| Strużycka et al., 2017 [58] | Poland | 29 (NR), 18 | n/a | EDs | BEWE scoring system |
| Szupiany-Janeczek et al., 2023 [59] | Poland | 59 (45/14), mean 30.6 | 60 (45/15), mean 30.7 | EDs | NR |
| Touyz et al., 1993 [60] | Australia | 30 (30/0), mean 19.6 | 15 (15/0), mean 22.1 | AN (n = 15), BN (n = 15) | NR |
| Uhlen et al., 2014 [61] | Norway | 66 (63/3), mean 27.7 | n/a | EDs | VEDE system |
| Ximenes et al., 2010 [62] | Brazil | 215 (NR) according to EAT-26, 248 (NR) according to BITE; range 12–16 | n/a | EDs | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijakowski, K.; Jankowski, J.; Gruszczyński, D.; Surdacka, A. Eating Disorders and Dental Erosion: A Systematic Review. J. Clin. Med. 2023, 12, 6161. https://doi.org/10.3390/jcm12196161
Nijakowski K, Jankowski J, Gruszczyński D, Surdacka A. Eating Disorders and Dental Erosion: A Systematic Review. Journal of Clinical Medicine. 2023; 12(19):6161. https://doi.org/10.3390/jcm12196161
Chicago/Turabian StyleNijakowski, Kacper, Jakub Jankowski, Dawid Gruszczyński, and Anna Surdacka. 2023. "Eating Disorders and Dental Erosion: A Systematic Review" Journal of Clinical Medicine 12, no. 19: 6161. https://doi.org/10.3390/jcm12196161
APA StyleNijakowski, K., Jankowski, J., Gruszczyński, D., & Surdacka, A. (2023). Eating Disorders and Dental Erosion: A Systematic Review. Journal of Clinical Medicine, 12(19), 6161. https://doi.org/10.3390/jcm12196161










