Deiodinase Types 1 and 3 and Proinflammatory Cytokine Values May Discriminate Depressive Disorder Patients from Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Collection and Estimation of Gene Expression and Serum Concentration
2.3. Detection of Gene Expression Using Real-Time PCR
2.4. Measurement of the Levels of the Iodothyronine Deiodinases and Cytokine Proteins with an Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis
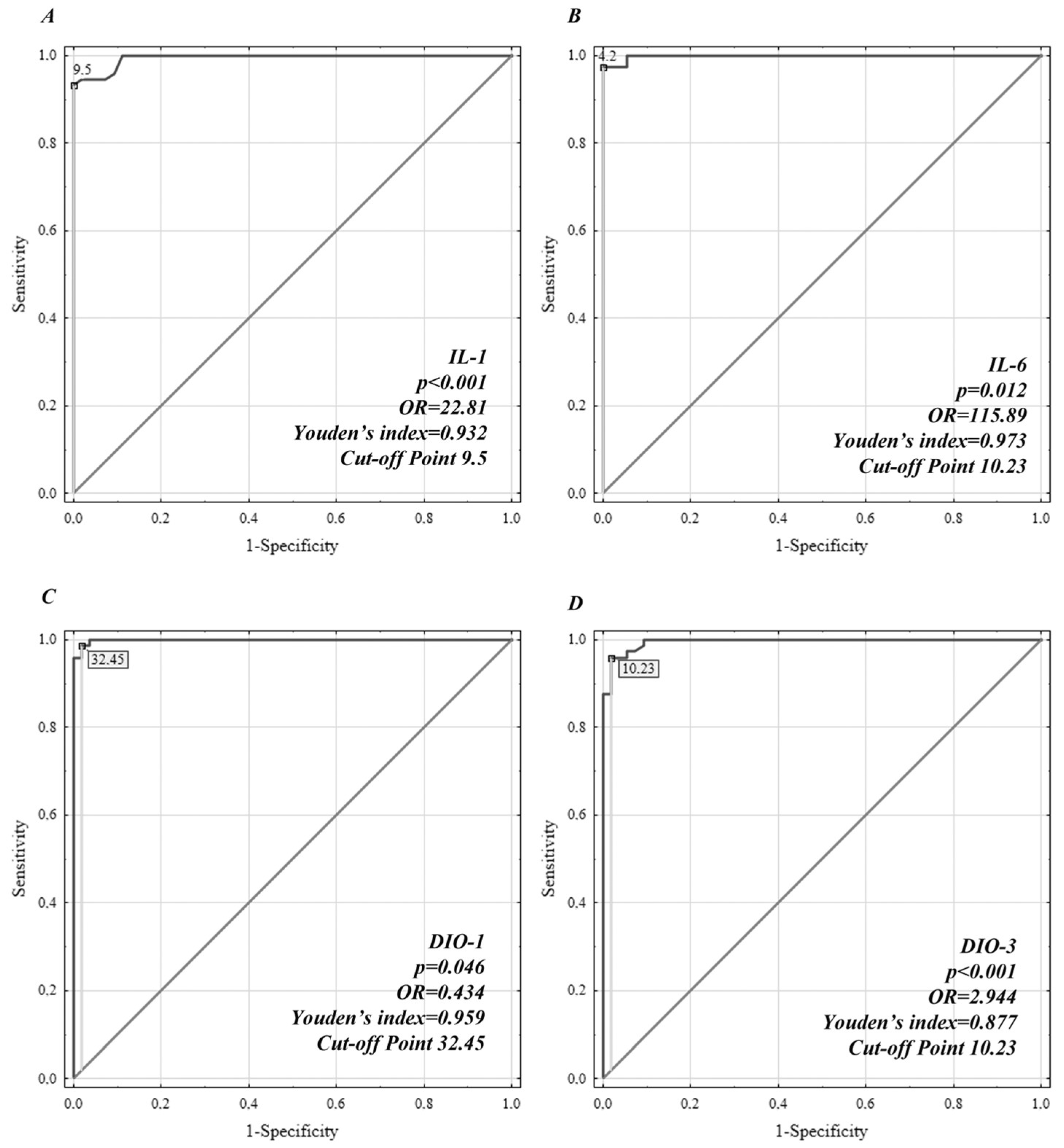
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Nedic Erjavec, G.; Sagud, M.; Nikolac Perkovic, M.; Svob Strac, D.; Konjevod, M.; Tudor, L.; Uzun, S.; Pivac, N. Depression: Biological markers and treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110139. [Google Scholar] [CrossRef]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2022, 24, 578. [Google Scholar] [CrossRef]
- Mohammad, M.Y.H.; Bushulaybi, N.A.; AlHumam, A.S.; AlGhamdi, A.Y.; Aldakhil, H.A.; Alumair, N.A.; Shafey, M.M. Prevalence of depression among hypothyroid patients attending the primary healthcare and endocrine clinics of King Fahad Hospital of the University (KFHU). J. Family Med. Prim. Care. 2019, 8, 2708–2713. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, J.; Liu, S.; Zhang, S.; Chen, P.; Jiang, T. The Role of Cytokines in the Peripheral Blood of Major Depressive Patients. Clin. Lab. 2017, 63, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Anzolin, A.P.; Feiten, J.G.; Bristot, G.; Possebon, G.M.P.; Fleck, M.P.A.; Caldieraro, M.A.; Kauer-Sant’Anna, M. Earlier age of onset is associated with a pro-inflammatory state in major depressive disorder. Psychiatry Res. 2022, 314, 114601. [Google Scholar] [CrossRef]
- Dong, Z.; Kuang, W.; Shen, X.; Tian, L. Plasma levels of interleukin-6 and antidepressant response to Paroxetine in Chinese depressive patients. Psychiatry Res. 2021, 297, 113723. [Google Scholar] [CrossRef] [PubMed]
- Nowak, W.; Grendas, L.N.; Sanmarco, L.M.; Estecho, I.G.; Arena, Á.R.; Eberhardt, N.; Rodante, D.E.; Aoki, M.P.; Daray, F.M.; Carrera Silva, E.A.; et al. Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR. EBioMedicine 2019, 50, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Scharpé, S.; Meltzer, H.Y.; Okayli, G.; Bosmans, E.; D’Hondt, P.; Vanden Bossche, B.V.; Cosyns, P. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: Further evidence for an immune response. Psychiatry Res. 1994, 54, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Gräf, K.J.; Kürten, I.; Meinhold, H. The hypothalamic-pituitary-thyroid axis in psychiatric patients and healthy subjects: Parts 1–4. Psychiatry Res. 1988, 24, 271–332. [Google Scholar] [CrossRef]
- Linnoila, M.; Lamberg, B.A.; Potter, W.Z.; Gold, P.W.; Goodwin, F.K. High reverse T3 levels in manic an unipolar depressed women. Psychiatry Res. 1982, 3, 271–276. [Google Scholar] [CrossRef]
- Musselman, D.L.; Nemeroff, C.B. Depression and endocrine disorders: Focus on the thyroid and adrenal system. Br. J. Psychiatry Suppl. 1996, 168, 123–128. [Google Scholar] [CrossRef]
- Cooper-Kazaz, R.; Lerer, B. Efficacy and safety of triiodothyronine supplementation in patients with major depressive disorder treated with specific serotonin reuptake inhibitors. Int. J. Neuropsychopharmacol. 2008, 11, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.; Broedel, O.; Eravci, M.; Stoltenburg-Didinger, G.; Plueckhan, H.; Fuxius, S.; Meinhold, H.; Baumgartner, A. Thyroid hormones in the rat amygdala as common targets for antidepressant drugs, mood stabilizers, and sleep deprivation. Biol. Psychiatry 2003, 54, 1049–1059. [Google Scholar] [CrossRef]
- Fliers, E.; Boelen, A. An update on non-thyroidal illness syndrome. J. Endocrinol. Invest. 2021, 44, 1597–1607. [Google Scholar] [CrossRef]
- Premachandra, B.N.; Kabir, M.A.; Williams, I.K. Low T3 syndrome in psychiatric depression. J. Endocrinol. Invest. 2006, 29, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.; Hollenberg, A.N. New insights into thyroid hormone action. Pharmacol. Ther. 2017, 173, 135–145. [Google Scholar] [CrossRef]
- Bauer, M.; Goetz, T.; Glenn, T.; Whybrow, P.C. The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol. 2008, 20, 1101–1114. [Google Scholar] [CrossRef]
- Wenzek, C.; Boelen, A.; Westendorf, A.M.; Engel, D.R.; Moeller, L.C.; Führer, D. The interplay of thyroid hormones and the immune system—Where we stand and why we need to know about it. Eur. J. Endocrinol. 2022, 186, R65–R77. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [PubMed]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Braun, D.; Pflug, S.; Brol, A.; Sylvester, M.; Steegborn, C.; Schweizer, U. Insights into the Mechanism of Human Deiodinase 1. Int. J. Mol. Sci. 2022, 23, 5361. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Vassalle, C.; Del Seppia, C.; Iervasi, G. Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions. Endocrinol. Metab. 2022, 36, 952–964. [Google Scholar] [CrossRef] [PubMed]
- van der Spek, A.H.; Fliers, E.; Boelen, A. Thyroid hormone metabolism in innate immune cells. J. Endocrinol. 2017, 232, R67–R81. [Google Scholar] [CrossRef]
- Boelen, A.; Maas, M.A.; Lowik, C.W.; Platvoet, M.C.; Wiersinga, W.M. Induced illness in interleukin-6 (IL-6) knock-out mice: A causal role of IL-6 in the development of the low 3,5,3′-triiodothyronine syndrome. Endocrinology 1996, 137, 5250–5254. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, J.; Wiersinga, W.M.; Boelen, A. Differential involvement of nuclear factor-kappaB and activator protein-1 pathways in the interleukin-1beta-mediated decrease of deiodinase type 1 and thyroid hormone receptor beta1 mRNA. J. Endocrinol. 2006, 189, 37–44. [Google Scholar] [CrossRef]
- Kwakkel, J.; Surovtseva, O.V.; de Vries, E.M.; Stap, J.; Fliers, E.; Boelen, A. A novel role for the thyroid hormone-activating enzyme type 2 deiodinase in the inflammatory response of macrophages. Endocrinology 2014, 155, 2725–2734. [Google Scholar] [CrossRef]
- Boelen, A.; Boorsma, J.; Kwakkel, J.; Wieland, C.W.; Renckens, R.; Visser, T.J.; Fliers, E.; Wiersinga, W.M. Type 3 deiodinase is highly expressed in infiltrating neutrophilic granulocytes in response to acute bacterial infection. Thyroid 2008, 18, 1095–1103. [Google Scholar] [CrossRef]
- Boelen, A.; Kwakkel, J.; Wieland, C.W.; St Germain, D.L.; Fliers, E.; Hernandez, A. Impaired bacterial clearance in type 3 deiodinase-deficient mice infected with Streptococcus pneumoniae. Endocrinology 2009, 150, 1984–1990. [Google Scholar] [CrossRef]
- Chaalal, A.; Poirier, R.; Blum, D.; Laroche, S.; Enderlin, V. Thyroid Hormone Supplementation Restores Spatial Memory, Hippocampal Markers of Neuroinflammation, Plasticity-Related Signaling Molecules, and β-Amyloid Peptide Load in Hypothyroid Rats. Mol. Neurobiol. 2019, 56, 722–735. [Google Scholar] [CrossRef]
- Talhada, D.; Feiteiro, J.; Costa, A.R.; Talhada, T.; Cairrão, E.; Wieloch, T.; Englund, E.; Santos, C.R.; Gonçalves, I.; Ruscher, K. Triiodothyronine modulates neuronal plasticity mechanisms to enhance functional outcome after stroke. Acta Neuropathol. Commun. 2019, 7, 216. [Google Scholar] [CrossRef]
- Seyedhosseini Tamijani, S.M.; Beirami, E.; Ahmadiani, A.; Dargahi, L. Thyroid hormone treatment alleviates the impairments of neurogenesis, mitochondrial biogenesis and memory performance induced by methamphetamine. Neurotoxicology 2019, 74, 7–18. [Google Scholar] [CrossRef]
- Wittenberg, G.M.; Greene, J.; Vértes, P.E.; Drevets, W.C.; Bullmore, E.T. Major Depressive Disorder Is Associated with Differential Expression of Innate Immune and Neutrophil-Related Gene Networks in Peripheral Blood: A Quantitative Review of Whole-Genome Transcriptional Data From Case-Control Studies. Biol. Psychiatry 2020, 88, 625–637. [Google Scholar] [CrossRef]
- Das, R.; Emon, M.P.Z.; Shahriar, M.; Nahar, Z.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, S.N.; Islam, M.R. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. 2021, 295, 113568. [Google Scholar] [CrossRef]
- Mehta, D.; Menke, A.; Binder, E. Gene expression studies in Major Depression. Curr. Psychiatry Rep. 2010, 12, 135–144. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th ed.; World Health Organization: Genewa, Switzerland, 2015. [Google Scholar]
- Patten, S.B. Performance of the Composite International Diagnostic Interview Short Form for major depression in community and clinical samples. Chronic Dis. Can. 1997, 18, 109–112. [Google Scholar] [PubMed]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Gałecka, E.; Talarowska, M.; Maes, M.; Su, K.P.; Górski, P.; Kumor-Kisielewska, A.; Szemraj, J. Expression levels of interferon-ɣ and type 2 deiodinase in patients diagnosed with recurrent depressive disorders. Pharmacol. Rep. 2018, 70, 133–138. [Google Scholar] [CrossRef]
- Gałecka, E.; Kumor-Kisielewska, A.; Orzechowska, A.; Maes, M.; Górski, P.; Szemraj, J. Assessment of type 1 and type 3 deiodinase expression levels in depressive disorders. Acta. Neurobiol. Exp. 2017, 77, 225–235. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Ruiz, N.A.L.; Del Ángel, D.S.; Brizuel, N.O.; Peraza, A.V.; Olguín, H.J.; Soto, M.P. Guzmán DC. Inflammatory Process and Immune System in Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2022, 25, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rizavi, H.S.; Ren, X.; Zhang, H.; Bhaumik, R.; Pandey, G.N. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res. 2016, 240, 314–320. [Google Scholar] [CrossRef]
- Anisman, H.; Merali, Z. Cytokines, stress and depressive illness: Brain-immune interactions. Ann. Med. 2003, 35, 2–11. [Google Scholar] [CrossRef]
- Ogłodek, E. Changes in the Serum Levels of Cytokines: IL-1β, IL-4, IL-8 and IL-10 in Depression with and without Posttraumatic Stress Disorder. Brain Sci. 2022, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ren, X.; Wang, X.; Tian, F. Associations between Autoimmunity and Depression: Serum IL-6 and IL-17 Have Directly Impact on the HAMD Scores in Patients with First-Episode Depressive Disorder. J. Immunol. Res. 2022, 2022, 6724881. [Google Scholar] [CrossRef] [PubMed]
- Daria, S.; Proma, M.A.; Shahriar, M.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Serum interferon-gamma level is associated with drug-naïve major depressive disorder. SAGE Open Med. 2020, 8, 2050312120974169. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Yuan, Y. The Combination of Serum BDNF, Cortisol and IFN-Gamma Can Assist the Diagnosis of Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2021, 17, 2819–2829. [Google Scholar] [CrossRef]
- Debnath, M.; Berk, M.; Maes, M. Translational evidence for the Inflammatory Response System (IRS)/Compensatory Immune Response System (CIRS) and neuroprogression theory of major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110343. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Carvalho, A.F. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol. Neurobiol. 2018, 55, 8885–8903. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Basile, M.S.; Lenzo, V.; Quattropani, M.C.; Bendtzen, K.; Nicoletti, F. Pathogenic contribution of the Macrophage migration inhibitory factor family to major depressive disorder and emerging tailored therapeutic approaches. J. Affect. Disord. 2020, 263, 15–24. [Google Scholar] [CrossRef]
- Hernandez, A.; Stohn, J.P. The Type 3 Deiodinase: Epigenetic Control of Brain Thyroid Hormone Action and Neurological Function. Int. J. Mol Sci. 2018, 19, 1804. [Google Scholar] [CrossRef] [PubMed]
- Ittermann, T.; Jürgens, C. Thyroid function: A new road to understanding age-related macular degeneration? BMC Med. 2015, 13, 95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, N.S.; Kuo, S.I.; Aliev, F.; McCutcheon, V.V.; Meyers, J.M.; Chan, G.; Hesselbrock, V.; Kamarajan, C.; Kinreich, S.; Kramer, J.R.; et al. Alcohol use disorder, psychiatric comorbidities, marriage and divorce in a high-risk sample. Psychol. Addict. Behav. 2022, 36, 364–374. [Google Scholar] [CrossRef]
- Baumgartner, A.; Heyne, A.; Campos-Barros, A.; Köhler, R.; Müller, F.; Meinhold, H.; Rommelspacher, H.; Wolffgramm, J. Hypothalamic-pituitary-thyroid axis in chronic alcoholism. II. Deiodinase activities and thyroid hormone concentrations in brain and peripheral tissues of rats chronically exposed to ethanol. Alcohol Clin. Exp. Res. 1994, 18, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Markova, N.; Chernopiatko, A.; Schroeter, C.A.; Malin, D.; Kubatiev, A.; Bachurin, S.; Costa-Nunes, J.; Steinbusch, H.M.; Strekalova, T. Hippocampal gene expression of deiodinases 2 and 3 and effects of 3,5-diiodo-L-thyronine T2 in mouse depression paradigms. Biomed. Res. Int. 2013, 2013, 565218. [Google Scholar] [CrossRef] [PubMed]
- Balzano, S.; Bergmann, B.M.; Gilliland, M.A.; Silva, J.E.; Rechtschaffen, A.; Refetoff, S. Effect of total sleep deprivation on 5′-deiodinase activity of rat brown adipose tissue. Endocrinology 1990, 127, 882–890. [Google Scholar] [CrossRef]
- Wu, Z.; Su, G.; Lu, W.; Liu, L.; Zhou, Z.; Xie, B. Clinical symptoms and their relationship with cognitive impairment in elderly patients with depressive disorder. Front. Psychiatry 2022, 13, 1009653. [Google Scholar] [CrossRef]
- Gevezova, M.; Sarafian, V.; Anderson, G.; Maes, M. Inflammation and Mitochondrial Dysfunction in Autism Spectrum Disorder. CNS Neurol. Disord. Drug Targets 2020, 19, 320–333. [Google Scholar] [CrossRef]
- Khan, A.; Harney, J.W.; Zavacki, A.M.; Sajdel-Sulkowska, E.M. Disrupted brain thyroid hormone homeostasis and altered thyroid hormone-dependent brain gene expression in autism spectrum disorders. J. Physiol. Pharmacol. 2014, 65, 257–272. [Google Scholar]
- Bárez-López, S.; Montero-Pedrazuela, A.; Bosch-García, D.; Venero, C.; Guadaño-Ferraz, A. Increased anxiety and fear memory in adult mice lacking type 2 deiodinase. Psychoneuroendocrinology 2017, 84, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Premachandra, B.N.; Radparvar, A.; Burman, K.; Williams, I.K. Apparent increase in type I 5′-deiodinase activity induced by antiepileptic medication in mentally retarded subjects. Horm. Res. 2002, 58, 273–278. [Google Scholar] [CrossRef]
- Köhrle, J.; Frädrich, C. Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic. Biol. Med. 2022, 193, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Bocco, B.M.; Louzad, R.; Silvestre, D.H.; Santos, M.C.; Anne-Palmer, E.; Rangel, I.F.; Abdalla, S.; Ferreira, A.C.; Ribeiro, M.O.; Gereben, B.; et al. Thyroid hormone activation by type 2 deiodinase mediates exercise-induced peroxisome proliferator-activated receptor-γ coactivator-1α expression in skeletal muscle. J. Physiol. 2016, 594, 5255–5269. [Google Scholar] [CrossRef]
- Cheng, A.W.M.; Bolognesi, M.; Kraus, V.B. DIO2 modifies inflammatory responses in chondrocytes. Osteoarthr. Cartil. 2012, 20, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Francija, E.; Lukic, I.; Petrovic, Z.; Brkic, Z.; Mitic, M.; Radulovic, J.; Adzic, M. GluN2A-ERK-mTOR pathway confers a vulnerability to LPS-induced depressive-like behaviour. Behav. Brain Res. 2022, 417, 113625. [Google Scholar] [CrossRef]
- Fischer, S.; Ehlert, U. Hypothalamic-pituitary-thyroid (HPT) axis functioning in anxiety disorders. A systematic review. Depress. Anxiety. 2018, 35, 98–110. [Google Scholar] [CrossRef]
- Boelen, A.; Mikita, J.; Boiziau, C.; Chassande, O.; Fliers, E.; Petry, K.G. Type 3 deiodinase expression in inflammatory spinal cord lesions in rat experimental autoimmune encephalomyelitis. Thyroid 2009, 19, 1401–1406. [Google Scholar] [CrossRef]
| rDD n = 73 | Controls n = 54 | t/χ2 | p | |
|---|---|---|---|---|
| Rows | 48.56 ± 5.23 | 48.50 ± 5.67 | 0.06 | 0.95 |
| Sex (female/male) | 34/39 | 25/29 | 0.001 | 0.98 |
| Disease duration | 5.30 ± 7.5 | |||
| Number of depressive episodes | 3.32 ± 3.56 | |||
| Number of hospitalizations | 2 ± 1.48 | |||
| Hamilton depression rating scale | 23.53 ± 6.39 |
| Variable | rDD n = 73 (mean ± SD) | Controls n = 54 (mean ± SD) | t | p | Size Effect | Power |
|---|---|---|---|---|---|---|
| IL1 pg/mL | 11.55 ± 1.44 | 6.81 ± 1.32 | 19.04 | <0.0001 | 3.44 | 1.00 |
| IL1B mRNA 2−ΔCt | 0.70 ± 0.09 | 0.43 ± 0.08 | 13.82 | <0.0001 | 3.20 | 1.00 |
| IL-6 pg/ml | 5.63 ± 0.89 | 2.11 ± 0.57 | 25.53 | <0.0001 | 4.73 | 1.00 |
| IL6 mRNA 2−ΔCt | 0.33 ± 0.06 | 0.12 ± 0.04 | 18.51 | <0.0001 | 4.49 | 1.00 |
| TNF-α pg/ml | 11.08 ± 1.25 | 4.86 ± 1.02 | 29.95 | <0.0001 | 5.46 | 1.00 |
| TNFA mRNA 2−ΔCt | 0.66 ± 0.07 | 0.29 ± 0.07 | 22.74 | <0.0001 | 5.31 | 1.00 |
| IFNG mRNA 2−ΔCt | 0.17 ± 0.08 | 0.18 ± 0.07 | −0.26 | 0.80 | ||
| IFN-γ pg/ml | 4.97 ± 0.89 | 4.79 ± 0.81 | 1.16 | 0.25 | ||
| DIO1 mRNA 2−ΔCt | 0.07 ± 0.02 | 0.08 ± 0.01 | −1.19 | 0.24 | ||
| DIO2 mRNA 2−ΔCt | 0.12 ± 0.2 | 0.32 ± 0.06 | −20.55 | <0.0001 | 4.21 | 1.00 |
| DIO3 mRNA 2−ΔCt | 0.06 ± 0.01 | 0.06 ± 0.03 | −1.63 | 0.11 | ||
| DIO1 U/L | 21.21 ± 5.25 | 55.96 ± 11.88 | −22.25 | <0.0001 | 3.78 | 1.00 |
| DIO2 U/L | 13.58 ± 5.77 | 37.32 ± 4.51 | −25.08 | <0.0001 | 4.58 | 1.00 |
| DIO3 U/L | 15.75 ± 2.87 | 4.44 ± 2.46 | 23.32 | <0.0001 | 4.23 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małujło-Balcerska, E.; Pietras, T. Deiodinase Types 1 and 3 and Proinflammatory Cytokine Values May Discriminate Depressive Disorder Patients from Healthy Controls. J. Clin. Med. 2023, 12, 6163. https://doi.org/10.3390/jcm12196163
Małujło-Balcerska E, Pietras T. Deiodinase Types 1 and 3 and Proinflammatory Cytokine Values May Discriminate Depressive Disorder Patients from Healthy Controls. Journal of Clinical Medicine. 2023; 12(19):6163. https://doi.org/10.3390/jcm12196163
Chicago/Turabian StyleMałujło-Balcerska, Elżbieta, and Tadeusz Pietras. 2023. "Deiodinase Types 1 and 3 and Proinflammatory Cytokine Values May Discriminate Depressive Disorder Patients from Healthy Controls" Journal of Clinical Medicine 12, no. 19: 6163. https://doi.org/10.3390/jcm12196163
APA StyleMałujło-Balcerska, E., & Pietras, T. (2023). Deiodinase Types 1 and 3 and Proinflammatory Cytokine Values May Discriminate Depressive Disorder Patients from Healthy Controls. Journal of Clinical Medicine, 12(19), 6163. https://doi.org/10.3390/jcm12196163






