Determination of the Effects of a Series of Ten Whole-Body Cryostimulation Sessions on Physiological Responses to Exercise and Skin Temperature Behavior following Exercise in Elite Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
2.3. Experimental Design
2.3.1. Preliminary Study
2.3.2. Experimental Exercise Test and Whole-Body Cryostimulation
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. The Basic Characteristics of the Study Participants were Presented in Table 1
3.2. The Effect of WBC on Physiological Responses to Exercise
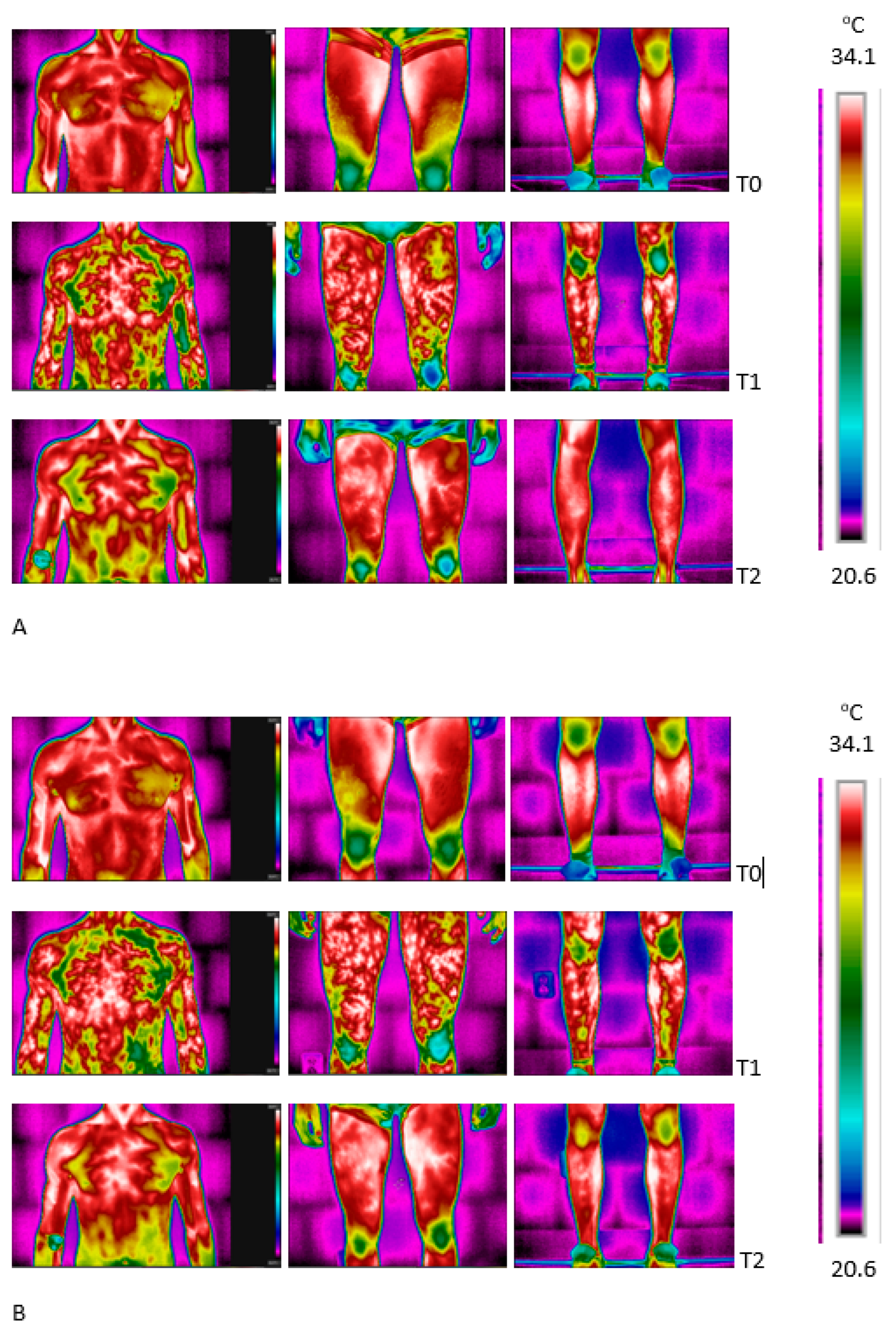
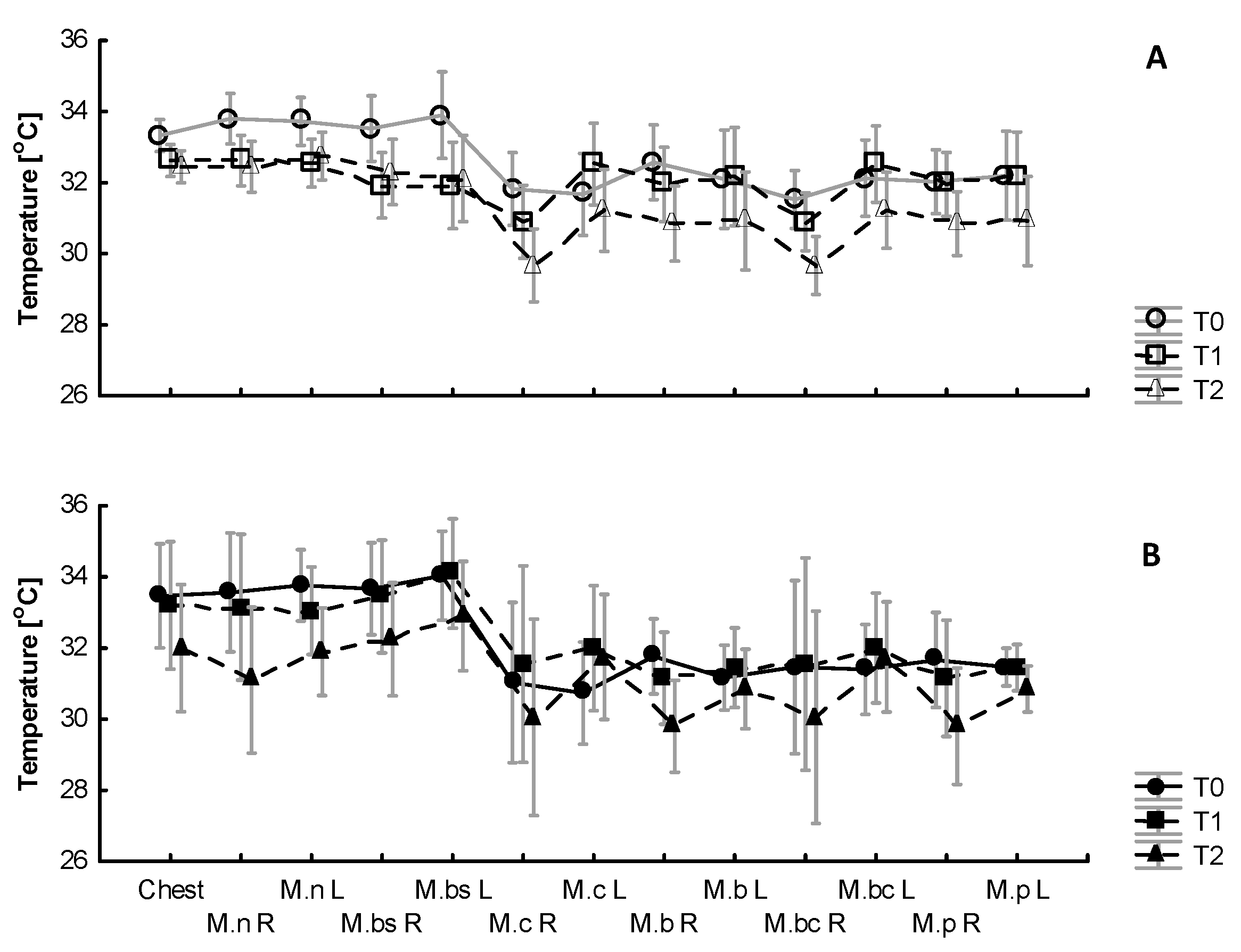
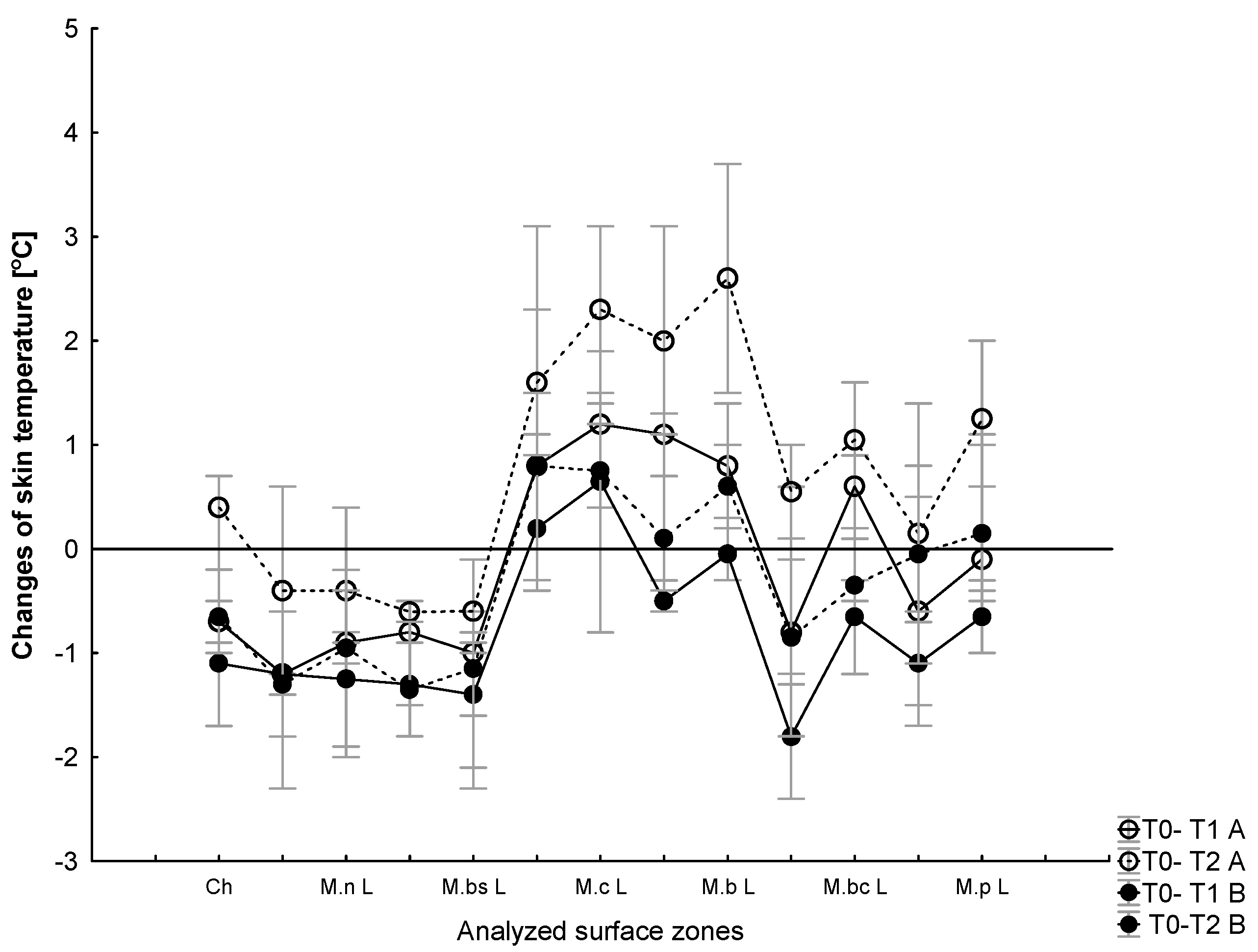
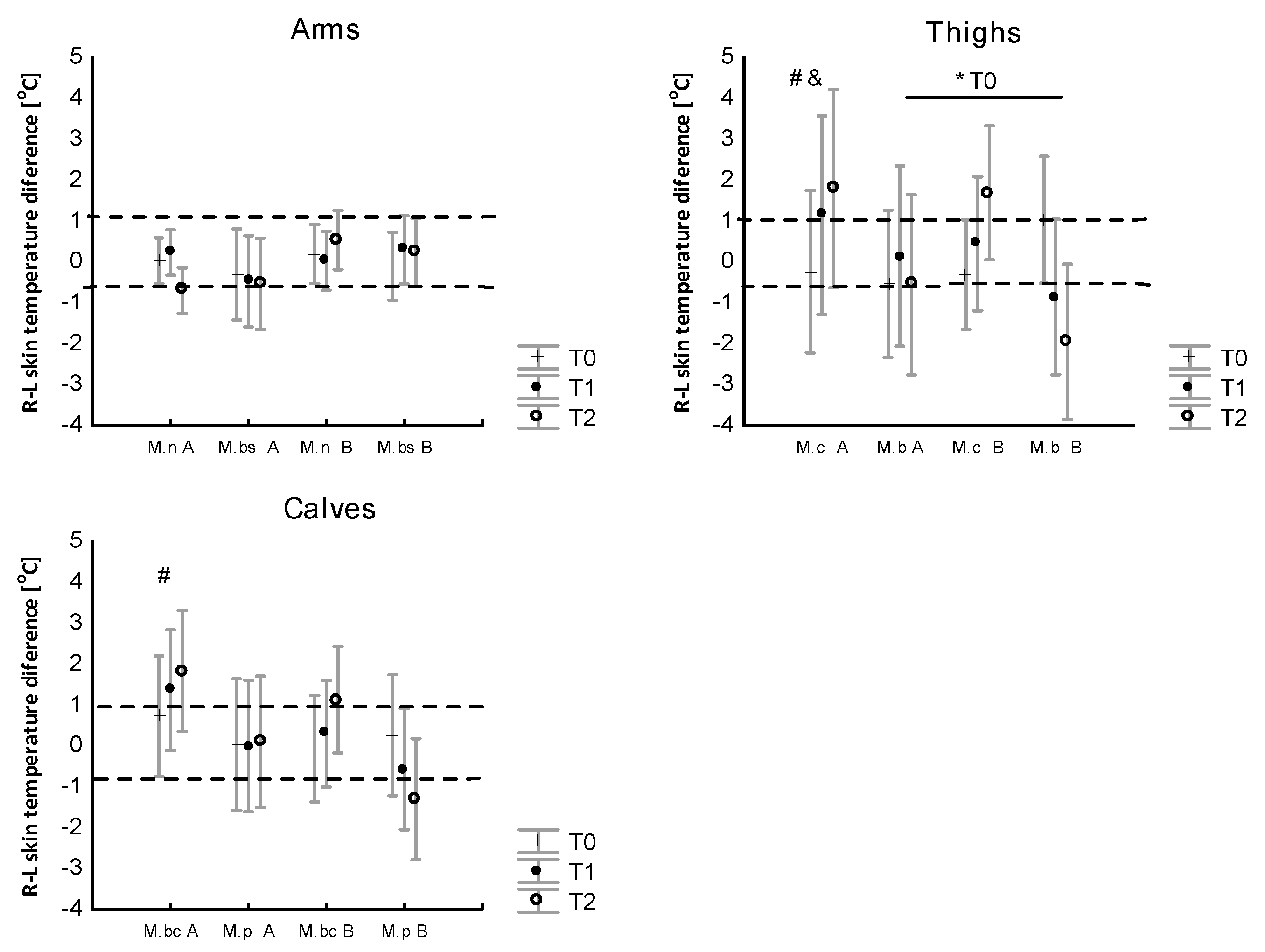
3.3. The Effect of WBC and Exercise on Skin Temperature Behavior following Exercise
4. Discussion
4.1. Changes in Body Composition, Metabolism and Cardiovascular Function Induced by a Series of Ten WBC Treatments
4.2. Changes in Metabolism, Cardiovascular Function and the Body’s Temperature in Response to Exercise after a Series of Ten WBC Treatments
4.3. Skin Surface Temperature Behavior (IRT) following Exercise Test under Thermoneutral Conditions after a Series of Ten WBCs
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hausswirth, C.; Louis, J.; Bieuzen, F. Effects of Whole-Body Cryotherapy vs. Far-Infrared vs. Passive Modalities on Recovery from Exercise-Induced Muscle Damage in Highly-Trained Runners. PLoS ONE 2011, 6, e27749. [Google Scholar] [CrossRef]
- Costello, J.T.; Culligan, K.; Selfe, J.; Donnelly, A.E. Muscle, skin and core temperature after -110°c cold air and 8°c water treatment. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.T.; Baker, P.R.; Minett, G.M.; Bieuzen, F.; Stewart, I.B.; Bleakley, C. Whole-body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. Cochrane Database Syst. Rev. 2015, 18, CD010789. [Google Scholar] [CrossRef]
- Castellani, J.W.; Young, A.J.; Degroot, D.W.; Stulz, D.A.; Cadarette, B.S.; Rhind, S.G.; Zamecnik, J.; Shek, P.N.; Sawka, M.N. Thermoregulation during cold exposure after several days of exhaustive exercise. J. Appl. Physiol. 2001, 90, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Bouzigon, R.; Dupuy, O.; Tiemessen, I.; De Nardi, M.; Bernard, J.P.; Mihailovic, T.; Theurot, D.; Miller, E.D.; Lombardi, G.; Dugué, B.M. Cryostimulation for Post-exercise Recovery in Athletes: A Consensus and Position Paper. Front. Sports Act. Living 2021, 3, 1–14. [Google Scholar] [CrossRef]
- Laza, V. Cryotherapy in athletes. Health Sports Rehabil. Med. 2019, 20, 85–91. [Google Scholar] [CrossRef]
- Fonda, B.; De Nardi, M.; Sarabon, N. Effects of whole-body cryotherapy duration on thermal and cardio-vascular response. J. Therm. Biol. 2014, 42, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-Body Cryotherapy in Athletes: From therapy to stimulation. An updated review of the literature. Front. Physiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A. Cryotherapy: Physiological Considerations and Applications to Physical Therapy. In Physical Therapy Perspectives in the 21st Century—Challenges and Possibilities; InTech: Houston TX, USA, 2012. [Google Scholar] [CrossRef][Green Version]
- Pournot, H.; Bieuzen, F.; Louis, J.; Filliard, J.R.; Barbiche, E.; Hausswirth, C. Time-Course of Changes in Inflammatory Response after Whole-Body Cryotherapy Multi Exposures following Severe Exercise. PLoS ONE 2011, 6, e22748. [Google Scholar] [CrossRef]
- Rose, C.; Edwards, K.M.; Siegler, J.; Graham, K.; Caillaud, C. Whole-body cryotherapy as a recovery technique after exercise: A review of the literature. Int. J. Sports Med. 2017, 38, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Olek, R.A.; Kujach, S.; Grzywacz, T.; Antosiewicz, J.; Garsztka, T.; Laskowski, R. 2012. Five-day whole-body cryostimulation, blood inflammatory markers, and performance in high-ranking professional tennis players. J. Athl. Train. 2012, 47, 664–672. [Google Scholar] [CrossRef]
- Ziemann, E.; Olek, R.A.; Grzywacz, T.; Kaczor, J.J.; Antosiewicz, J.; Skrobot, W.; Kujach, S.; Laskowski, R. Whole-body cryostimulation as an effective way of reducing exercise-induced inflammation and blood cholesterol in young men. Eur. Cytokine Netw. 2014, 25, 14–23. [Google Scholar] [CrossRef]
- Kröger, M.; De Mareés, M.; Dittmar, K.H.; Sperlich, B.; Mester, J. Whole-body Cryotherapy’s enhancement of acute recovery of running performance in well-trained athletes. Int. J. Sports Physiol. Perform. 2015, 10, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, J.H.; Hill, H.M.; Miller, M.G.; Cheatham, C.C.; Michael, T.J.; Baker, R.J. Comparisons of cubed ice, crushed ice, and wetted ice on intramuscular and surface temperature changes. J. Athl. Train. 2009, 44, 136–141. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, Y.; Louis, J.; Schaal, K.; Bieuzen, F.; Filliard, J.-R.; Brisswalter, J.; Hausswirth, C. Whole-Body Cryotherapy multi exposures speed up performance supercompensation during the taper in functionally overreached endurance athletes. Sports Perform. Sci. Rep. 2017, 6, 1–3. [Google Scholar]
- Nédélec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in soccer: Part II-recovery strategies. Sports Med. 2013, 43, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef]
- Siqueira, A.F.; Vieira, A.; Bottaro, M.; Ferreira-Júnior, J.B.; Nóbrega, O.; de Souza, V.C.; Marqueti, R.d.C.; Babault, N.; Durigan, J.L.Q. Multiple cold-water immersions attenuate muscle damage but not alter systemic inflammation and muscle function recovery: A parallel randomized controlled trial. Sci. Rep. 2018, 8, 10961. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, S.Y.; McHugh, M.P.; Howatson, G. Don’t Lose Your Cool with Cryotherapy: The Application of Phase Change Material for Prolonged Cooling in Athletic Recovery and Beyond. Front. Sports Act. Living 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Roberts, L.A.; Muthalib, M.; Stanley, J.; Lichtwark, G.; Nosaka, K.; Coombes, J.S.; Peake, J.M. Effects of cold water immersion and active recovery on hemodynamics and recovery of muscle strength following resistance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R389–R398. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Micielska, K.; Kozłowska, M.; Wnorowski, K.; Skrobecki, J.; Radzimiński, L.; Babińska, A.; Rodziewicz, E.; Lombardi, G.; Ziemann, E. A 2-week specific volleyball training supported by the whole body cryostimulation protocol induced an increase of growth factors and counteracted deterioration of physical performance. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Selfe, J.; Alexander, J.; Costello, J.T.; May, K.; Garratt, N.; Atkins, S.; Dillon, S.; Hurst, H.; Davison, M.; Przybyla, D.; et al. The effect of three different (−135 °C) whole body cryotherapy exposure durations on elite rugby league players. PLoS ONE 2014, 9, e86420. [Google Scholar] [CrossRef]
- Merrick, M.A.; Jutte, L.S.; Smith, M.E. Cold Modalities with Different Thermody. J. Athl. Train. 2003, 38, 28–33. [Google Scholar] [PubMed]
- White, G.E.; Wells, G.D. Cold-water immersion and other forms of cryotherapy: Physiological changes potentially affecting recovery from high-intensity exercise. Extrem. Physiol. Med. 2013, 2, 1. [Google Scholar] [CrossRef]
- Hausswirth, C.; Schaal, K.; Le Meur, Y.; Bieuzen, F.; Filliard, J.R.; Volondat, M.; Louis, J. Parasympathetic Activity and Blood Catecholamine Responses following a Single Partial-Body Cryostimulation and a Whole-Body Cryostimulation. PLoS ONE 2013, 8, e72658. [Google Scholar] [CrossRef]
- Westerlund, T.; Smolander, J.; Uusitalo-Koskinen, A.; Mikkelsson, M. The blood pressure responses to an acute and long-term whole-body cryotherapy (−110 °C) in men and women. J. Therm. Biol. 2004, 29, 285–290. [Google Scholar] [CrossRef]
- Young, A.J. Homeostatic responses to prolonged cold exposure: Human cold acclimatization. In Handbook of physiology: Environmental Physiology; Fregly, M.J., Blatteis, C.M., Eds.; American Physiological Society: Bethesda, MD, USA, 1996; pp. 419–438. [Google Scholar]
- Castellani, J.W.; Young, A.J. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton. Neurosci. 2016, 196, 63–74. [Google Scholar] [CrossRef]
- Kim, K.; Suzuki, K.; Peake, J.; Ahn, N.; Ogawa, K.; Hong, C.; Kim, S.; Lee, I.; Park, J. Physiological and leukocyte subset responses to exercise and cold exposure in cold-acclimatized skaters. Biol. Sport 2014, 31, 39–48. [Google Scholar] [CrossRef][Green Version]
- Leppäluoto, J.; Korhonen, I.; Hassi, J. Habituation of thermal sensations, skin temperatures, and norepinephrine in men exposed to cold air. J. Appl. Physiol. 2001, 90, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.M.; Mäntysaari, M.; Pääkkönen, T.; Jokelainen, J.; Palinkas, L.A.; Hassi, J.; Leppäluoto, J.; Tahvanainen, K.; Rintamäki, H. Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Aviat. Space Environ. Med. 2008, 79, 875–882. [Google Scholar] [CrossRef]
- Zalewski, P.; Bitner, A.; Słomko, J.; Szrajda, J.; Klawe, J.J.; Tafil-Klawe, M.; Newton, J.L. Whole-body cryostimulation increases parasympathetic outflow and decreases core body temperature. J. Therm. Biol. 2014, 45, 75–80. [Google Scholar] [CrossRef]
- Kenny, G.P.; Journeay, W.S. Human thermoregulation: Separating thermal and nonthermal effects on heat loss. Front. Biosci. 2010, 15, 259–290. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G. Skin temperature measurements by infrared thermography during running exercise. Exp. Therm. Fluid Sci. 2016, 71, 103–113. [Google Scholar] [CrossRef]
- Merla, A.; Mattei, P.A.; Di Donato, L.; Romani, G.L. Thermal imaging of cutaneous temperature modifications in runners during graded exercise. Ann. Biomed. Eng. 2010, 38, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Formenti, D.; Merla, A.; Priego Quesada, J.I. The Use of Infrared Thermography in the Study of Sport and Exercise Physiology. In Application of Infrared Thermography in Sports Science; Jose Ignacio Priego Quesada, Springer International Publishing AG: Cham, Switzerland, 2017; pp. 111–136. [Google Scholar] [CrossRef]
- Priego Quesada, J.I.; Vardasca, R. Issues and Future Developments of Infrared Thermography in Sports Science. In Application of Infrared Thermography in Sports Science; Jose Ignacio Priego Quesada, Springer International Publishing AG: Cham, Switzerland, 2017; pp. 297–319. [Google Scholar] [CrossRef]
- Binek, M.; Drzazga, Z.; Socha, T.; Pokora, I. Do exist gender differences in skin temperature of lower limbs following exercise test in male and female cross-country skiers? J. Therm. Anal. Calorim. 2022, 147, 7373–7383. [Google Scholar] [CrossRef]
- Chudecka, M.; Lubkowska, A. Temperature changes of selected body’s surfaces of handball players in the course of training estimated by thermovision, and the study of the impact of physiological and morphological factors on the skin temperature. J. Therm. Biol. 2010, 35, 379–385. [Google Scholar] [CrossRef]
- Drzazga, Z.; Binek, M.; Pokora, I.; Sadowska-Krępa, E. A preliminary study on infrared thermal imaging of cross-country skiers and swimmers subjected to endurance exercise. J. Therm. Anal. Calorim. 2018, 134, 701–710. [Google Scholar] [CrossRef]
- Tanda, G. Total body skin temperature of runners during treadmill exercise: A pilot study. J. Therm. Anal. Calorim. 2018, 131, 1967–1977. [Google Scholar] [CrossRef]
- Boerner, E.; Podbielska, H. Application of thermal imaging to assess the superficial skin temperature distribution after local cryotherapy and ultrasound. J. Therm. Anal. Calorim. 2018, 131, 2049–2055. [Google Scholar] [CrossRef]
- Cholewka, A.; Stanek, A.; Sieroń, A.; Drzazga, Z. Thermography study of skin response due to whole-body cryotherapy. Skin. Res. Technol. 2012, 18, 180–187. [Google Scholar] [CrossRef]
- Chudecka, M.; Lubkowska, A.; Kempinska-Podhorodecka, A. Body surface temperature distribution in relation to body composition in obese women. J. Therm. Biol. 2014, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pokora, I.; Sadowska-Krepa, E.; Wolowski, Ł.; Wyderka, P.; Michnik, A.; Drzazga, Z. The Effect of Medium-Term Sauna-Based Heat Acclimation (MPHA) on Thermophysiological and Plasma Volume Responses to Exercise Performed under Temperate Conditions in Elite Cross-Country Skiers. Int. J. Environ. Res. Public Health 2021, 18, 6906. [Google Scholar] [CrossRef]
- Czuba, M.; Maszczyk, A.; Gerasimuk, D.; Roczniok, R.; Fidos-Czuba, O.; Zając, A.; Gołaś, A.; Mostowik, A.; Langfort, J. The effects of hypobaric hypoxia on erythropoiesis, Maximal oxygen uptake and energy cost of exercise under normoxia in elite biathletes. J. Sports Sci. Med. 2014, 13, 912–920. [Google Scholar]
- Cheng, B.; Kuipers, H.; Snyder, A.C.; Keizer, H.A.; Jeukendrup, A.; Hesselink, M. A new approach for the determination of ventilator and lactate thresholds. Int. J. Sport Med. 1992, 13, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Cuttell, S.; Hammond, L.; Langdon, D.; Costello, J. Individualising the exposure of −110 °C whole body cryotherapy: The effects of sex and body composition. J. Therm. Biol. 2017, 65, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dugué, B.; Smolander, J.; Westerlund, T.; Oksa, J.; Nieminen, R.; Moilanen, E.; Mikkelsson, M. Acute and long-term effects of winter swimming and whole-body cryotherapy on plasma antioxidative capacity in healthy women. Scand. J. Clin. Lab. Investig. 2005, 65, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Klimek, A.T.; Lubkowska, A.; Szyguła, Z.; Frączek, B.; Chudecka, M. The influence of single whole body cryostimulation treatment on the dynamics and the level of maximal anaerobic power. Int. J. Occup. Med. Environ. Health 2011, 24, 184–191. [Google Scholar] [CrossRef]
- Burton, A. The application of the theory of heat flow to the study of energy metabolism. J. Nutr. 1934, 7, 497–533. [Google Scholar] [CrossRef]
- Stolwijk, J.A.; Hardy, J.D. Partitional calorimetric studies of responses of man to thermal transients. J. Appl. Physiol. 1966, 61, 302–308. [Google Scholar] [CrossRef]
- Moran, D.S.; Shitzer, A.; Pandolf, K.B. A physiological strain index to evaluate heat stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R129–R134. [Google Scholar] [CrossRef] [PubMed]
- Pokora, I.; Żebrowska, A. Application of A Physiological Strain Index in Evaluating Responses to Exercise Stress—A Comparison Between Endurance and High Intensity Intermittent Trained Athletes. J. Hum. Kinet. 2016, 50, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ammer, K. The Glamorgan protocol for recording and evaluation of thermal images of the human body. Thermol. Int. 2008, 18, 125–144. [Google Scholar]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.; Costa, C.M.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consen- sus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Fernàndez-Cuevas, I.; Marins, J.C.B.; Lastras, J.A.; Carmona, P.M.G.; Cano, S.P.; Garcìa- Concepción, M.A.; Sillero-Quintana, M. Classification of factor influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- Pilch, W.; Wyrostek, J.; Major, P.; Zuziak, R.; Piotrowska, A.; Czerwinska-Ledwig, O.; Grzybkowska, A.; Zasada, M.; Ziemann, E.; Zychowska, M. The effect of whole-body cryostimulation on body composition and leukocyte expression of HSPA1A, HSPB1, and CRP in obese men. Cryobiology 2020, 94, 100–106. [Google Scholar] [CrossRef]
- Pilch, W.; Piotrowska, A.; Wyrostek, J.; Czerwińska-Ledwig, O.; Ziemann, E.; Antosiewicz, J.; Zasada, M.; Kulesa-Mrowiecka, M.; Żychowska, M. Different Changes in Adipokines, Lipid Profile, and TNF-Alpha Levels between 10 and 20 Whole Body Cryostimulation Sessions in Individuals with I and II Degrees of Obesity. Biomedicines 2022, 10, 269. [Google Scholar] [CrossRef]
- Filliard, J.; Faria, F.C.; Bieuzen, F.; Volondat, M. The effects of the whole-body cryotherapy on the body composition. Br. J. Sports Med. 2016, 50, 38. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Sproull, J.; Szygula, Z. Whole-body cryotherapy is an effective method of reducing abdominal obesity in menopausal women with metabolic syndrome. J. Clin. Med. 2020, 9, 2797. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Yes, even human brown fat is on fire! J. Clin. Investig. 2012, 122, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Obesity and Thermoregulation, 1st ed.; Elsevier, B.V.: Amsterdam, The Netherlands, 2018; Volume 156. [Google Scholar] [CrossRef]
- Gordon, K.; Blondin, D.P.; Friesen, B.J.; Tingelstad, H.C.; Kenny, G.P.; Haman, F. Seven days of cold acclimation substantially reduces shivering intensity and increases nonshivering thermogenesis in adult humans. J. Appl. Physiol. 2019, 126, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Iwen, K.A.; Backhaus, J.; Cassens, M.; Waltl, M.; Hedesan, O.C.; Merkel, M.; Heeren, J.; Sina, C.; Rademacher, L.; Windjäger, A.; et al. Cold-induced brown adipose tissue activity alters plasma fatty acids and improves glucose metabolism in men. J. Clin. Endocr. Metab. 2017, 102, 4226–4234. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lans, A.A.J.J.; Hoeks, J.; Brans, B.; Vijgen, G.H.E.J.; Visser, M.G.W.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef]
- Lubkowska, A.; Szyguła, Z. Changes in blood pressure with compensatory heart rate decrease and in the level of aerobic capacity in response to repeated whole-body cryostimulation in normotensive, young and physically active men. Int. J. Occup. Med. Environ. Health 2010, 23, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Remie, C.M.E.; Moonen, M.P.B.; Roumans, K.H.M.; Nascimento, E.B.M.; Gemmink, A.; Havekes, B.; Schaart, G.; Kornips, E.; Joris, P.J.; Schrauwen-Hinderling, V.B.; et al. Metabolic responses to mild cold acclimation in type 2 diabetes patients. Nat. Commun. 2021, 12(1), 1516. [Google Scholar] [CrossRef]
- Kenefick, R.W.; Mahood, N.V.; Hazzard, M.P.; Quinn, T.J.; Castellani, J.W. Hypohydration effects on thermoregulation during moderate exercise in the cold. Eur. J. Appl. Physiol. 2004, 92, 565–570. [Google Scholar] [CrossRef]
- Reis, H.H.T.; Brito, C.J.; Sillero-Quintana, M.; Silva, A.G.; Fernández-Cuevas, I.; Cerqueira, M.S.; Werneck, F.Z.; Marins, J.C.B. Can Adipose Tissue Influence the Evaluation of Thermographic Images in Adolescents? Int. J. Environ. Res. Public Health 2023, 20, 4405. [Google Scholar] [CrossRef]
- Chudecka, M.; Lubkowska, A. Thermal Imaging of Body Surface Temperature Distribution in Women with Anorexia Nervosa. Eur. Eat Disord. Rev. 2016, 24, 57–61. [Google Scholar] [CrossRef]
- Neves, E.B.; Salamunes, A.C.C.; de Oliveira, R.M.; Stadnik, A.M.W. Effect of Body Fat and Gender on Body Temperature Distribution. J. Therm. Biol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Salamunes, A.C.C.; Stadnik, A.M.W.; Neves, E.B. The effect of body fat percentage and body fat distribution on skin surface temperature with infrared thermography. J. Therm. Biol. 2017, 66, 1–9. [Google Scholar] [CrossRef]
- Weigert, M.; Nitzsche, N.; Kunert, F.; Lösch, C.; Schulz, H. The influence of body composition on exercise-associated skin temperature changes after resistance training. J. Therm. Biol. 2018, 75, 112–119. [Google Scholar] [CrossRef]
- Neves, E.B.; Moreira, T.R.; Lemos, R.J.; Vilaça-Alves, J.; Rosa, C.; Reis, V.M. The influence of subcutaneous fat in the skin temperature variation rate during exercise. Res. Biomed. Eng. 2015, 31, 307–312. [Google Scholar] [CrossRef]
- Abate, M.; Di Carlo, L.; Di Donato, L.; Romani, G.L.; Merla, A. Comparison of cutaneous termic response to a standarised warm up in trained and untrained individuals. J. Sports Med. Phys. Fit. 2013, 53, 209–215. [Google Scholar]
- Formenti, D.; Ludwig, N.; Gargano, M.; Gondola, M.; Dellerma, N.; Caumo, A. Thermal imaging of exercise-associated skin temperature changes in trained and untrained female subjects. Ann. Biomed. Eng. 2013, 41, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, R.G.; Coyle, E.F. Cutaneous blood flow during exercise is higher in endurance-trained humans. J. Appl. Physiol. 2000, 88, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Szyguła, R.; Dybek, T.; Klimek, A.; Tubek, S. Impact of 10 sessions of whole body cryostimulation on cutaneous microcirculation measured by laser doppler flowmetry. J. Hum. Kinet. 2011, 30, 75–83. [Google Scholar] [CrossRef]
- Stanek, A.; Cholewka, A. Whole-Body Cryostimulation Improves Inflammatory Endothelium Parameters and Decreases Oxidative Stress in Healthy Subjects. Antioxidants 2020, 9, 1308. [Google Scholar] [CrossRef]
- Yang, A.L.; Tsai, S.J.; Jiang, M.J.; Jen, C.J.; Chen, H.I. Chronic exercise increases both inducible and endothelial nitric oxide synthase gene expression in endothelial cells of rat aorta. J. Biomed. Sci. 2002, 9, 149–155. [Google Scholar] [CrossRef]
- Wiecek, M.; Szygula, Z.; Gradek, J.; Kusmierczyk, J.; Szymura, J. Whole-Body Cryotherapy Increases the Activity of Nitric Oxide Synthase in Older Men. Biomolecules 2021, 11, 1041. [Google Scholar] [CrossRef]
- Brothers, R.M.; Wingo, J.E.; Hubing, K.A.; Crandall, C.G. Methodological assessment of skin and limb blood flows in the human forearm during thermal and baroreceptor provocations. J. Appl. Physiol. 2010, 109, 895–900. [Google Scholar] [CrossRef]
- Minson, C.T.; Berry, L.T.; Joyner, M.J. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J. Appl. Physiol. 2001, 91, 1619–1626. [Google Scholar] [CrossRef]
- Fanger, P.O.; Ipsen, B.M.; Langkilde, G.; Olessen, B.W.; Christensen, N.K.; Tanabe, S. Comfort limits for asymmetric thermal radiation. Energy Build. 1985, 8, 225–236. [Google Scholar] [CrossRef]
- Lubkowska, A.; Suska, M. The increase in systolic and diastolic blood pressure after exposure to cryogenic temperatures in normotensive men as a contraindication for whole-body cryostimulation. J. Therm. Biol. 2011, 36, 264–268. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Haymes, E.M. Thermoregulation and marathon running: Biological and environmental influences. Sports Med. 2001, 31, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Kenefick, R.W.; Cheuvront, S.N.; Sawka, M.N. Thermoregulatory function during the marathon. Sports Med. 2007, 37, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L.; Byrne, C.; Lee, J.K.W. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann. Acad. Med. Singap. 2008, 37, 347–353. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (Hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Davis, J.D.; Secher, N.H.; Lieshout, J.J.; Arce-Esquivel, A.A.; Simmons, G.H.; Bender, S.B.; Padilla, J.; Bache, R.J.; Merkus, D.; et al. Peripheral circulation. Compr. Physiol. 2012, 2, 321–447. [Google Scholar] [CrossRef]
- Mortensen, S.P.; Saltin, B. Regulation of the skeletal muscle blood flow in humans. Exp. Physiol. 2014, 99, 1552–1558. [Google Scholar] [CrossRef]
- Silva, A.G.d.; Albuquerque, M.R.; Brito, C.J.; Stroppa, G.M.; Oliveira, S.A.F.; Sillero-Quintana, M.; Marins, J.C.B. Effect of Whole-, Upper-, and Lower-Body High-Intensity Rowing Exercise on Skin Temperature Measured by Thermography. Res. Q. Exerc. Sport 2022, 94, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Delavier, F. Strength Training Anatomy; Human Kinetics: Champaign, IL, USA, 2010; ISBN-13 978-0736092265. [Google Scholar]
- Cheon, S.; Lee, J.-H.; Jun, H.-P.; An, Y.W.; Chang, E. Acute Effects of Open Kinetic Chain Exercise Versus Those of Closed Kinetic Chain Exercise on Quadriceps Muscle Thickness in Healthy Adults. Int. J. Environ. Res. Public Health 2020, 17, 4669. [Google Scholar] [CrossRef]
- Knyszyńska, A.; Radecka, A.; Lubkowska, A. Thermal imaging of exercise-associated skin temperature changes in swimmers subjected to 2-min intensive exercise on a vasa swim bench ergometer. Int. J. Environ. Res. Public Health 2021, 18, 6493. [Google Scholar] [CrossRef]
- Oliveira, S.A.F.; Marins, J.C.; Silva, A.G.; Brito, C.J.; Moreira, D.G.; Sillero-Quintana, M. Measuring of skin temperature via infra- red thermography after an upper body progressive aerobic exercise. J. Phys. Educ. Sport 2018, 18, 184–192. [Google Scholar] [CrossRef]
- Pokora, I. Relationships Between Electromyographic Characteristics, Mechanical Work Efficiency and Body Temperatures During Running Exercise Test in Men. J. Hum. Kinet. 2004, 11, 3–14. [Google Scholar]
- Silberstein, B.; Kaitan, J. Thermographically measured normal skin temperature asymmetry in the human male. Cancer 1975, 36, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, F.; Neves, E.B.; Moura, M.A.M.d.; Nohama, P. The thermography in support for the diagnosis of muscle injury in sport. Rev. Bras. Med. Esporte 2014, 20, 59–64. [Google Scholar] [CrossRef]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Rodrìguez-Sanz, D.; Losa-Iglesias, M.E.; López-López, D.; Calvo-Lobo, C.; Palomo-López, P.; Becerro-de-Bengoa-Vallejo, R. Infrared thermography applied to lower limb muscles in elite soccer players with functional ankle equinus and non-equinus condition. Peer J. 2017, 25, e3388. [Google Scholar] [CrossRef]
- Schmitt, L.C.; Paterno, M.V.; Hewett, T.E. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cru-ciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 2012, 42, 750–759. [Google Scholar] [CrossRef]
- Bishop, C.; Turner, A.; Read, P. Effects of inter-limb asymmetries on physical and sports performance: A systematic review. J. Sports Sci. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Carabello, R.J.; Reid, K.F.; Clark, D.J.; Phillips, E.M.; Fielding, R.A. Lower extremity strength and power asymmetry assessment in healthy and mobility-limited populations: Reliability and association with physical functioning. Aging Clin. Exp. Res. 2010, 22, 324–329. [Google Scholar] [CrossRef]
- Liu, T.; Jensen, J.L. Age-related differences in bilateral asymmetry in cycling performance. Res. Q. Exerc. Sport 2012, 83, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Vardasca, R.; Ring, E.F.J.; Plassmann, P.; Jones, C.D. Thermal symmetry of the upper and lower extremities in healthy subjects. Thermol. Int. 2012, 22, 53–60. [Google Scholar]
- Zaproudina, N.; Varmavuo, V.; Airaksinen, O.; Närhi, M. Reproducibility of infrared thermography measurements in healthy individuals. Physiol. Meas. 2008, 29, 515–524. [Google Scholar] [CrossRef]
- Al-Nakhli, H.H.; Petrofsky, J.S.; Laymon, M.S.; Berk, L.S. The use of ther- mal infrared imaging to detect delayed onset muscle soreness. J. Vis. Exp. 2012, 59, e3551. [Google Scholar] [CrossRef]
- Chudecka, M.; Lubkowska, A.; Leźnicka, K.; Krupecki, K. The use of thermal imaging in the evaluation of the symmetry of muscle activity in various types of exercises (Symmetrical and Asymmetrical). J. Hum. Kinet. 2015, 49, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bini, R.R.; Hume, P.A. Relationship between pedal force asymmetry and performance in cycling time trial. J. Sports Med. Phys. Fit. 2015, 55, 892–898. [Google Scholar]
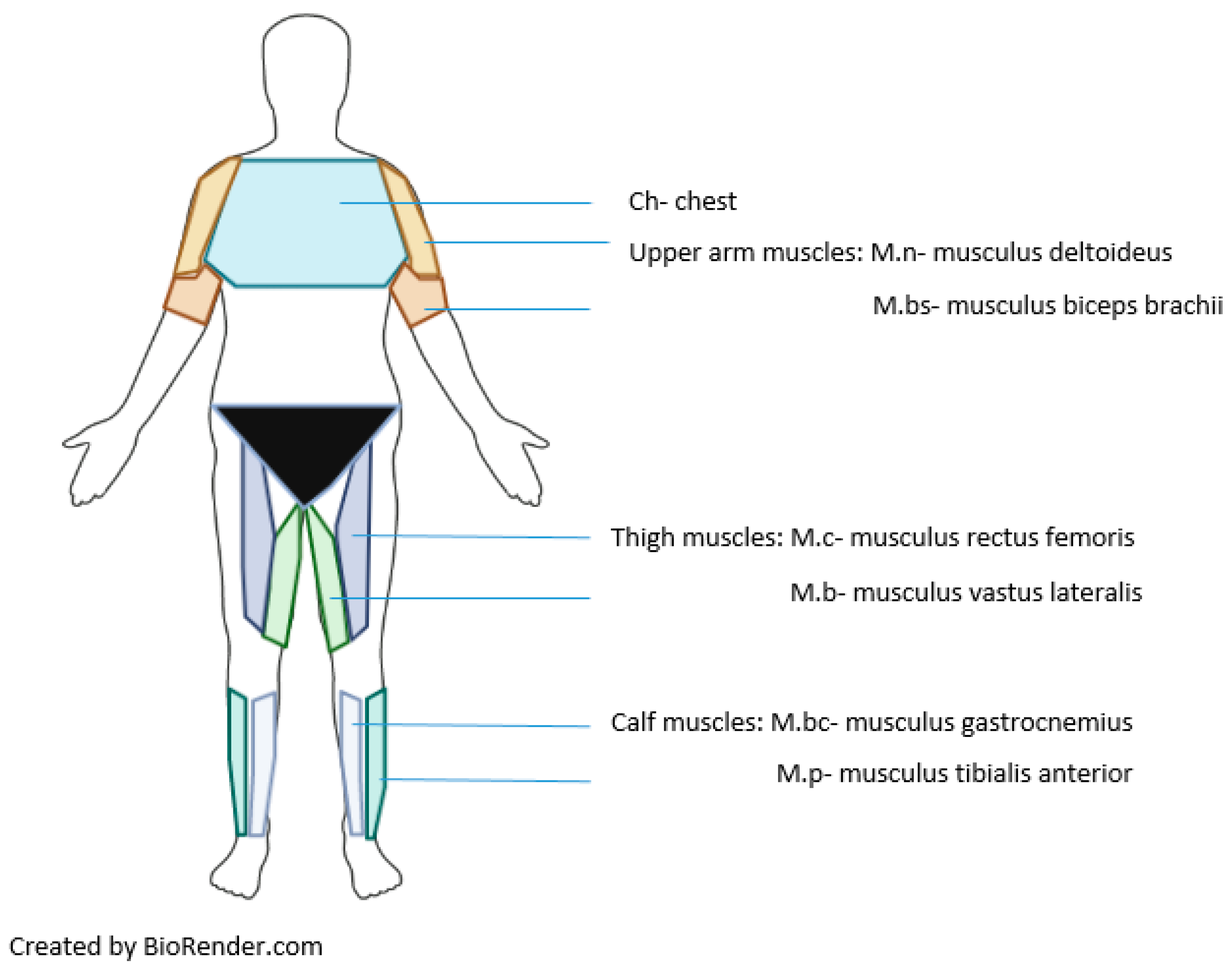
| Indicators | ||
|---|---|---|
| N = 17 | x ± SD | |
| Age | 21.5 ± 2.2 | |
| Height (cm) | 179.5 ± 5.7 | |
| Body mass (kg) | 75.18 ± 5.8 A | 74.91 ± 5.9 B |
| Body mass index (kg·m−2) | 22.05 ± 1.9 | |
| Fat mass (kg) | 7.78 ± 1.7 A | 6.94 ± 1.9 B* |
| Fat mass (%) | 10.09 ± 2.2 A | 9.31 ± 2.6 B |
| Fat-free mass (kg) | 67.4 ± 5.6 A | 67.9 ± 6.1 B |
| Body surface area (m2) | 1.93 ± 0.1 | |
| Training status (years) | 9.2 ± 10.5 | |
| Powermax (W) | 395.7 ± 9.1 | |
| VO2max (ml·kg−1·min−1) | 67.5 ± 6.4 | |
| HRmax (bs·min−1) | 191.5 ± 12.8 | |
| VLT (km·h−1) | 14.0 ± 0.3 | |
| GLT (%) | 1.6 ± 0.6 | |
| HRLT (bs·min−1) | 179.8 ± 3.1 | |
| Preliminary Study | Series A | 10 × WBC | Series B | |
|---|---|---|---|---|
| Exercise test | GXT test | SM test | SM test | |
| Measurements | VO2max LT HRLT | HR, VO2, RER, SBP, DBP, Ti, Tsk | HR, VO2, RER, SBP, DBP, Ti, Tsk | |
| IRT | IRT | |||
| Regions of interest in IRT | Anterior view | Anterior view |
| Indicators | A ± SD | B ± SD | Effect of Intervention (B) Effect of Exercise (t) Interaction (B × t); p; η2 |
|---|---|---|---|
| VO2rest (L·min−1) | 0.51 ± 0.12 | 0.55 ± 0.09 | (B); p = 0.8 (t); p = 0.001; 0.9 (B × t); p = 0.25 |
| VO2mean (L·min−1) | 3.44 ± 0.37 | 3.47 ± 0.34 | |
| HRrest (bs·min−1) | 79.2 ± 14.2 | 77.4 ± 14.3 | (B); p = 0.29 (t); p = 0.001; 0.9 (B × t); p = 0.57 |
| HRmean (bs·min−1) | 167 ± 7.7 | 169.5 ± 8.5 | |
| VO2max (%) | 71.6 ± 10.9 | 72.0 ± 5.98 | p > 0.05 |
| Indicators | A ± SD | B ± SD | Effect of Intervention (B) Effect of Exercise (t) Interaction (B × t) p; η2 |
|---|---|---|---|
| Tirest (°C) | 36.72 ± 0.31 | 36.49 ± 0.25 | (B); p = 0.11 (t); p = 0.000; 0.6 (B × t); p = 0.26 |
| Tilast (°C) | 37.96 ± 0.39 | 37.60 ± 0.17 * | |
| SBPrest (mmHg) | 137.5 ± 3.1 | 139.3 ± 3.4 | (B); p = 0.97 (t); p = 0.005; 0.3 (B × t); p = 0.53 |
| SBPlast (mmHg) | 129.0 ± 4.2 | 126.8 ± 4.6 | |
| DBPrest (mmHg) | 75.5 ± 2.9 | 73.5 ± 10.7 | (B); p = 0.38 (t); p = 0.005; 0.2 (B × t); p = 0.71 |
| DBPlast (mmHg) | 72.2 ± 5.3 | 68.8 ± 8.3 | |
| MAPrest (mmHg) | 94.76 ± 8.05 | 96.06 ± 9.34 | (B); p = 0.59 (t); p = 0.004; 0.3 (B × t); p = 0.89 |
| MAPlast (mmHg) | 88.10 ± 8.69 | 89.93 ± 6.79 | |
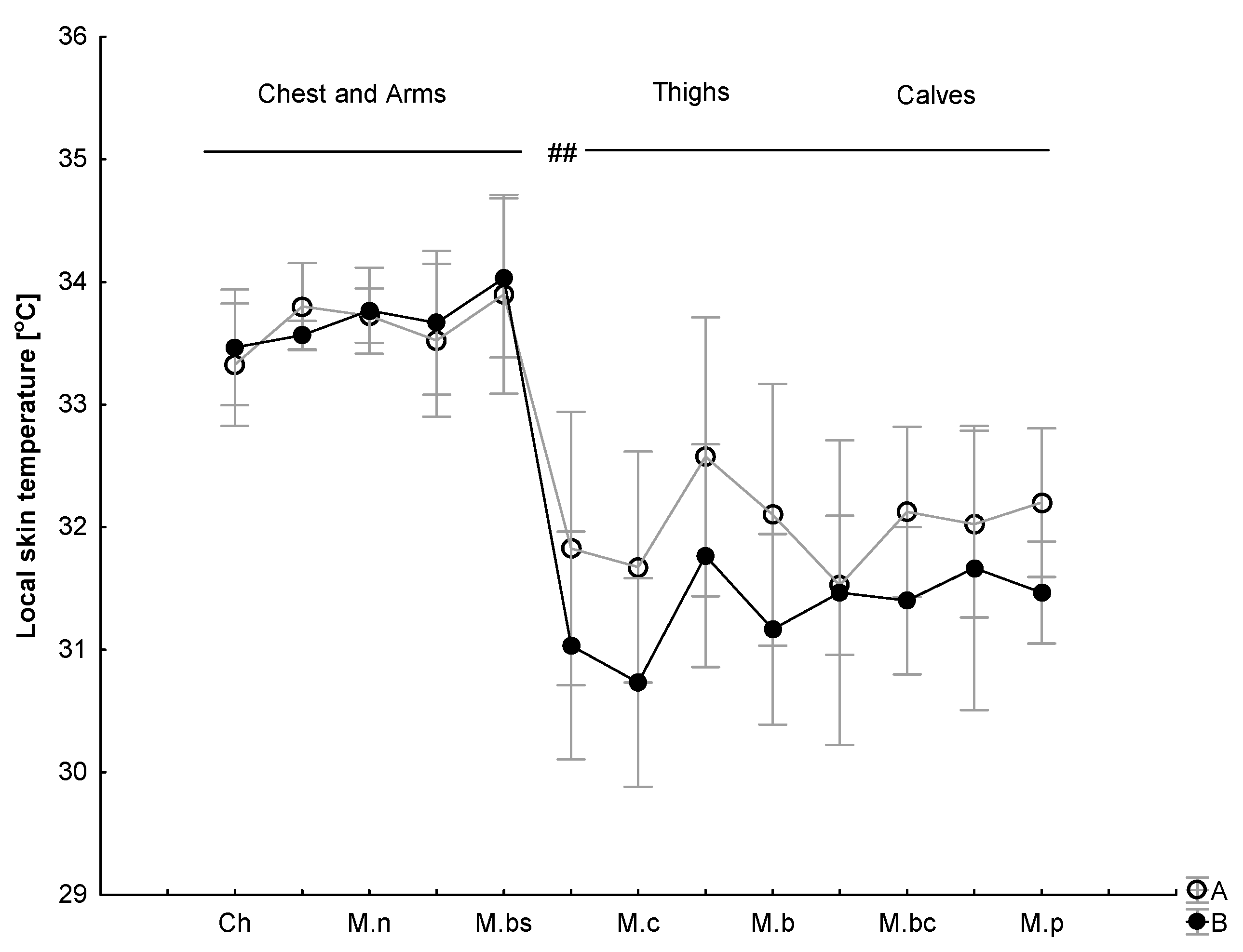
| (°C) | 34.62 ± 0.22 | 34.88 ± 0.28 | (B); p = 0.12 (t); p = 0.000; 0.65 (B × t); p = 0.12 |
| (°C) | 35.91 ± 0.28 | 35.92 ± 0.30 | |
| (°C) | 31.62 ± 0.23 | 32.52 ± 0.45 * | (B); p = 0.003; 0.6 (t); p = 0.001; 0.8 (B × t); p = 0.34 |
| (°C) | 33.09 ± 0.29 | 33.76 ± 0.68 * | |
| Mrest (W·m−2) | 137.5 ± 3.1 | 145.2 ± 3.4 | (B); p = 0.58 (t); p = 0.000; 0.3 (B × t); p = 0.26 |
| Mlast (W·m−2) | 423.6 ± 24.2 | 451.9 ± 21.6 | |
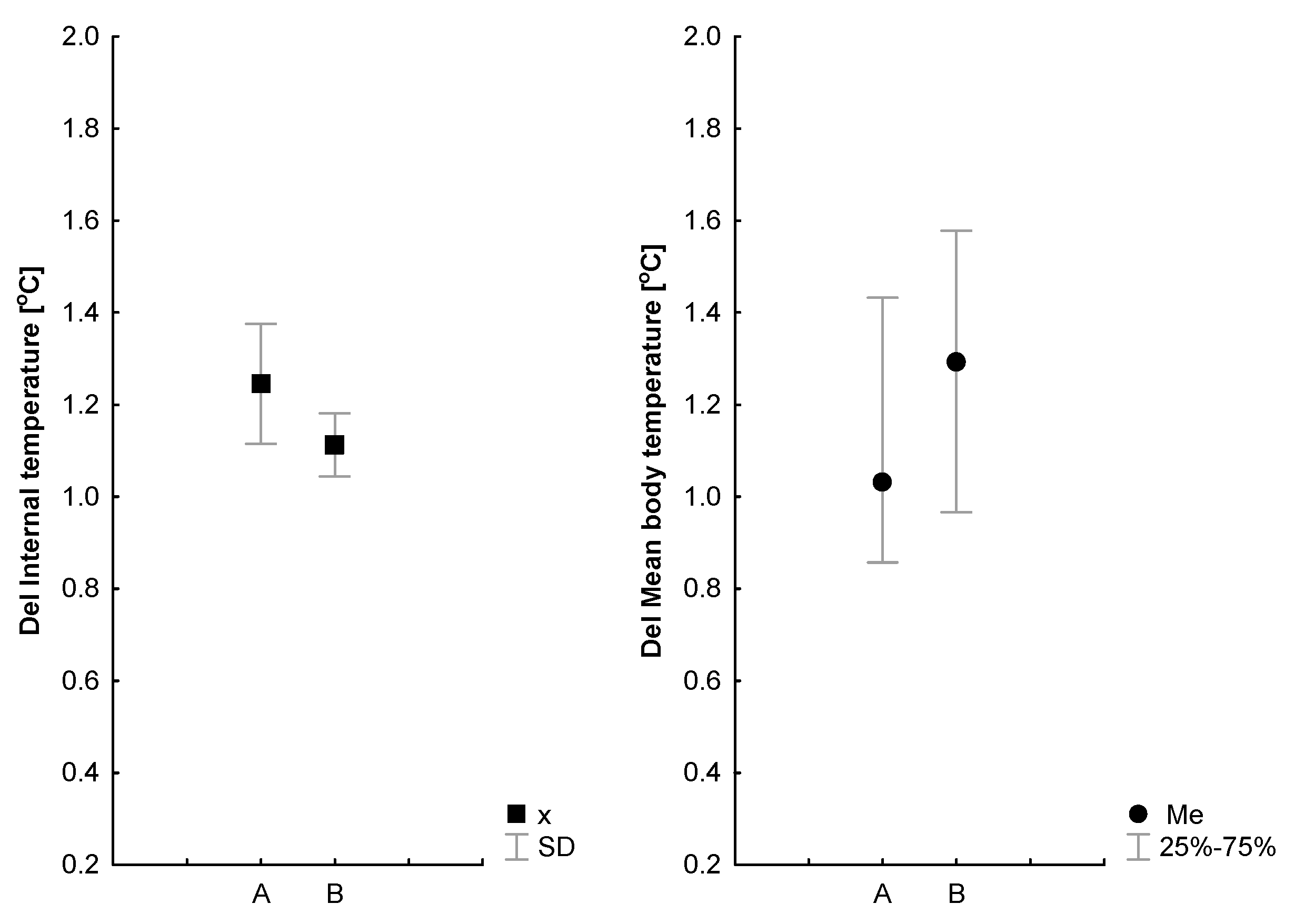
| Δgrest = Ti-MTsk (°C) | 4.31 ± 0.8 (+1.05) | 2.87 ± 1.16 *(+0.9) | (B); p = 0.000; 0.65 (t); p = 0.02; 0.2 (B × t); p = 0.35 |
| Δglast = Ti-MTsk (°C) | 5.36 ± 0.74 | 3.77 ± 0.48 *# | |
| PSI | 7.04 ± 0.73 | 6.42 ± 0.27 * | p < 0.05 |
| ƒHR PSI | 0.64 ± 0.07 | 0.68 ± 0.03 | p > 0.05 |
| ƒTi PSI | 0.35 ± 0.07 | 0.34 ± 0.03 | p > 0.05 |
| Indicators | A ± SD | B ± SD | Effect of Intervention (B) Effect of Exercise (t) Interaction (B × t); p; η2 |
|---|---|---|---|
| CORTrest (nmol·L−1) | 702 ± 279 | 525 ± 191 | (B); p = 0.18; (t); p = 0.003; 0.3 (B × t); p = 0.03; 0.2 |
| CORTlast (nmol·L−1) | 892 ± 319 | 695 ± 273 | |
| NErest (pg·mL−1) | 78 ± 93 | 77 ± 45 | (B); p = 0.29 (t); p = 0.001; 0.9 (B × t); p = 0.57 |
| NElast (pg·mL−1) | 91 ± 39 | 90 ± 56 | |
| EPIrest (pg·mL−1) | 61 ± 23 | 32 ± 15 | (B); p = 0.11 (t); p = 0.000; 0.6 (B × t); p = 0.26 |
| EPIlast (pg·mL−1) | 72 ± 22 | 55 ± 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokora, I.; Drzazga, Z.; Wyderka, P.; Binek, M. Determination of the Effects of a Series of Ten Whole-Body Cryostimulation Sessions on Physiological Responses to Exercise and Skin Temperature Behavior following Exercise in Elite Athletes. J. Clin. Med. 2023, 12, 6159. https://doi.org/10.3390/jcm12196159
Pokora I, Drzazga Z, Wyderka P, Binek M. Determination of the Effects of a Series of Ten Whole-Body Cryostimulation Sessions on Physiological Responses to Exercise and Skin Temperature Behavior following Exercise in Elite Athletes. Journal of Clinical Medicine. 2023; 12(19):6159. https://doi.org/10.3390/jcm12196159
Chicago/Turabian StylePokora, Ilona, Zofia Drzazga, Piotr Wyderka, and Mariusz Binek. 2023. "Determination of the Effects of a Series of Ten Whole-Body Cryostimulation Sessions on Physiological Responses to Exercise and Skin Temperature Behavior following Exercise in Elite Athletes" Journal of Clinical Medicine 12, no. 19: 6159. https://doi.org/10.3390/jcm12196159
APA StylePokora, I., Drzazga, Z., Wyderka, P., & Binek, M. (2023). Determination of the Effects of a Series of Ten Whole-Body Cryostimulation Sessions on Physiological Responses to Exercise and Skin Temperature Behavior following Exercise in Elite Athletes. Journal of Clinical Medicine, 12(19), 6159. https://doi.org/10.3390/jcm12196159









