The Effects of Combined Motor Control and Isolated Extensor Strengthening versus General Exercise on Paraspinal Muscle Morphology, Composition, and Function in Patients with Chronic Low Back Pain: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Participant Recruitment
2.4. Randomization and Blinding
2.5. Procedure
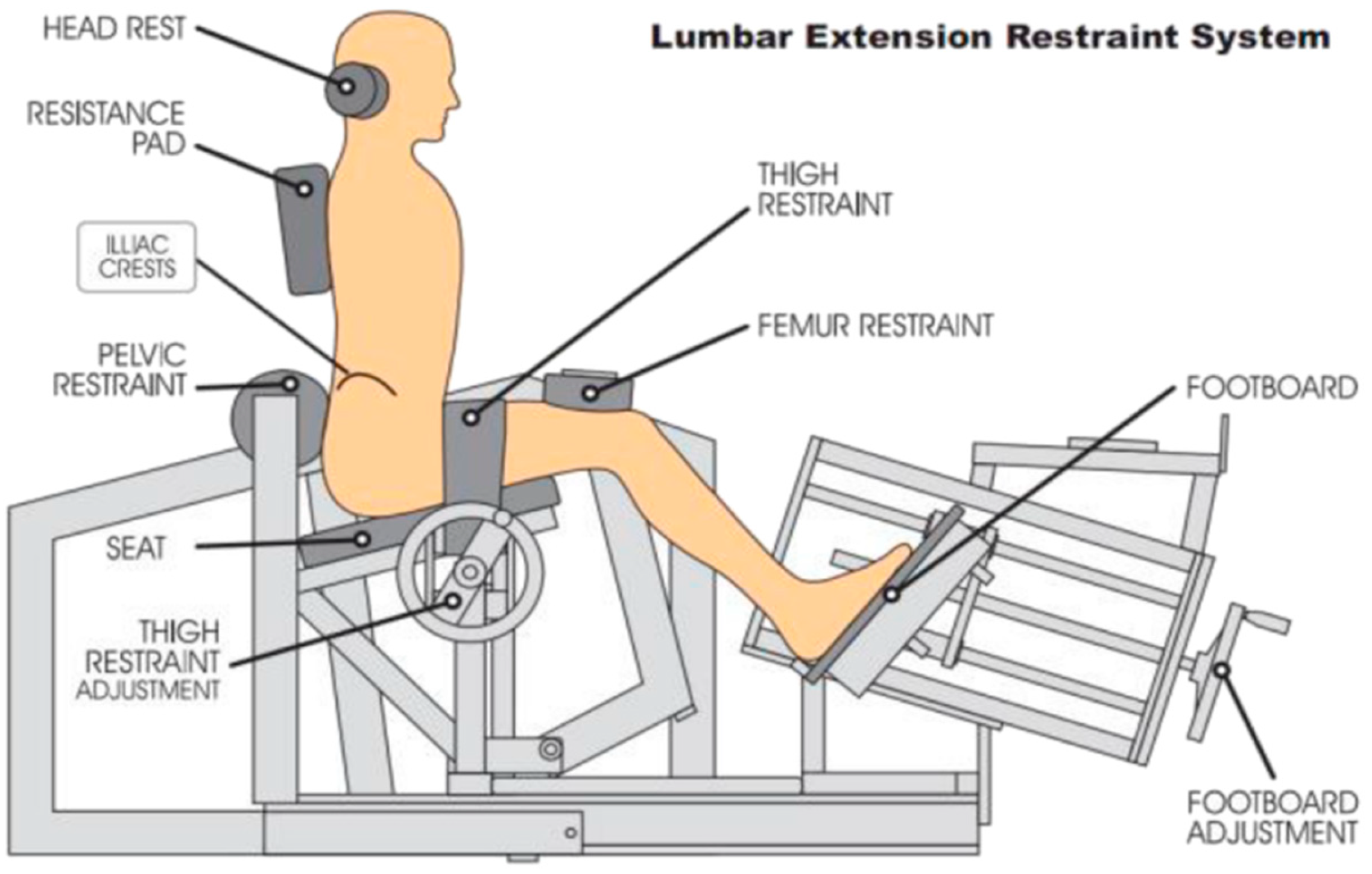
2.6. Intervention Protocols
2.6.1. General Exercise Group (GE)
2.6.2. Combined Motor Control and Isolated Lumbar Extension Group (MC+ILEX)
2.6.3. Phase 1: Cognitive Phase
2.6.4. Phase 2: Autonomous Activation Phase
2.7. Outcome Measures
2.8. Primary Outcome
Multifidus Muscle Morphology
2.9. Secondary Outcomes
2.9.1. Multifidus Muscle Function
2.9.2. Erector Spinae Muscle Morphology
2.9.3. Disability
2.9.4. Health-Related Quality of Life
2.9.5. Pain
2.9.6. Adherence
2.10. Statistical Analysis
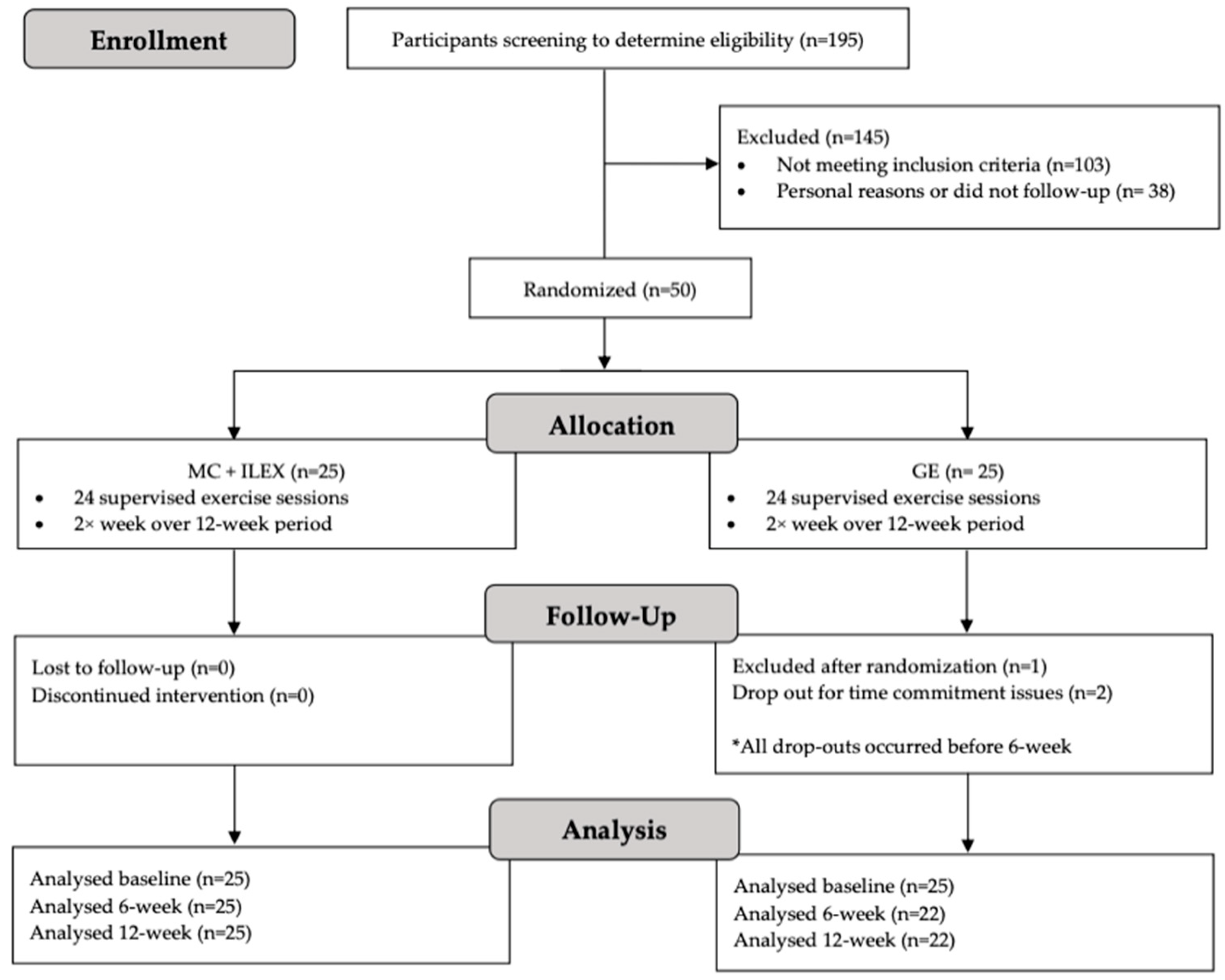
3. Results
3.1. Demographics and Adherence
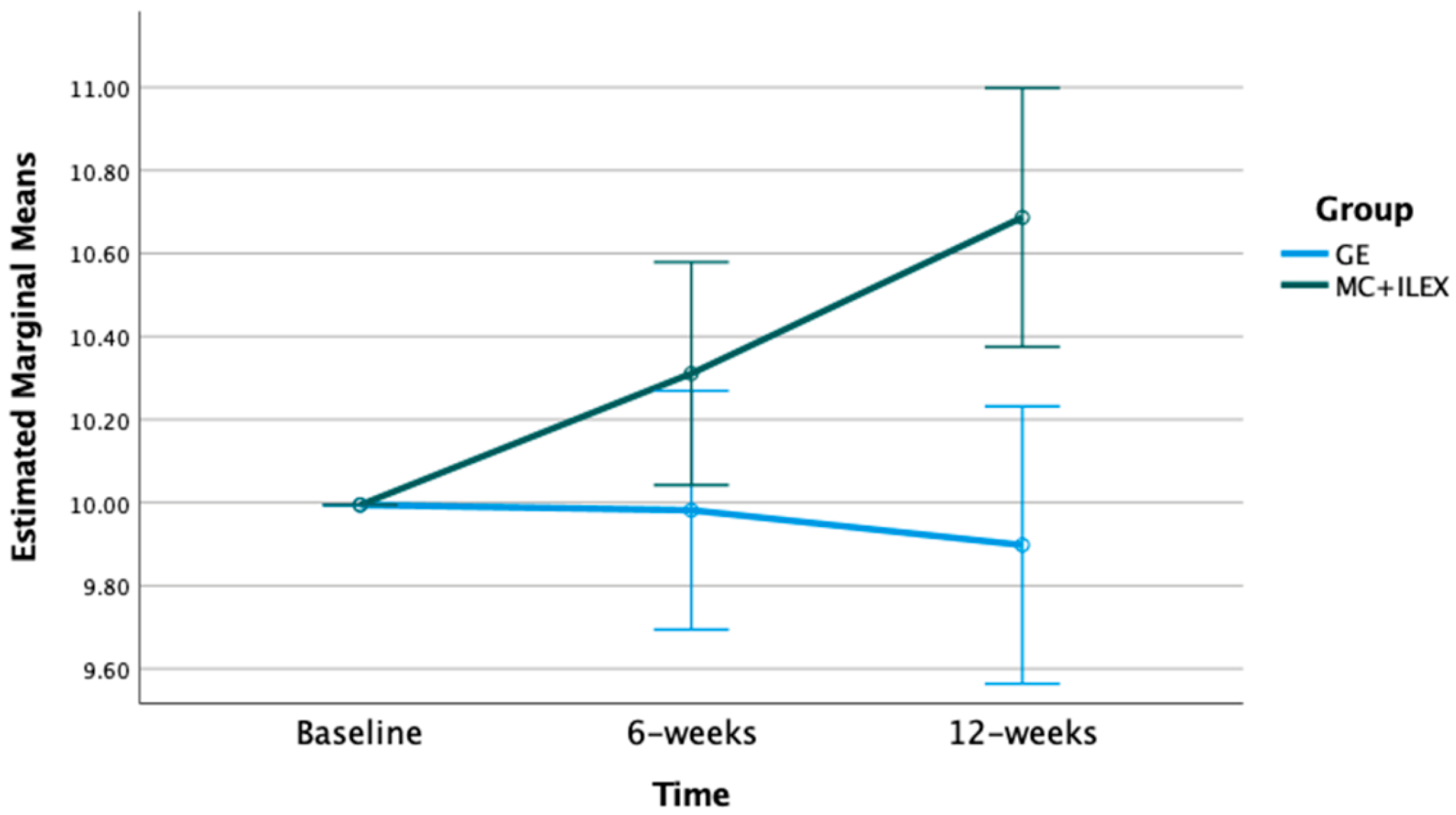
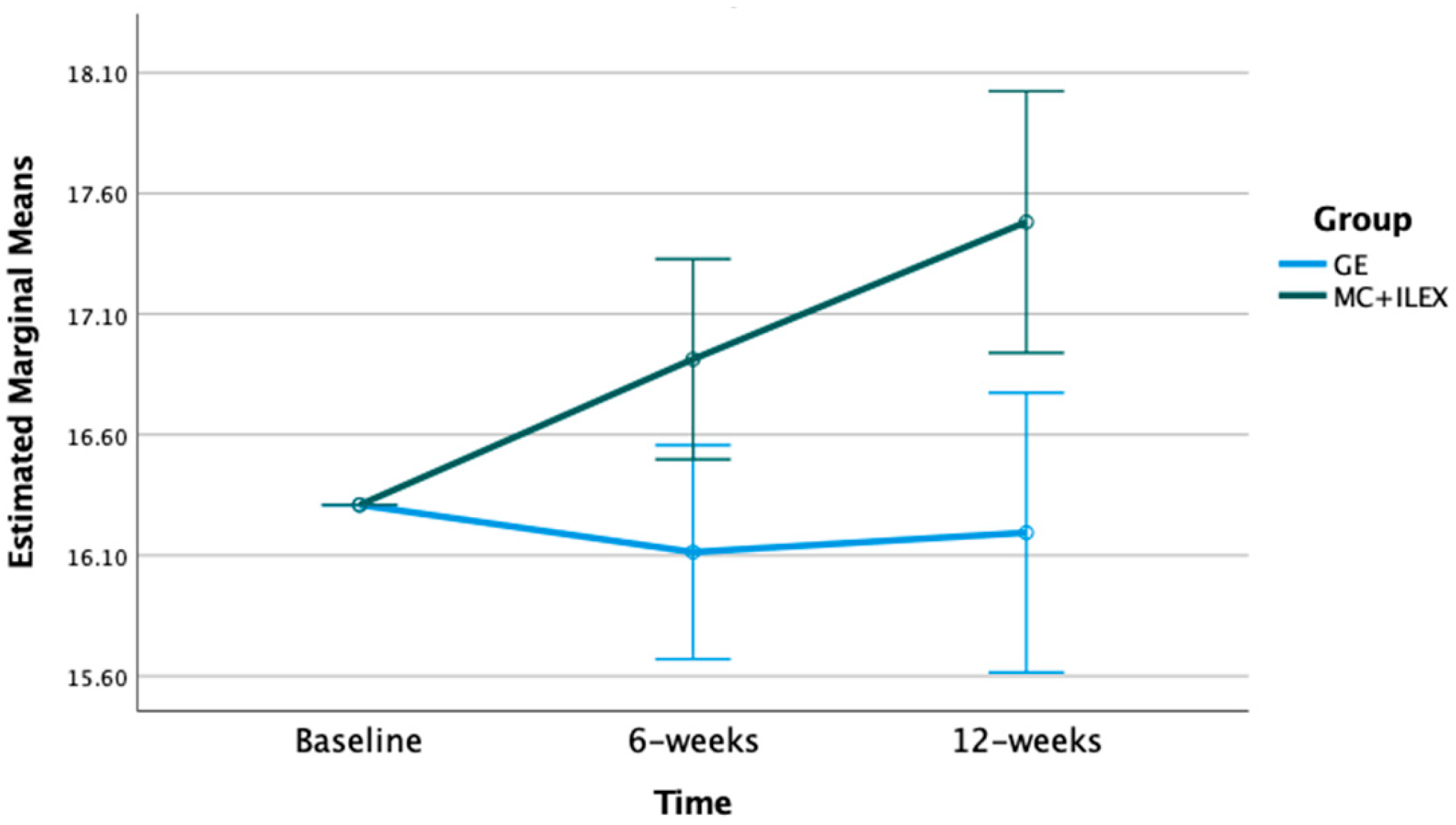
3.2. Effect of MC+ILEX and GE on Muscle Cross-Sectional Area (CSA)
3.3. Effect of MC+ILEX and GE on Fatty Infiltration (% Fat Fraction)
3.4. Effect of MC+ILEX and GE on Multifidus Thickness and Function
3.5. Effect of MC+ILEX and GE on Self-Reported Outcomes
3.6. Correlation between Muscle Morphology and Clinical Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Day 1 | Day 2 |
|---|---|
| Hip extension (multi-hip machine) * | Goblet squat |
| Prone leg curl * | Step up |
| Lat pull-down * | Leg extension * |
| Seated row * | Peck deck * |
| Hip abduction * | Lying side hip raises |
| Hip adduction | Abdominal curl |
| Multifidus Activation | |
|---|---|
| Positions | Prone or on hands and knees (depending on individuals preference) |
| Place fingers on either side of spinous process; assess various spinal levels from T1/T2 to L5/S1 | |
| Cues | Attempt to swell muscle up towards fingers |
| Mentally visualize tilting pelvis without physically executing the movement | |
| Visualize contracting a cable that runs from your pelvis through your spine | |
| Ideal | Symmetrical contraction |
| response | Absence of activation of the global muscles |
| Normal breathing | |
| Able to hold 10 × 10 s | |
| Transverse Abdominis Activation | |
| Positions | Initial position or crook-lying |
| Find neutral pelvis | |
| Position fingers slightly towards the midline and below the anterior superior iliac spine (ASIS) | |
| Cues | Attempt to draw your navel downwards to the table |
| Attempt to move your fingers together (medially) | |
| Ideal | Gradually increase tension; exerting a 10–15% level of effort |
| response | Symmetrical contraction |
| Absence of activation of the global muscles | |
| Normal breathing | |
| Able to hold 10 × 10 s | |
References
- Fortin, M.; Rye, M.; Roussac, A.; Naghdi, N.; Macedo, L.G.; Dover, G.; Elliott, J.M.; DeMont, R.; Weber, M.H.; Pepin, V. The effects of combined motor control and isolated extensor strengthening versus general exercise on paraspinal muscle morphology and function in patients with chronic low back pain: A randomised controlled trial protocol. BMC Musculoskelet. Disord. 2021, 22, 472. [Google Scholar] [CrossRef] [PubMed]
- Freburger, J.K.; Holmes, G.M.; Agans, R.P.; Jackman, A.M.; Darter, J.D.; Wallace, A.S.; Castel, L.D.; Kalsbeek, W.D.; Carey, T.S. The rising prevalence of chronic low back pain. Arch. Intern. Med. 2009, 169, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- Juniper, M.; Le, T.K.; Mladsi, D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: A literature-based review. Expert Opin. Pharmacother. 2009, 10, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Parthan, A.; Evans, C.J.; Le, K. Chronic low back pain: Epidemiology, economic burden and patient-reported outcomes in the USA. Expert Rev. Pharmacoecon. Outcomes Res. 2006, 6, 359–369. [Google Scholar] [CrossRef]
- Tousignant-Laflamme, Y.; Martel, M.O.; Joshi, A.B.; Cook, C.E. Rehabilitation management of low back pain—It’s time to pull it all together! J. Pain Res. 2017, 10, 2373–2385. [Google Scholar] [CrossRef]
- Caneiro, J.P.; Smith, A.; Bunzli, S.; Linton, S.; Moseley, G.L.; O’Sullivan, P. From Fear to Safety: A Roadmap to Recovery from Musculoskeletal Pain. Phys. Ther. 2022, 102, 271. [Google Scholar] [CrossRef] [PubMed]
- Pomarensky, M.; Macedo, L.; Carlesso, L.C. Management of Chronic Musculoskeletal Pain Through a Biopsychosocial Lens. J. Athl. Train. 2021, 57, 312–318. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Fortin, M.; Macedo, L.G. Multifidus and Paraspinal Muscle Group Cross-Sectional Areas of Patients with Low Back Pain and Control Patients: A Systematic Review with a Focus on Blinding. Phys. Ther. 2013, 93, 873–888. [Google Scholar] [CrossRef]
- Ranger, T.A.; Cicuttini, F.M.; Jensen, T.S.; Peiris, W.L.; Hussain, S.M.; Fairley, J.; Urquhart, D.M. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017, 17, 1729–1748. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, P.; Bendix, T.; Sorensen, J.S.; Korsholm, L.; Leboeuf-Yde, C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007, 5, 2. [Google Scholar] [CrossRef]
- Goubert, D.; Oosterwijck, J.V.; Meeus, M.; Danneels, L. Structural Changes of Lumbar Muscles in Non-specific Low Back Pain: A Systematic Review. Pain Physician 2016, 19, 985–1000. [Google Scholar]
- Seyedhoseinpoor, T.; Taghipour, M.; Dadgoo, M.; Sanjari, M.A.; Takamjani, I.E.; Kazemnejad, A.; Khoshamooz, Y.; Hides, J. Alteration of lumbar muscle morphology and composition in relation to low back pain: A systematic review and meta-analysis. Spine J. 2022, 22, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Ranson, C.A.; Burnett, A.F.; Kerslake, R.; Batt, M.E.; O’Sullivan, P.B. An investigation into the use of MR imaging to determine the functional cross sectional area of lumbar paraspinal muscles. Eur. Spine J. 2006, 15, 764–773. [Google Scholar] [CrossRef]
- Hodges, P.W.; Danneels, L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 464–476. [Google Scholar] [CrossRef]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4 European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15, 192–300. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287. [Google Scholar] [CrossRef]
- Nicol, V.; Verdaguer, C.; Daste, C.; Bisseriex, H.; Lapeyre, É.; Lefèvre-Colau, M.M.; Rannou, F.; Roren, A.; Facione, J.; Nguyen, C. Chronic Low Back Pain: A Narrative Review of Recent International Guidelines for Diagnosis and Conservative Treatment. J. Clin. Med. 2023, 12, 1685. [Google Scholar] [CrossRef]
- De Sire, A.; Lippi, L.; Marotta, N.; Ferrillo, M.; Folli, A.; Turco, A.; Ammendolia, A.; Invernizzi, M. Myths and truths on biophysics-based approach in rehabilitation of musculoskeletal disorders. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ketterer, E.; Peñacoba-Puente, C.; Ortega-Santiago, R.; Galán-del-Río, F.; Valera-Calero, J.A. Consideration of Psychosocial Factors in Acute Low Back Pain by Physical Therapists. J. Clin. Med. 2023, 12, 3865. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Bruce-Low, S.; Smith, D. A Review of the Clinical Value of Isolated Lumbar Extension Resistance Training for Chronic Low Back Pain. PM&R 2015, 7, 169–187. [Google Scholar]
- Rainville, J.; Hartigan, C.; Martinez, E.; Limke, J.; Jouve, C.; Finno, M. Exercise as a treatment for chronic low back pain. Spine J. 2004, 4, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Bloxham, S. A Systematic Review of the Effects of Exercise and Physical Activity on Non-Specific Chronic Low Back Pain. Healthcare 2016, 4, 22. [Google Scholar] [CrossRef]
- Van Middelkoop, M.; Rubinstein, S.M.; Verhagen, A.P.; Ostelo, R.W.; Koes, B.W.; van Tulder, M.W. Exercise therapy for chronic nonspecific low-back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.; Spink, M.; Ho, A.; Chuter, V. Exercise interventions for the treatment of chronic low back pain: A systematic review and meta-analysis of randomised controlled trials. Clin. Rehabil. 2015, 29, 1155–1167. [Google Scholar] [CrossRef]
- Koes, B.W.; van Tulder, M.W.; Thomas, S. Diagnosis and treatment of low back pain. BMJ 2006, 332, 1430–1434. [Google Scholar] [CrossRef]
- Shnayderman, I.; Katz-Leurer, M. An aerobic walking programme versus muscle strengthening programme for chronic low back pain: A randomized controlled trial. Clin. Rehabil. 2013, 27, 207–214. [Google Scholar] [CrossRef]
- Cuellar, W.A.; Wilson, A.; Blizzard, C.L.; Otahal, P.; Callisaya, M.L.; Jones, G.; Hides, J.A.; Winzenberg, T.M. The assessment of abdominal and multifidus muscles and their role in physical function in older adults: A systematic review. Physiotherapy 2017, 103, 21–39. [Google Scholar] [CrossRef]
- Prins, M.R.; Griffioen, M.; Veeger, T.T.J.; Kiers, H.; Meijer, O.G.; van der Wurff, P.; Bruijn, S.M.; van Dieen, J.H. Evidence of splinting in low back pain? A systematic review of perturbation studies. Eur. Spine J. 2018, 27, 40–59. [Google Scholar] [CrossRef]
- Berry, D.B.; Padwal, J.; Johnson, S.; Englund, E.K.; Ward, S.R.; Shahidi, B. The effect of high-intensity resistance exercise on lumbar musculature in patients with low back pain: A preliminary study. BMC Musculoskelet. Disord. 2019, 20, 290. [Google Scholar] [CrossRef]
- Welch, N.; Moran, K.; Antony, J.; Richter, C.; Marshall, B.; Coyle, J.; Falvey, E.; Franklyn-Miller, A. The effects of a free-weight-based resistance training intervention on pain, squat biomechanics and MRI-defined lumbar fat infiltration and functional cross-sectional area in those with chronic low back. BMJ Open Sport Exerc. Med. 2015, 1, e000050. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.M.; Boghra, S.B.; Macedo, L.G.; Zheng, Y.P.; Pang, M.Y.C.; Cheung, J.P.Y.; Karppinen, J.; Samartzis, D.; Wong, A.Y.L. Does Motor Control Exercise Restore Normal Morphology of Lumbar Multifidus Muscle in People with Low Back Pain?—A Systematic Review. J. Pain Res. 2021, 14, 2543. [Google Scholar] [CrossRef]
- Van Middelkoop, M.; Rubinstein, S.M.; Kuijpers, T.; Verhagen, A.P.; Ostelo, R.; Koes, B.W.; van Tulder, M.W. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur. Spine J. 2011, 20, 19–39. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Malmivaara, A.; Tulder, M.W. Exercise therapy for chronic low back pain. Cochrane Database Syst. Rev. 2021, 9, 1–550. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Pagé, G.M.; Lacasse, A.; Beaudet, N.; Choinière, M.; Deslauriers, S.; Diatchenko, L.; Dupuis, L.; Gregoire, S.; Hovey, R.; Leclair, R.; et al. The Quebec Low Back Pain Study: A protocol for an innovative 2-tier provincial cohort. Pain Rep. 2019, 5, e799. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Iversen, V.M.; Vasseljen, O.; Mork, P.J.; Berthelsen, I.R.; Børke, J.B.B.; Berheussen, G.F.; Tveter, A.T.; Salvesen, O.; Fimland, M.S. Resistance training in addition to multidisciplinary rehabilitation for patients with chronic pain in the low back: Study protocol. Contemp. Clin. Trials Commun. 2017, 6, 115–121. [Google Scholar] [CrossRef]
- Bayles, M.P.; Swank, A.M. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2021. [Google Scholar]
- Hodges, P.W. Core stability exercise in chronic low back pain. Orthop. Clin. N. Am. 2003, 34, 245–254. [Google Scholar] [CrossRef]
- Hodges, P.W.; Ferreira, P.; Ferreira, M.L. Lumbar Spine: Treatment of instability and disorders of movement control. In Pathology and Intervention in Musculoskeletal Rehabilitation, 1st ed.; Elsevier: New York, NY, USA, 2009; pp. 389–425. [Google Scholar]
- Ferreira, M.L.; Ferreira, P.H.; Latimer, J.; Herbert, R.D.; Hodges, P.W.; Jennings, M.D.; Maher, C.G.; Refshauge, K.M. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: A randomized trial. Pain 2007, 131, 31–37. [Google Scholar] [CrossRef]
- O’Sullivan, P.B.; Phyty, G.D.M.; Twomey, L.T.; Allison, G.T. Evaluation of Specific Stabilizing Exercise in the Treatment of Chronic Low Back Pain with Radiologic Diagnosis of Spondylolysis or Spondylolisthesis. Spine 1997, 22, 2959–2967. [Google Scholar] [CrossRef]
- Macedo, L.G.; Latimer, J.; Maher, C.G.; Hodges, P.W.; McAuley, J.H.; Nicholas, M.K.; Tonkin, L.; Stanton, C.J.; Stanton, T.R.; Stafford, R. Effect of Motor Control Exercises Versus Graded Activity in Patients with Chronic Nonspecific Low Back Pain: A Randomized Controlled Trial. Phys. Ther. 2012, 92, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.O.P.; Maher, C.G.; Latimer, J.; Hodges, P.W.; Herbert, R.D.; Refshauge, K.M.; McAuley, J.H.; Jennings, M.D. Motor control exercise for chronic low back pain: A randomized placebo-controlled trial. Phys. Ther. 2009, 89, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Jull, G.; Hodges, P.W.; Hides, J.A. Therapeutic Exercise for Spinal Segmental Stabilization in Low Back Pain: Scientific Basis and Clinical Approach, 1st ed.; Churchill Livingstone: Edinburgh, UK, 1999; pp. 1–185. [Google Scholar]
- Conway, R.; Behennah, J.; Fisher, J.; Osborne, N.; Steele, J. Associations between Trunk Extension Endurance and Isolated Lumbar Extension Strength in Both Asymptomatic Participants and Those with Chronic Low Back Pain. Healthcare 2016, 4, 70. [Google Scholar] [CrossRef]
- Steele, J.; Bruce-Low, S.; Smith, D.; Jessop, D.; Osborne, N. A Randomized Controlled Trial of Limited Range of Motion Lumbar Extension Exercise in Chronic Low Back Pain. Spine 2013, 38, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Pollock, M.L.; Leggett, S.H.; Graves, J.E.; Jones, A.; Fulton, M.; Cirulli, J. Effect of resistance training on lumbar extension strength. Am. J. Sports Med. 1989, 17, 624–629. [Google Scholar] [CrossRef]
- Kiesel, K.B.; Uhl, T.L.; Underwood, F.B.; Rodd, D.W.; Nitz, A.J. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man. Ther. 2007, 12, 161–166. [Google Scholar] [CrossRef]
- Fortin, M.; Rizk, A.; Frenette, S.; Boily, M.; Rivaz, H. Ultrasonography of multifidus muscle morphology and function in ice hockey players with and without low back pain. Phys. Ther. Sport 2019, 37, 77–85. [Google Scholar] [CrossRef]
- Larivière, C.; Gagnon, D.; De Oliveira, E., Jr.; Henry, S.M.; Mecheri, H.; Dumas, J.P. Ultrasound Measures of the Lumbar Multifidus: Effect of Task and Transducer Position on Reliability. PM&R 2013, 5, 678–687. [Google Scholar]
- Skeie, E.J.; Borge, J.A.; Leboeuf-Yde, C.; Bolton, J.; Wedderkopp, N. Reliability of diagnostic ultrasound in measuring the multifidus muscle. Chiropr. Man. Ther. 2015, 23, 15. [Google Scholar] [CrossRef]
- Saltychev, M.; Mattie, R.; McCormick, Z.; Bärlund, E.; Laimi, K. Psychometric properties of the Oswestry Disability Index. Int. J. Rehabil. Res. 2017, 40, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto, A.; Boers, M.; Deyo, R.A.; Buchbinder, R.; Corbin, T.P.; Costa, L.O.P.; Foster, N.E.; Grotle, M.; Koes, B.W.; Kovacs, F.M.; et al. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain 2018, 159, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Guo, Y.; Shenkman, E.; Muller, K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: A report from the wellness incentive and navigation (WIN) study. Health Qual. Life Outcomes 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Jensen, M.P.; Turner, J.A.; Romano, J.M.; Fisher, L.D. Comparative reliability and validity of chronic pain intensity measures. Pain 1999, 83, 157–162. [Google Scholar] [CrossRef]
- Childs, J.D.; Piva, S.R.; Fritz, J.M. Responsiveness of the Numeric Pain Rating Scale in Patients with Low Back Pain. Spine 2005, 30, 1331–1334. [Google Scholar] [CrossRef]
- Hides, J.A.; Stanton, W.R.; Mendis, M.D.; Gildea, J.; Sexton, M.J. Effect of Motor Control Training on Muscle Size and Football Games Missed from Injury. Med. Sci. Sports Exerc. 2012, 44, 1141–1149. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; pp. 1–567. [Google Scholar]
- Fortin, M.; Omidyeganeh, M.; Battié, M.C.; Ahmad, O.; Rivaz, H. Evaluation of an automated thresholding algorithm for the quantification of paraspinal muscle composition from MRI images. Biomed. Eng. OnLine 2017, 16, 61. [Google Scholar] [CrossRef]
- Shahtahmassebi, B.; Hebert, J.J.; Hecimovich, M.; Fairchild, T.J. Trunk exercise training improves muscle size, strength, and function in older adults: A randomized controlled trial. Scand. J. Med. Sci. Sports 2019, 29, 980–991. [Google Scholar] [CrossRef]
- Macedo, L.G.; Hodges, P.W.; Bostick, G.; Hancock, M.; Laberge, M.; Hanna, S.; Spadoni, G.; Gross, A.; Schneider, J. Which Exercise for Low Back Pain? (WELBack) trial predicting response to exercise treatments for patients with low back pain: A validation randomised controlled trial protocol. BMJ Open 2021, 11, e042792. [Google Scholar] [CrossRef]
- Wesselink, E.O.; Pool, J.J.M.; Mollema, J.; Weber, K.A.; Elliott, J.M.; Coppieters, M.W.; Pool-Goudzwaard, A.L. Is fatty infiltration in paraspinal muscles reversible with exercise in people with low back pain? A systematic review. Eur. Spine J. 2023, 32, 787–796. [Google Scholar] [CrossRef]
- Addison, O.; Marcus, R.L.; LaStayo, P.C.; Ryan, A.S. Intermuscular Fat: A Review of the Consequences and Causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, 1–26. [Google Scholar] [CrossRef]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef]
- Alabousi, A.; Al-Attar, S.; Joy, T.R.; Hegele, R.A.; McKenzie, C.A. Evaluation of adipose tissue volume quantification with IDEAL fat–water separation. J. Magn. Reson. Imaging 2011, 34, 474–479. [Google Scholar] [CrossRef]
- Hodges, P.W.; Bailey, J.F.; Fortin, M.; Battié, M.C. Paraspinal muscle imaging measurements for common spinal disorders: Review and consensus-based recommendations from the ISSLS degenerative spinal phenotypes group. Eur. Spine J. 2021, 30, 3428–3441. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van Tulder, M.W.; Malmivaara, A.; Koes, B.W. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst. Rev. 2005, 3, 1–82. [Google Scholar] [CrossRef]
- Hildebrandt, M.; Fankhauser, G.; Meichtry, A.; Luomajoki, H. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Musculoskelet. Disord. 2017, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Teichtahl, A.J.; Urquhart, D.M.; Wang, Y.; Wluka, A.E.; Wijethilake, P.; O’Sullivan, R.; Cicuttini, F.M. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J. 2015, 15, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Gibbons, L.E.; Videman, T.; Battié, M.C. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand. J. Med. Sci. Sports 2015, 25, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Lazáry, À.; Varga, P.P.; Battié, M.C. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur. Spine J. 2017, 26, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Sions, J.M.; Coyle, P.C.; Velasco, T.O.; Elliott, J.M.; Hicks, G.E. Multifidi Muscle Characteristics and Physical Function among Older Adults with and without Chronic Low Back Pain. Arch. Phys. Med. Rehabil. 2017, 98, 51–57. [Google Scholar] [CrossRef]
- James, G.; Chen, X.; Diwan, A.; Hodges, P.W. Fat infiltration in the multifidus muscle is related to inflammatory cytokine expression in the muscle and epidural adipose tissue in individuals undergoing surgery for intervertebral disc herniation. Eur. Spine J. 2021, 30, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Schryver, A.; Rivaz, H.; Rizk, A.; Frenette, S.; Boily, M.; Fortin, M. Ultrasonography of Lumbar Multifidus Muscle in University American Football Players. Med. Sci. Sports Exerc. 2020, 52, 1495. [Google Scholar] [CrossRef]
- Schlaeger, S.; Inhuber, S.; Rohrmeier, A.; Dieckmeyer, M.; Freitag, F.; Klupp, E.; Weidlich, D.; Feuerriegel, G.; Kreuzpointner, F.; Schwirtz, A.; et al. Association of paraspinal muscle water-fat MRI-based measurements with isometric strength measurements. Eur. Radiol. 2019, 29, 599–608. [Google Scholar] [CrossRef]
- Khan, A.B.; Weiss, E.H.; Khan, A.W.; Omeis, I.; Verla, T. Back Muscle Morphometry: Effects on Outcomes of Spine Surgery. World Neurosurg. 2017, 103, 174–179. [Google Scholar] [CrossRef]
- Gengyu, H.; Jinyue, D.; Chunjie, G.; Bo, Z.; Yu, J.; Jiaming, L.; Weishi, L. The predictive value of preoperative paraspinal muscle morphometry on complications after lumbar surgery: A systematic review. Eur. Spine J. 2022, 31, 364–379. [Google Scholar] [CrossRef]
- Crawford, R.J.; Fortin, M.; Weber, K.A.; Smith, A.; Elliott, J.M. Are Magnetic Resonance Imaging Technologies Crucial to Our Understanding of Spinal Conditions? J. Orthop. Sports Phys. Ther. 2019, 49, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, A.A.; Sra, A.; Do, O. Effect of Stabilization Exercise on Lumbar Multifidus Muscle Thickness in patients with non-specific Chronic Low Back Pain. Iran. Rehabil. J. 2014, 12, 5. [Google Scholar]
- Olsen, M.F.; Bjerre, E.; Hansen, M.D.; Tendal, B.; Hilden, J.; Hróbjartsson, A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: Systematic review of empirical studies. J. Clin. Epidemiol. 2018, 101, 87–106. [Google Scholar] [CrossRef]
- Maughan, E.F.; Lewis, J.S. Outcome measures in chronic low back pain. Eur. Spine J. 2010, 19, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Copay, A.G.; Cher, D.J. Is the Oswestry Disability Index a valid measure of response to sacroiliac joint treatment? Qual. Life Res. 2016, 25, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Bissell, G.; Bruce-Low, S.; Wakefield, C. The effect of lumbar extension training with and without pelvic stabilization on lumbar strength and low back pain1. J. Back Musculoskelet. Rehabil. 2011, 24, 241–249. [Google Scholar] [CrossRef]
- Kovacs, F.M.; Abraira, V.; Royuela, A.; Corcoll, J.; Alegre, L.; Tomás, M.; Mir, M.A.; Cano, A.; Muriel, A.; Zamora, J.; et al. Minimum detectable and minimal clinically important changes for pain in patients with nonspecific neck pain. BMC Musculoskelet. Disord. 2008, 9, 43. [Google Scholar] [CrossRef]
- Kovacs, F.M.; Abraira, V.; Royuela, A.; Corcoll, J.; Alegre, L.; Cano, A.; Muriel, A.; Zamora, J.; Gil del Real, M.T.; Gestoso, M.; et al. Minimal Clinically Important Change for Pain Intensity and Disability in Patients with Nonspecific Low Back Pain. Spine 2007, 32, 2915–2920. [Google Scholar] [CrossRef]
- Hägg, O.; Fritzell, P.; Nordwall, A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur. Spine J. 2003, 12, 12–20. [Google Scholar] [CrossRef]
- Sharma, A.; Madaan, V.; Petty, F.D. Exercise for Mental Health. Prim. Care Companion J. Clin. Psychiatry 2006, 8, 106. [Google Scholar] [CrossRef]
- Strickland, J.C.; Smith, M.A. The anxiolytic effects of resistance exercise. Front. Psychol. 2014, 5, 753. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Report from the Canadian Chronic Disease Surveillance System: Mood and Anxiety Disorders in Canada. 2016. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/report-canadian-chronic-disease-surveillance-system-mood-anxiety-disorders-canada-2016.html (accessed on 13 December 2022).
- Canadian Mental Health Association. The Relationship between Mental Health, Mental Illness and Chronic Physical Conditions. Available online: https://ontario.cmha.ca/documents/the-relationship-between-mental-health-mental-illness-and-chronic-physical-conditions/ (accessed on 12 December 2022).
- Kim, D. Correlation between physical function, cognitive function, and health-related quality of life in elderly persons. J. Phys. Ther. Sci. 2016, 28, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Noonan, A.M.; Brown, S.H.M. Paraspinal muscle pathophysiology associated with low back pain and spine degenerative disorders. JOR Spine 2021, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yan, B.; Jiao, Y.; Chen, Z.; Zheng, Y.; Lin, Y.; Cao, P. Correlation between the fatty infiltration of paraspinal muscles and disc degeneration and the underlying mechanism. BMC Musculoskelet. Disord. 2022, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, B.; Hubbard, J.C.; Gibbons, M.C.; Ruoss, S.; Zlomislic, V.; Allen, R.T.; Garfin, S.R.; Ward, S.R. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J. Orthop. Res. 2017, 35, 2700–2706. [Google Scholar] [CrossRef] [PubMed]

| Group | MC+ILEX n = 25 | GE n = 25 | p-Value |
|---|---|---|---|
| Age (year) (mean ± SD) | 45.16 ± 10.66 | 37.60 ± 11.60 | 0.020 # |
| Sex (male) | 5 (33.3%) | 10 (66.7%) | 0.123 * |
| Sex (female) | 20 (57.1%) | 15 (42.9%) | |
| Height (cm) | 169.68 ± 10.93 | 169.29 ± 7.86 | 0.887 # |
| Weight (kg) | 75.08 ± 16.39 | 76.39 ± 19.58 | 0.805 # |
| BMI | 26.09 ± 5.01 | 26.40 ± 5.22 | 0.834 # |
| LBP Length (months) | 73.52 ± 82.81 | 101.69 ± 105.62 | 0.299 # |
| NPR Scores | |||
| Baseline | 5.26 ± 1.75 | 5.19 ± 1.72 | 0.887 # |
| 6 weeks | 3.58 ± 1.78 | 3.63 ± 1.07 | 0.890 # |
| 12 weeks | 2.80 ± 1.81 | 3.41 ± 1.62 | 0.225 # |
| ODI Scores | |||
| Baseline | 29.40 ± 9.85 | 26.04 ± 10.03 | 0.238 # |
| 6 weeks | 22.96 ± 11.47 | 21.45 ± 10.05 | 0.637 # |
| 12 weeks | 19.70 ± 10.66 | 18.27 ± 7.05 | 0.756 # |
| SF-12 Scores | |||
| Baseline | 87.10 ± 12.88 | 79.62 ± 27.09 | 0.218 # |
| 6 weeks | 89.39 ± 12.92 | 85.60 ± 29.43 | 0.558 # |
| 12 weeks | 90.77 ± 24.33 | 83.53 ± 33.75 | 0.389 # |
| Variables | Measurement Period | MC+ILEX n = 25 | GE n = 25 | Main Effect of Group | Interaction Effect between Time and Group |
|---|---|---|---|---|---|
| L4/L5 MF CSA (cm2) | Baseline | 10.00 | 10.00 | p-value = 0.009 F = 7.55 df = 1 | p-value = 0.001 F = 8.33 df = 1.72 |
| 6 weeks (std. error) | 10.31 (0.13) * | 9.98 (0.14) | |||
| 12 weeks | 10.69 (0.15) * | 9.90 (0.17) | |||
| MD (95% CI) | 0.69 (0.38 to 1.00) * | −0.10 (−0.43 to 0.24) | |||
| Main effect of time | p-value = <0.001 F = 11.60 df = 2 | p-value = 0.71 F = 0.35 df = 2 | |||
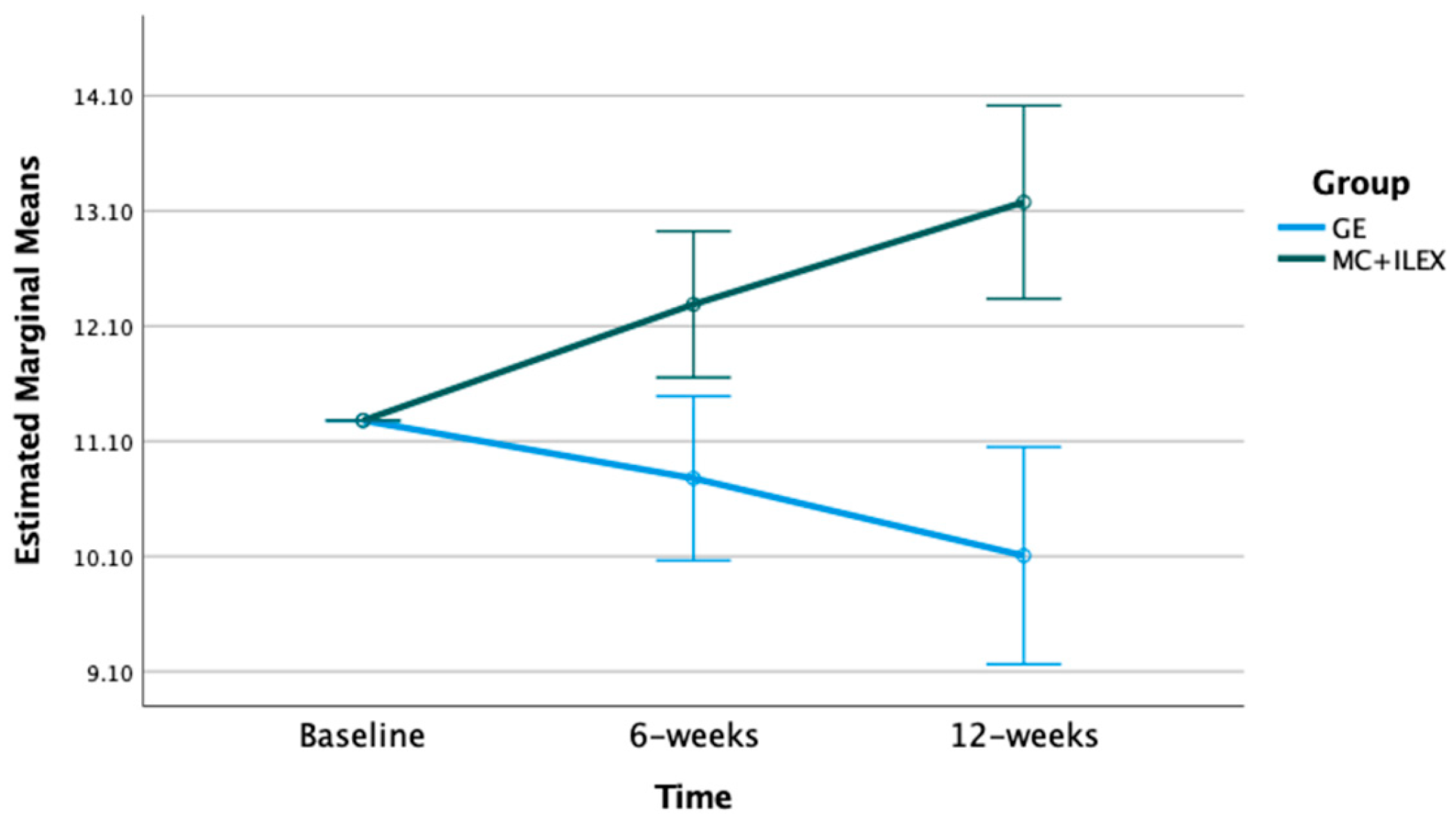
| L4/L5 ES CSA (cm2) | Baseline | 16.31 | 16.31 | p-value = 0.001 F = 12.49 df = 1 | p-value = 0.002 F = 6.53 df = 2 |
| 6 weeks (std. error) | 16.91 (0.21) * | 16.11 (0.22) | |||
| 12 weeks | 17.48 (0.27) * | 16.20 (0.29) | |||
| MD (95% CI) | 1.17 (0.63 to 1.71) * | −0.16 (−0.70 to −0.47) | |||
| Main effect of time | p-value = <0.001 F = 9.94 df = 2 | p-value = 0.68 F = 0.39 df = 2 | |||
| L5/S1 MF CSA (cm2) | Baseline | 11.88 | 11.88 | p-value = <0.001 F = 7.27 df = 1 | p-value = <0.001 F = 14.47 df = 2 |
| 6 weeks (std. error) | 12.10 (0.15) | 11.74 (0.17) | |||
| 12 weeks | 12.57 (0.15) * | 11.43 (0.17) * | |||
| MD (95% CI) | 0.69 (0.39 to 1.00) * | −0.45 (−0.80 to −0.10) * | |||
| Main effect of time | p-value = <0.001 F = 13.08 df = 2 | p-value = 0.013 F = 4.99 df = 2 | |||
| L5/S1 ES CSA (cm2) | Baseline | 11.28 | 11.28 | p-value = <0.001 F = 23.88 df = 1 | p-value = <0.001 F = 14.54 df = 2 |
| 6 weeks (std. error) | 12.29 (0.31) * | 10.78 (0.35) | |||
| 12 weeks | 13.18 (0.42) * | 10.11 (0.47) | |||
| MD (95% CI) | 1.90 (1.06 to 2.73) * | −1.174 (−2.12 to −0.23) * | |||
| Main effect of time | p-value = <0.001 F = 11.04 df = 2 | p-value = 0.054 F = 3.14 df = 2 |
| Variables | Measurement Period | MC+ILEX n = 25 | GE n = 25 | Main Effect of Group | Interaction Effect between Time and Group |
|---|---|---|---|---|---|
| L4/L5 MF FF (cm2) | Baseline | 25.91 | 25.91 | p-value = 0.891 F = 0.02 df = 1 | p-value = 0.388 F = 0.96 df = 2 |
| 6 weeks (std. error) | 25.89 (0.53) | 25.15 (0.52) | |||
| 12 weeks | 24.95 (0.67) | 25.52 (0.65) | |||
| MD (95% CI) | −0.96 (−2.31 to 0.39) | −0.39 (−1.71 to 0.92) | |||
| Main effect of time | p-value = 0.37 F = 1.11 df = 2 | p-value = 0.34 F = 1.11 df= 2 | |||
| L4/L5 ES FF (cm2) | Baseline | 33.48 | 33.48 | p-value = 0.96 F = 0.002 df = 1 | p-value = 0.97 F = 0.03 df = 2 |
| 6 weeks (std. error) | 33.21 (0.81) | 33.11 (0.79) | |||
| 12 weeks | 32.96 (0.59) | 33.14 (0.57) | |||
| MD (95% CI) | −0.52 (−1.70 to 0.67) | −0.34 (−1.50 to 0.82) | |||
| Main effect of time | p-value = 0.68 F = 0.389 df = 2 | p-value = 0.79 F = 0.235 df = 2 | |||
| L5/S1 MF FF (cm2) | Baseline | 27.92 | 27.92 | p-value = 0.37 F = 0.82 df = 1 | p-value = 0.46 F = 0.80 df = 2 |
| 6 weeks (std. error) | 27.80 (0.38) | 27.56 (0.39) | |||
| 12 weeks | 28.23 (0.53) | 27.43 (0.54) | |||
| MD (95% CI) | 0.32 (−0.76 to 1.40) | −0.49 (−1.59 to 0.62) | |||
| Main effect of time | p-value = 0.008 F = 5.62 df = 2 | p-value = 0.69 F = 0.39 df = 2 | |||
| L5/S1 ES FF (cm2) | Baseline | 41.38 | 41.38 | p-value = 0.12 F = 2.56 df = 1 | p-value = 0.24 F = 1.47 df = 2 |
| 6 weeks (std. error) | 39.34 (0.62) * | 40.94 (0.63) | |||
| 12 weeks | 39.28 (0.79) | 40.68 (0.81) | |||
| MD (95% CI) | −2.09 (−3.70 to −0.49) * | −0.70 (−2.35 to 0.95) | |||
| Main effect of time | p-value = 0.01 F = 5.62 df = 2 | p-value = 0.69 F = 0.38 df = 2 |
| Variables | Measurement Period | MC+ILEX n = 25 | GE n = 25 | Main Effect of Group | Interaction Effect between Time and Group |
|---|---|---|---|---|---|
| L4 | Baseline | 2.95 | 2.95 | p-value = 0.03 F = 4.81 df = 1 | p-value = <0.001 F = 8.72 df = 2 |
| 6 weeks (std. error) | 3.09 (0.03) * | 3.00 (0.03) | |||
| 12 weeks | 3.17 (0.04) * | 2.95 (0.04) | |||
| MD (95% CI) | 0.22 (0.15 to 0.29) * | −0.002 (−0.08 to 0.73) | |||
| Main effect of time | p-value = <0.001 F = 20.91 df = 2 | p-value = 0.28 F = 1.32 df = 2 | |||
| L5 | Baseline | 2.83 | 2.83 | p-value = <0.001 F = 19.04 df = 1 | p-value = <0.001 F = 11.04 df = 2 |
| 6 weeks (std. error) | 2.99 (0.03) * | 2.85 (0.03) | |||
| 12 weeks | 3.08 (0.04) * | 2.87 (0.04) | |||
| MD (95% CI) | 0.25 (0.18 to 0.32) * | 0.35 (−0.04 to 0.11) | |||
| Main effect of time | p-value = <0.001 F = 27.06 df = 2 | p-value = 0.65 F = 0.43 df = 2 |
| Variables | Measurement Period | MC+ILEX n = 25 | GE n = 25 | Main Effect of Group | Interaction Effect between Time and Group |
|---|---|---|---|---|---|
| L4 | Baseline | 15.76 | 15.76 | p-value = 0.96 F = 0.002 df = 1 | p-value = 0.79 F = 0.24 df = 2 |
| 6 weeks (std. error) | 15.36 (1.31) | 14.83 (1.39) | |||
| 12 weeks | 15.99 (0.91) | 16.65 (0.97) | |||
| MD (95% CI) | 0.22 (−1.60 to 2.05) | 0.88 (−1.06 to 2.83) | |||
| Main effect of time | p-value = 0.88 F = 0.13 df = 2 | p-value = 0.37 F = 1.03 df = 2 | |||
| L5 | Baseline | 11.10 | 11.10 | p-value = 0.93 F = 0.007 df = 2 | p-value = 0.98 F = 0.03 df = 2 |
| 6 weeks (std. error) | 10.71 (0.95) | 10.43 (1.01) | |||
| 12 weeks | 11.06 (1.14) | 11.12 (1.21) | |||
| MD (95% CI) | −0.04 (−2.33 to 2.25) | 0.02 (−2.43 to 2.46) | |||
| Main effect of time | p-value = 0.91 F = 0.10 df = 2 | p-value = 0.77 F= 0.26 df = 2 |
| Variables | Measurement Period | MC + ILEX n = 25 | GE n = 25 | Main Effect of Group | Interaction Effect between Time and Group |
|---|---|---|---|---|---|
| Pain score (NPR) | Baseline | 5.23 ± 0.34 | 5.20 ± 0.42 | p-value = 0.52 F = 0.43 df = 1 | p-value = 0.34 F = 1.10 df = 2 |
| 6 weeks (std. error) | 3.58 ± 0.33 * | 3.73 ± 0.41 * | |||
| 12 weeks | 2.80 ± 0.38 * | 3.56 ± 0.46 | |||
| MD (95% CI) | −2.43 (−3.26 to 1.61) * | −1.64 (−2.64 to 0.63) | |||
| Main effect of time | p-value = <0.001 F = 20.69 df = 2 | p-value = <0.001 F = 8.46 df = 2 | |||
| Disability score (ODI) | Baseline | 29.54 ± 2.05 | 27.52 ± 2.19 | p-value = 0.62 F = 0.25 df = 1 | p-value = 0.84 F = 0.11 df = 1.49 |
| 6 weeks (std. error) | 23.08 ± 2.23 * | 22.00 ± 2.38 * | |||
| 12 weeks | 19.08 ± 1.95 * | 18.19 ± 2.08 * | |||
| MD (95% CI) | −10.46 (−14.55 to −6.37) * | −9.33 (−13.71 to −4.96) * | |||
| Main effect of time | p-value = <0.001 F = 14.46 df = 2 | p-value = <0.001 F = 10.40 df = 2 | |||
| SF-12 physical (PCS) | Baseline | 38.78 ± 1.76 | 40.75 ± 1.88 | p-value = 0.29 F = 1.17 df = 1 | p-value = 0.32 F = 1.14 df = 2 |
| 6 weeks (std. error) | 42.26 ± 1.53 | 46.16 ± 1.64 * | |||
| 12 weeks | 45.20 ± 1.51 * | 45.44 ± 1.61 | |||
| MD (95% CI) | 6.42 (2.67 to 10.18) * | 4.70 (0.68 to 8.71) * | |||
| Main effect of time | p-value = 0.004 F = 6.23 df = 2 | p-value = 0.03 F = 4.04 df = 2 | |||
| SF-12 mental (MCS) | Baseline | 48.83 ± 2.12 | 45.67 ± 2.27 | p-value = 0.71 F = 0.14 df = 1 | p-value = 0.37 F = 1.02 df = 2 |
| 6 weeks (std. error) | 47.03 ± 1.97 | 46.43 ± 2.11 | |||
| 12 weeks | 49.34 ± 2.49 | 49.85 ± 2.67 | |||
| MD (95% CI) | 0.52 (−3.07 to 4.10) | 4.17 (0.35 to 8.01) * | |||
| Main effect of time | p-value = 0.41 F = 0.90 df = 2 | p-value = 0.07 F = 2.78 df = 2 |
| Group | ΔNPR [95% CI] | ΔODI [95% CI] | ΔSF12-M [95% CI] | ΔSF12-P [95% CI] | ΔSF12 [95% CI] |
|---|---|---|---|---|---|
| ΔMF CSA L4/L5 | 0.08 [−0.22 to 0.36] | 0.21 [−0.09 to 0.47] | −0.10 [−0.38 to 0.20] | −0.15 [−0.43 to 0.15] | −0.16 [−0.44 to 0.14] |
| ΔMF CSA L5/S1 | −0.13 [−0.42 to 0.18] | −0.09 [−0.38 to 0.22] | 0.01 [−0.29 to 0.31] | −0.07 [−0.37 to 0.23] | −0.04 [−0.34 to 0.26] |
| ΔES CSA L4/L5 | 0.02 [−0.29 to 0.31] | −0.07 [−0.36 to 0.22] | −0.14 [−0.42 to 0.16] | 0.12 [−0.18 to 0.40] | −0.01 [−0.30 to 0.29] |
| ΔES CSA L5/S1 | −0.15 [−0.43 to 0.16] | 0.16 [−0.19 to 0.40] | −0.29 [−0.55 to 0.01] | −0.05 [−0.35 to 0.25] | −0.22 [−0.49 to 0.09] |
| ΔMF FF L4/5 | −0.00 [−0.31 to 0.30] | 0.22 [−0.10 to 0.49] | 0.03 [−0.28 to 0.33] | −0.10 [−0.39 to 0.22] | −0.05 [−0.35 to 0.27] |
| ΔMF FF L5/S1 | −0.16 [−0.45 to 0.17] | −0.32 [−0.57 to 0.00] | 0.06 [−0.26 to 0.37] | 0.18 [−0.15 to 0.47] | 0.15 [−0.17 to 0.45] |
| ΔES FF L4/L5 | −0.02 [−0.32 to 0.29] | 0.21 [−0.11 to 0.49] | −0.08 [−0.38 to 0.24] | −0.13 [−0.42 to 0.18] | −0.14 [−0.43 to 0.18] |
| ΔES FF L5/S1 | 0.12 [−0.20 to 0.42] | 0.14 [−0.18 to 0.44] | 0.03 [−0.29 to 0.34] | −0.07 [−0.38 to 0.25] | −0.03 [−0.34 to 0.29] |
| ΔMF Thickness L4 | −0.05 [−0.34 to 0.25] | −0.12 [−0.40 to 0.18] | −0.31 * [−0.55 to −0.01] | 0.06 [−0.24 to 0.34] | −0.16 [−0.43 to 0.14] |
| ΔMF Thickness L5 | 0.02 [−0.27 to 0.31] | −0.08 [−0.37 to 0.22] | −0.37 * [−0.60 to −0.09] | 0.16 [−0.14 to 0.43] | −0.13 [−0.41 to 0.17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortin, M.; Rye, M.; Roussac, A.; Montpetit, C.; Burdick, J.; Naghdi, N.; Rosenstein, B.; Bertrand, C.; Macedo, L.G.; Elliott, J.M.; et al. The Effects of Combined Motor Control and Isolated Extensor Strengthening versus General Exercise on Paraspinal Muscle Morphology, Composition, and Function in Patients with Chronic Low Back Pain: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 5920. https://doi.org/10.3390/jcm12185920
Fortin M, Rye M, Roussac A, Montpetit C, Burdick J, Naghdi N, Rosenstein B, Bertrand C, Macedo LG, Elliott JM, et al. The Effects of Combined Motor Control and Isolated Extensor Strengthening versus General Exercise on Paraspinal Muscle Morphology, Composition, and Function in Patients with Chronic Low Back Pain: A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(18):5920. https://doi.org/10.3390/jcm12185920
Chicago/Turabian StyleFortin, Maryse, Meaghan Rye, Alexa Roussac, Chanelle Montpetit, Jessica Burdick, Neda Naghdi, Brent Rosenstein, Cleo Bertrand, Luciana G. Macedo, James M. Elliott, and et al. 2023. "The Effects of Combined Motor Control and Isolated Extensor Strengthening versus General Exercise on Paraspinal Muscle Morphology, Composition, and Function in Patients with Chronic Low Back Pain: A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 18: 5920. https://doi.org/10.3390/jcm12185920
APA StyleFortin, M., Rye, M., Roussac, A., Montpetit, C., Burdick, J., Naghdi, N., Rosenstein, B., Bertrand, C., Macedo, L. G., Elliott, J. M., Dover, G., DeMont, R., Weber, M. H., & Pepin, V. (2023). The Effects of Combined Motor Control and Isolated Extensor Strengthening versus General Exercise on Paraspinal Muscle Morphology, Composition, and Function in Patients with Chronic Low Back Pain: A Randomized Controlled Trial. Journal of Clinical Medicine, 12(18), 5920. https://doi.org/10.3390/jcm12185920







