Magnetic Resonance Imaging Evaluation of Multifidus Muscle in Patients with Low Back Pain after Microlumbar Discectomy Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Procedure
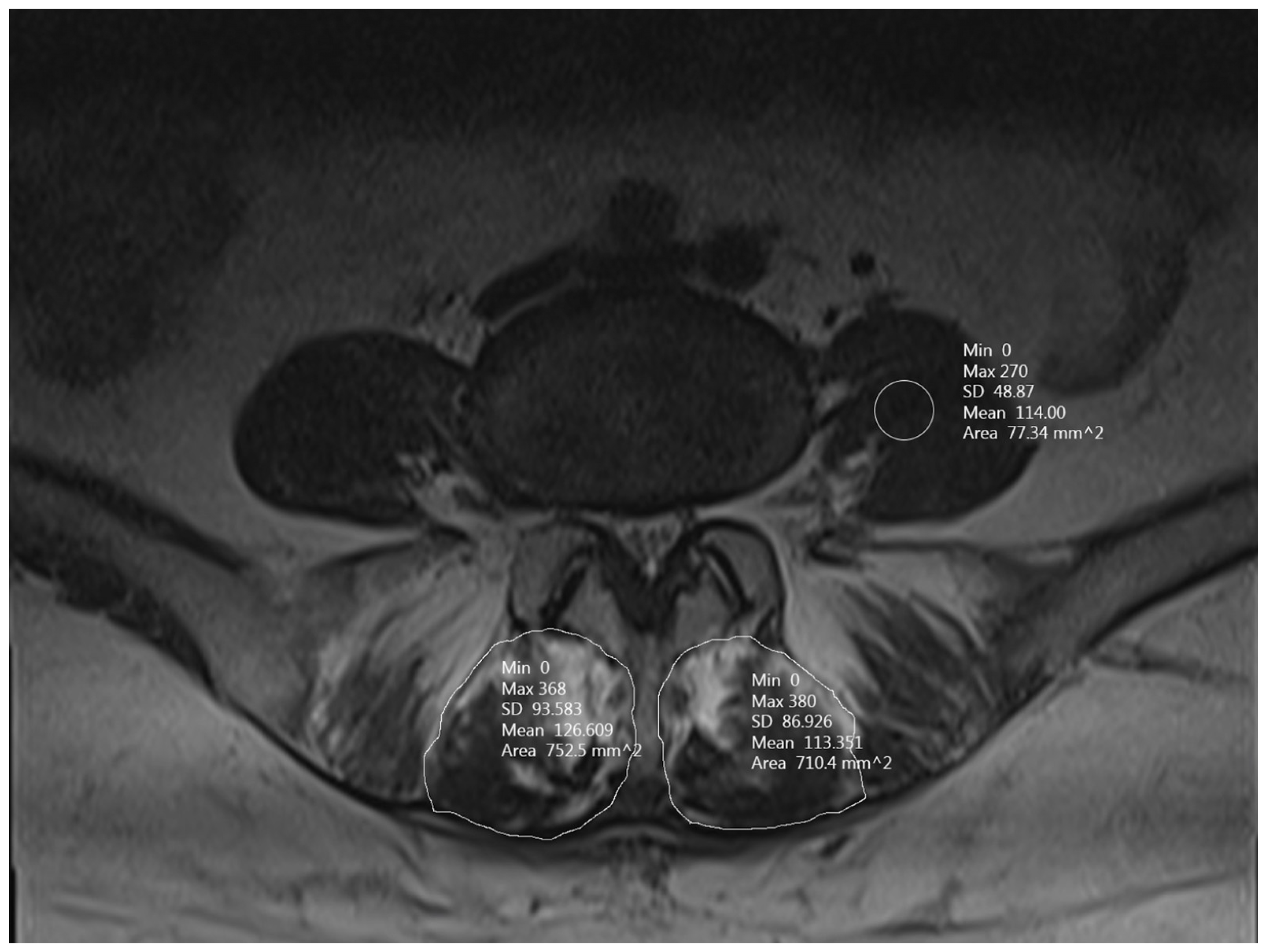
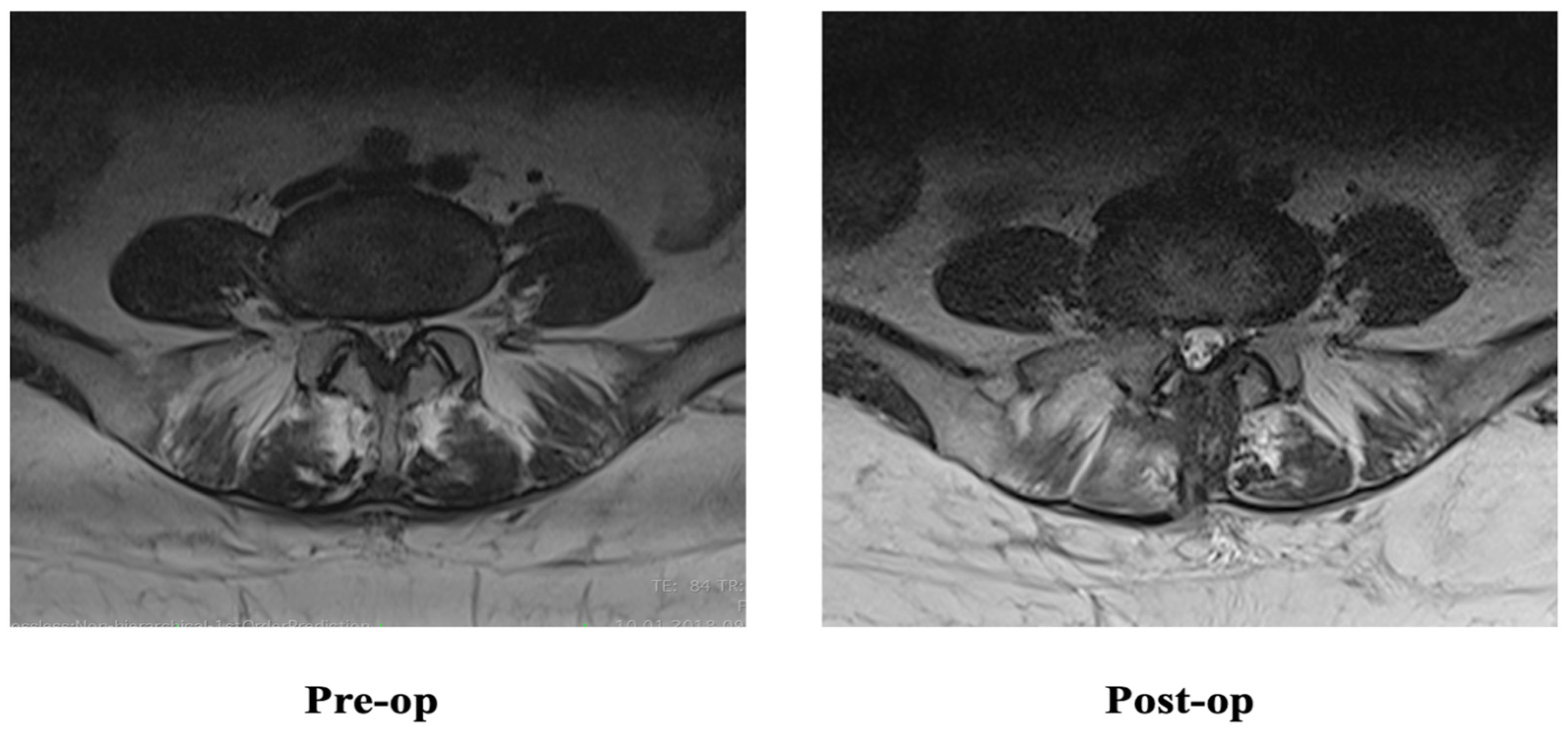
2.3. Radiological Evaluation
2.4. Outcome Data and Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Correlation
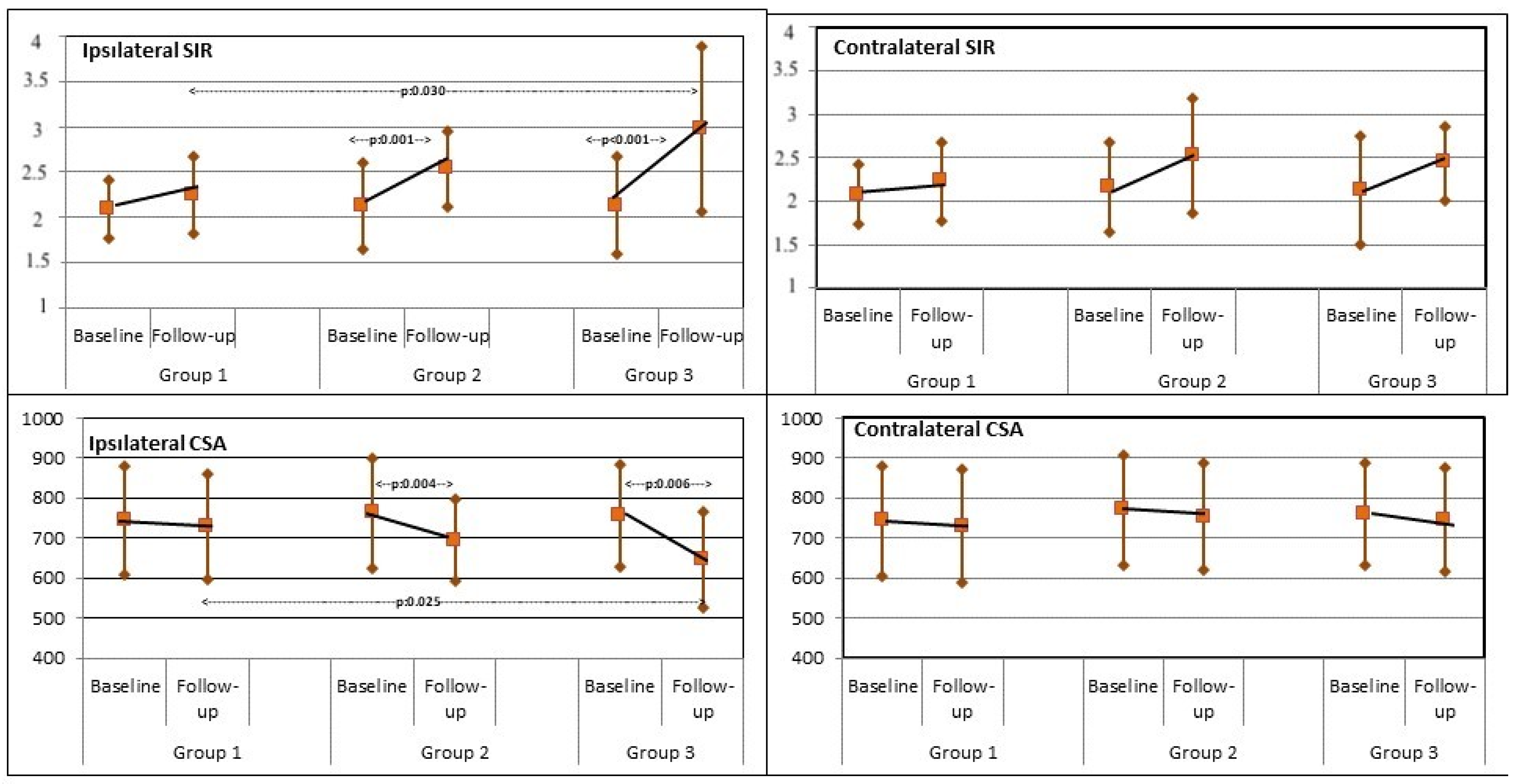
3.3. MFM SIR
3.4. MFM CSA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, S.; Horton, R. Low back pain: A major global challenge. Lancet 2018, 391, 2302. [Google Scholar] [CrossRef]
- Kjaer, P.; Tunset, A.; Boyle, E.; Jensen, T.S. Progression of lumbar disc herniations over an eight-year period in a group of adult Danes from the general population—A longitudinal MRI study using quantitative measures. BMC Musculoskelet. Disord. 2016, 17, 26. [Google Scholar] [CrossRef][Green Version]
- Zielinska, N.; Podgórski, M.; Haładaj, R.; Polguj, M.; Olewnik, Ł. Risk Factors of Intervertebral Disc Pathology—A Point of View Formerly and Today—A Review. J. Clin. Med. 2021, 10, 409. [Google Scholar] [CrossRef]
- Jin, L.; Yin, Y.; Chen, W. Role of the Lumbosacral Transition Vertebra and Vertebral Lamina in the Pathogenesis of Lumbar Disc Herniation. Orthop. Surg. 2021, 13, 2355–2362. [Google Scholar] [CrossRef]
- Foster, N.; Anema, J.; Cherkin, D. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Kang, S. Nonsurgical Treatments for Patients with Radicular Pain from Lumbosacral Disc Herniation. Spine J. 2019, 19, 1478–1489. [Google Scholar] [CrossRef]
- Atlas, S.J.; Keller, R.B.; Wu, Y.A.; Deyo, R.A.; Singer, D.E. Longterm outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine (Phila Pa 1976) 2005, 30, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, D.; Hwang, S.; Easa, J. An Evidence-based Clinical Guideline for the Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy. Spine J. 2014, 14, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Haines, S.; Jordan, N.; Boen, J. Discectomy Strategies for Lumbar Disc Herniation: Results of the LAPDOG Trial. J. Clin. Neurosci. 2002, 9, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Thomé, C.; Barth, M.; Scharf, J.; Schmiedek, P. Outcome after lumbar sequestrectomy compared with microdiscectomy: A prospective randomized study. J. Neurosurg. Spine 2005, 2, 271–278. [Google Scholar] [CrossRef]
- Silverplats, K.; Lind, B.; Zoëga, B. Clinical Factors of Importance for Outcome After Lumbar Disc Herniation Surgery: Long-term Follow-up. Eur. Spine J. 2010, 19, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Virk, S.; Diwan, A.; Phillips, F.; Sandhu, H.; Khan, S. What is the Rate of Revision Discectomies After Primary Discectomy on a National Scale? Clin. Orthop. 2017, 475, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Woodham, M.; Woodham, A. The Role of the Lumbar Multifidus in Chronic Low Back Pain: A Review. PM R 2010, 2, 142–167. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.; Wolf, S.; Claes, L.; Arand, M.; Wiesend, A. Stability of the lumbar spine with different muscle groups. Spine 1995, 20, 192–198. [Google Scholar] [CrossRef]
- Fan, S.; Hu, Z.; Zhao, F.; Zhao, X.; Huang, Y.; Fang, X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: Minimally invasive procedure versus conventional open approach. Eur. Spine J. 2010, 19, 316–324. [Google Scholar] [CrossRef]
- Tsutsumimoto, T.; Shimogata, M.; Ohta, H.; Misawa, H. Mini-open Versus Conventional Open Posterior Lumbar Interbody Fusion for the Treatment of Lumbar Degenerative Spondylolisthesis: Comparison of Paraspinal Muscle Damage and Slip Reduction. Spine 2009, 34, 1923–1928. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Park, E. Multifidus Muscle Changes After Biportal Endoscopic Spinal Surgery: Magnetic Resonance Imaging Evaluation. World Neurosurg. 2019, 130, e525–e534. [Google Scholar] [CrossRef]
- Sadeghi, S.; Bible, J.E.; Cortes, D.H. Quantifying Dysfunction of the Lumbar Multifidus Muscle After Radiofrequency Neurotomy and Fusion Surgery: A Preliminary Study. J. Eng. Sci. Med. Diagn. Ther. 2020, 3, 041001. [Google Scholar]
- Smuck, M.; Crisostomo, R.; Demirjian, R.; Fitch, D.; Kennedy, D.; Geisser, M. Morphologic Changes in the Lumbar Spine After Lumbar Medial Branch Radiofrequency Neurotomy: A Quantitative Radiological Study. Spine J. 2015, 15, 1415–1421. [Google Scholar] [CrossRef]
- Pishnamaz, M.; Schemmann, U.; Herren, C. Muscular Changes After Minimally Invasive Versus Open Spinal Stabilization of Thoracolumbar Fractures: A Literature Review. J. Musculoskelet. Neuronal Interact. 2018, 18, 62–70. [Google Scholar]
- Gold, G. Dynamic and Functional Imaging of the Musculoskeletal System. Semin. Musculoskelet. Radiol. 2003, 7, 245–248. [Google Scholar]
- Kikuchi, Y.; Nakamura, T.; Takayama, S.; Horiuchi, Y.; Toyama, Y. MR imaging in the diagnosis of denervated and reinnervated skeletal muscles: Experimental study in rats. Radiology 2003, 229, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Gejo, R.; Kawaguchi, Y.; Kondoh, T.; Tabuchi, E.; Matsui, H.; Torii, K.; Ono, T.; Kimura, T. Magnetic resonance imaging and histologic evidence of postoperative back muscle injury in rats. Spine 2000, 25, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Storheim, K.; Berg, L.; Hellum, C.; Gjertsen, Ø.; Neckelmann, G.; Espeland, A.; Keller, A.; Norwegian Spine Study Group. Fat in the lumbar multifidus muscles—Predictive value and change following disc prosthesis. surgery and multidisciplinary rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up of a randomized trial. BMC Musculoskelet. Disord. 2017, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Faur, C.; Patrascu, J.; Haragus, H.; Anglitoiu, B. Correlation between multifidus fatty atrophy and lumbar disc degeneration in low back pain. BMC Musculoskelet. Disord. 2019, 20, 414. [Google Scholar] [CrossRef]
- Koebbe, C.J.; Maroon, J.C.; Abla, A.; El-Kadi, H.; Bost, J. Lumbar microdiscectomy: A historical perspective and current technical considerations. Neurosurg. Focus. 2002, 13, E3. [Google Scholar] [CrossRef]
- Dowling, T.J.; Munakomi, S.; Dowling, T.J. Microdiscectomy. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tonomura, H.; Hatta, Y.; Mikami, Y.; Ikeda, T.; Harada, T.; Nagae, M.; Koike, H.; Hase, H.; Kubo, T. Magnetic Resonance Imaging Evaluation of the Effects of Surgical Invasiveness on Paravertebral Muscles After Muscle-preserving Interlaminar Decompression (MILD). Clin. Spine Surg. 2017, 30, E76–E82. [Google Scholar] [CrossRef]
- Langley, G.B.; Sheppeard, H. The visual analogue scale: Its use in pain measurement. Rheumatol. Int. 1985, 5, 145–148. [Google Scholar] [CrossRef]
- Stevens, K.; Spenciner, D.; Griffiths, K.; Kim, K.; ZwienenbergLee, M.; Alamin, T. Comparison of Minimally Invasive and Conventional Open Posterolateral Lumbar Fusion Using Magnetic Resonance Imaging and Retraction Pressure Studies. J. Spinal Disord. Tech. 2006, 19, 77–86. [Google Scholar] [CrossRef]
- Suwa, H.; Hanakita, J.; Ohshita, N.; Gotoh, K.; Matsuoka, N.; Morizane, A. Postoperative changes in paraspinal muscle thickness after various lumbar back surgery procedures. Neurol. Med. Chir. 2000, 40, 151–154. [Google Scholar] [CrossRef]
- Taylor, H.; McGregor, A.; Medhi-Zadeh, S.; Richards, S.; Kahn, N.; Zadeh, J. The Impact of Self-retaining Retractors on the Paraspinal Muscles During Posterior Spinal Surgery. Spine 2002, 27, 2758–2762. [Google Scholar] [CrossRef]
- Laasonen, E. Atrophy of Sacrospinal Muscle Groups in Patients with Chronic, Diffusely Radiating Lumbar Back Pain. Neuroradiology 1984, 26, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, T.; Herno, A.; Palja¨rvi, L.; Airaksinen, O.; Partanen, J.; Tapaninaho, A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine 1993, 18, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.; Brand, R.; van der Kallen, B.; Lycklama à Nijeholt, G.; Peul, W. Does minimally invasive lumbar disc surgery result in less muscle injury than conventional surgery? A randomized controlled trial. Eur. Spine J. 2011, 20, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ozalp, H. Disk Hernilerinde Altın Standart: Mikrodiskektomi. Turk Norosirurji Dergsi 2018, 28, 196–200. [Google Scholar]
- Kazemi, N.; Crew, L.K.; Tredway, T.L. The future of spine surgery: New horizons in the treatment of spinal disorders. Surg. Neurol. Int. 2013, 4 (Suppl. S1), S15–S21. [Google Scholar]
- Beneck, G.; Kulig, K. Multifidus atrophy is localized and bilateral in active persons with chronic unilateral low back pain. Arch. Phys. Med. Rehabil. 2012, 93, 300–306. [Google Scholar] [CrossRef]
- Wallwork, T.; Stanton, W.; Freke, M.; Hides, J. The Effect of Chronic Low Back Pain on Size and Contraction of the Lumbar Multifidus Muscle. Man Ther. 2009, 14, 496–500. [Google Scholar] [CrossRef]
- He, K.; Head, J.; Mouchtouris, N.; Hines, K.; Shea, P.; Schmidt, R.; Hoelscher, C.; Stricsek, G.; Harrop, J.; Sharan, A. The Implications of Paraspinal Muscle Atrophy in Low Back Pain, Thoracolumbar Pathology, and Clinical Outcomes After Spine Surgery: A Review of the Literature. Glob. Spine J. 2020, 10, 657–666. [Google Scholar] [CrossRef]
- Lu, H.; Wang, L.; Li, M.; Chen, X. The Association Between Changes in Multifidus Muscle Morphology and Back Pain Scores Following Discectomy Surgery for Lumbar Disc Herniation: A Systematic Review and Meta-analysis. Eur. Spine J. 2022, 31, 1784–1794. [Google Scholar] [CrossRef]
- Gejo, R.; Matsui, H.; Kawaguchi, Y.; Ishihara, H.; Tsuji, H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine 1999, 24, 1023–1028. [Google Scholar] [CrossRef]
- Gille, O.; Jolivet, E.; Dousset, V.; Degrise, C.; Obeid, I.; Vital, J.M.; Skalli, W. Erector spinae muscle changes on magnetic resonance imaging following lumbar surgery through a posterior approach. Spine 2007, 32, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Motosuneya, T.; Asazuma, T.; Tsuji, T.; Watanabe, H.; Nakayama, Y.; Nemoto, K. Postoperative change of the cross-sectional area of back musculature after 5 surgical procedures as assessed by magnetic resonance imaging. J. Spinal Disord. Tech. 2006, 19, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Datta, G.; Gnanalingham, K.; Peterson, D.; Mendoza, N.; O’Neill, K.; Van Dellen, J.; McGregor, A.; Hughes, S. Back pain and disability after lumbar laminectomy: Is there a relationship to muscle retraction? Neurosurgery 2004, 54, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.; Nieborg, A.; Brand, R.; Peul, W. Serum creatine phosphokinase as an indicator of muscle injury after various spinal and nonspinal surgical procedures. J. Neurosurg. Spine 2007, 7, 282–286. [Google Scholar] [CrossRef]
- Rosatelli, A.L.; Ravichandiran, K.; Agur, A.M. Threedimensional study of the musculotendinous architecture of lumbar multifidus and its functional implications. Clin. Anat. 2008, 21, 539–546. [Google Scholar] [CrossRef]
- Wang, X.; Jia, R.; Li, J. Research Progress on the Mechanism of Lumbarmultifidus Injury and Degeneration. Oxid. Med. Cell. Longev. 2021, 2021, 6629037. [Google Scholar] [CrossRef]
- Shahidi, B.; Hubbard, J.; Gibbons, M.; Ruoss, S.; Zlomislic, V.; Allen, R.; Garfin, S.; Ward, S. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J. Orthop. Res. 2017, 35, 2700–2706. [Google Scholar] [CrossRef]
| Variables | Value |
|---|---|
| Patients | 79 |
| Age | 55.89 ± 9.22 |
| Gender | |
| Female | 29 (36.7%) |
| Male | 50 (63.3%) |
| BMI | 28 ± 6.14 |
| Operation level | |
| L3–L4 | 16 (20.2%) |
| L4–L5 | 33 (41.7%) |
| L5–S1 | 30 (37.9%) |
| Variables | Group 1 | Group 2 | Group 3 | p |
|---|---|---|---|---|
| Patients (n) | 22 | 27 | 30 | |
| Age | 58.63 ± 5.94 | 53.25 ± 9.73 | 56.26 ± 10.29 | 0.121 |
| Gender | 0.87 | |||
| Female | 8 | 9 | 12 | |
| Male | 14 | 18 | 18 | |
| BMI | 27.18 ± 1.53 | 26.71 ± 1.68 | 27.42 ± 2.21 | |
| Surgery level | 0.45 | |||
| L3–L4 | 3 | 8 | 5 | |
| L4–L5 | 12 | 9 | 12 | |
| L5–S1 | 7 | 10 | 13 |
| Total | Group 1 (n = 22) | Group 2 (n = 27) | Group 3 (n = 30) | p | |
|---|---|---|---|---|---|
| Follow-up point (days) | 130.41 ± 58.13 | 11.64 ± 8.58 | 95.16 ± 44.25 | 284.43 ± 84.23 | <0.001 |
| Surgery time (minutes) | 75.11 ± 22.8 | 72.56 ± 22.57 | 75.65 ± 25.52 | 77.12 ± 20.14 | 0.773 |
| VAS score | |||||
| Baseline | 7.05 ± 1.18 | 7.13 ± 1.20 | 7.14 ± 1.26 | 6.9 ± 1.12 | 0.69 |
| Follow-up point | 4.59 ± 1.21 | 4.22 ± 1.34 | 4.77 ± 1.31 | 4.65 ± 0.98 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, H.C.; Aydin, S.O. Magnetic Resonance Imaging Evaluation of Multifidus Muscle in Patients with Low Back Pain after Microlumbar Discectomy Surgery. J. Clin. Med. 2023, 12, 6122. https://doi.org/10.3390/jcm12196122
Kose HC, Aydin SO. Magnetic Resonance Imaging Evaluation of Multifidus Muscle in Patients with Low Back Pain after Microlumbar Discectomy Surgery. Journal of Clinical Medicine. 2023; 12(19):6122. https://doi.org/10.3390/jcm12196122
Chicago/Turabian StyleKose, Halil Cihan, and Serdar Onur Aydin. 2023. "Magnetic Resonance Imaging Evaluation of Multifidus Muscle in Patients with Low Back Pain after Microlumbar Discectomy Surgery" Journal of Clinical Medicine 12, no. 19: 6122. https://doi.org/10.3390/jcm12196122
APA StyleKose, H. C., & Aydin, S. O. (2023). Magnetic Resonance Imaging Evaluation of Multifidus Muscle in Patients with Low Back Pain after Microlumbar Discectomy Surgery. Journal of Clinical Medicine, 12(19), 6122. https://doi.org/10.3390/jcm12196122





