Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSAS | obstructive sleep apnea syndrome |
| AHI | Apnea–Hypopnea Index |
| PG | polygraphy |
| PSG | polysomnography |
| HRP | home cardiorespiratory polygraphy |
| BMI | Body Mass Index |
| TST | total sleep time |
| IQR | interquartile range |
References
- American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am. J. Respir. Crit. Care Med. 1996, 153, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Gozal, D.; Kheirandish-Gozal, L. The Status of Pediatric Obstructive Sleep Apnea in 2015: Progress? YES!! More Questions? Definitely YES!! Curr. Sleep Med. Rep. 2016, 2, 20–30. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of pediatric obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Montgomery-Downs, H.; O’Brien, L.M.; Holbrook, C.R.; Gozal, D. Snoring and sleep-disordered breathing in young children: Subjective and objective correlates. Sleep 2004, 27, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.S.; Carroll, J.L.; Jeffries, J.L.; Grone, C.; Bean, J.A.; Chini, B.; Bokulic, R.; Daniels, S.R. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2004, 169, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Greene, M.G.; Carroll, J.L. Blood pressure in children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1998, 157, 1098–1103. [Google Scholar] [CrossRef]
- Amin, R.S.; Kimball, T.R.; Bean, J.A.; Jeffries, J.L.; Willging, J.P.; Cotton, R.T.; Witt, S.A.; Glascock, B.J.; Daniels, S.R. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 165, 1395–1399. [Google Scholar] [CrossRef]
- Amin, R.; Somers, V.K.; McConnell, K.; Willging, P.; Myer, C.; Sherman, M.; McPhail, G.; Morgenthal, A.; Fenchel, M.; Bean, J.; et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension 2008, 51, 84–91. [Google Scholar] [CrossRef]
- Gozal, D.; Lipton, A.J.; Jones, K.L. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep 2002, 25, 59–65. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L.; Serpero, L.D.; Sans Capdevila, O.; Dayyat, E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: Effect of adenotonsillectomy. Circulation 2007, 116, 2307–2314. [Google Scholar] [CrossRef]
- Gozal, D. Sleep-disordered breathing and school performance in children. Pediatrics 1998, 102, 616–620. [Google Scholar] [CrossRef]
- Gozal, D.; Crabtree, V.M.; Sans Capdevila, O.; Witcher, L.A.; Kheirandish-Gozal, L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am. J. Respir. Crit. Care Med. 2007, 176, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Sans Capdevila, O.; Crabtree, V.M.; Kheirandish-Gozal, L.; Gozal, D. Increased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep-disordered breathing: A communitybased study. Pediatrics 2008, 121, e1208–e1214. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P.; Löppönen, T.; Tolonen, U.; Lanning, P.; Knip, M.; Löppönen, H. Growth and biomedical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics 2002, 109, e55. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kheirandish-Gozal, L.; Spruyt, K.; Mitchell, R.B.; Promchiarak, J.; Simakajornboon, N.; Kaditis, A.G.; Splaingard, D.; Splaingard, M.; Brooks, L.; et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: A multicenter retrospective study. Am. J. Respir. Crit. Care Med. 2010, 182, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Kljajic, Z.; Roje, Z.; Becic, K.; Capkun, V. Obstructive sleep apnea in children: How it affects parental psychological status? Int. J. Pediatr. Otorhinolaryngol. 2019, 117, 157–162. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Yang, W.; Shen, T.; Xue, P.; Yan, X.; Chen, D.; Qiao, Y.; Chen, M.; Ren, R.; et al. Correlations between obstructive sleep apnea and adenotonsillar hypertrophy in children of different weight status. Sci. Rep. 2019, 9, 11455. [Google Scholar] [CrossRef]
- Alonso-Álvarez, M.L.; Terán-Santos, J.; Ordax Carbajo, E.; Cordero-Guevara, J.A.; Navazo-Egüia, A.I.; Kheirandish-Gozal, L.; Gozal, D. Reliability of home respiratory polygraphy for the diagnosis of sleep apnea in children. Chest 2015, 147, 1020–1028. [Google Scholar] [CrossRef]
- Baugh, R.F.; Archer, S.M.; Mitchell, R.B.; Rosenfeld, R.M.; Amin, R.; Burns, J.J.; Darrow, D.H.; Giordano, T.; Litman, R.S.; Li, K.K.; et al. Clinical practice guideline: Tonsillectomy in children. Otolaryngol. Head. Neck Surg. 2011, 144, S1–S30. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical practice guideline: Tonsillectomy in children (update) executive summary. Otolaryngol. Head Neck Surg. 2019, 160, 187–205. [Google Scholar] [CrossRef]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef]
- Goldstein, N.; Fatima, M.; Campbell, T.; Rosenfeld, R.M. Child behavior and quality of life before and after tonsillectomy and adenoidectomy. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Kelly, J.; Call, E.; Yao, N. Quality of life after adenotonsillectomy for obstructive sleep apnea in children. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 190–194. [Google Scholar] [CrossRef]
- Flanary, V. Long-term effect of adenotonsillectomy on quality of life in pediatric patients. Laryngoscope 2003, 113, 1639–1644. [Google Scholar] [CrossRef]
- Brietzke, S.E.; Gallagher, D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: A meta-analysis. Otolaryngol. Head Neck Surg. 2006, 134, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Wilson, M.; Lin, H.C.; Chang, H.W. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol. Head Neck Surg. 2009, 140, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Anthony, L.; Somers, V.; Fenchel, M.; McConnell, K.; Jefferies, J.; Willging, P.; Kalra, M.; Daniels, S. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am. J. Respir. Crit. Care Med. 2008, 177, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.B.; Kelly, J. Outcome of adenotonsillectomy for severe obstructive sleep apnea in children. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1375–1379. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Kelly, J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol. Head Neck Surg. 2007, 137, 43–48. [Google Scholar] [CrossRef]
- Guilleminault, C.; Li, K.; Quo, S.; Inouye, R.N. A prospective study on the surgical outcomes of children with sleep-disordered breathing. Sleep 2004, 27, 95–100. [Google Scholar] [PubMed]
- Guilleminault, C.; Li, K.K.; Khramtsov, A.; Pelayo, R.; Martinez, S. Sleep disordered breathing: Surgical outcomes in prepubertal children. Laryngoscope 2004, 114, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.B.; Kelly, J. Outcome of adenotonsillectomy for obstructive sleep apnea in children under 3 years. Otolaryngol. Head Neck Surg. 2005, 132, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Tauman, R.; Gulliver, T.E.; Krishna, J.; Montgomery-Downs, H.E.; O’Brien, L.M.; Ivanenko, A.; Gozal, D. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J. Pediatr. 2006, 149, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, C.M.; Mitchell, R.B.; Schubert, C.; Rudnick, E.F. Pediatric obstructive sleep apnea and quality of life: A metaanalysis. Otolaryngol. Head Neck Surg. 2008, 138, 265. [Google Scholar] [CrossRef]
- Øverland, B.; Berdal, H.; Akre, H. Obstructive sleep apnea in 2-6 year old children referred for adenotonsillectomy. Eur. Arch. Otorhinolaryngol. 2019, 276, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, H.; Zhang, G.H.; Li, P.; Yang, Q.T.; Liu, X.; Li, Y. Outcome of adenotonsillectomy for obstructive sleep apnea syndrome in children. Ann. Otol. Rhinol. Laryngol. 2010, 119, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- Barlow, S.E. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120, S164–S192. [Google Scholar] [CrossRef]
- Franco, R.A., Jr.; Rosenfeld, R.M.; Rao, M. First place–resident clinical science award 1999: Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000, 123, 9–16. [Google Scholar] [CrossRef]
- Arezzo, E.; Festa, P.; D’Antò, V.; Michelotti, A.; De Vincentiis, G.C.; Sitzia, E.; Giuliani, M.; Piga, S.; Galeotti, A. Linguistic adaptation and validation of Italian version of OSA-18, a quality of life questionnaire for evaluation of children with obstructive sleep apnea-hypopnea syndrome (OSAS). Int. J. Pediatr. Otorhinolaryngol. 2020, 129, 109727. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2012; p. 176. [Google Scholar]
- Kljajić, Z.; Roje, Ž.; Bečić, K.; Čapkun, V.; Vilović, K.; Ivanišević, P.; Marušić, E. Formula for the prediction of apnea/hypopnea index in children with obstructive sleep apnea without polysomnography according to the clinical parameters: Is it reliable? Int. J. Pediatr. Otorhinolaryngol. 2017, 100, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kljajic, Z.; Glumac, S.; Deutsch, J.A.; Lupi-Ferandin, S.; Dogas, Z.; Roje, Z. Feasibility study of determining a risk assessment model for obstructive sleep apnea in children based on local findings and clinical indicators. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110081. [Google Scholar] [CrossRef] [PubMed]
- Schechter, M.S. Technical report: Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002, 109, e69. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; McKean, M. Adenotonsillectomy for obstructive sleep apnea in children. Cochrane Libr. 2006. [Google Scholar]
- Costa, D.J.; Mitchell, R. Adenotonsillectomy for obstructive sleep apnea in obese children: A meta-analysis. Otolaryngol. Head Neck Surg. 2009, 140, 455–460. [Google Scholar] [CrossRef]
- Galluzzi, F.; Garavello, W. Impact of adenotonsillectomy in children with severe obstructive sleep apnea: A systematic review. Auris Nasus Larynx 2021, 48, 549–554. [Google Scholar] [CrossRef]
- Lipton, A.J.; Gozal, D. Treatment of obstructive sleep apnea in children: Do we really know how? Sleep. Med. Rev. 2003, 7, 61–80. [Google Scholar] [CrossRef]
- Montgomery-Downs, H.E.; Crabtree, V.M.; Gozal, D. Cognition, sleep and respiration in at risk children treated for obstructive sleep apnoea. Eur. Respir. J. 2005, 25, 336–342. [Google Scholar] [CrossRef]
- Chervin, R.D.; Ruzicka, D.L.; Giordani, B.J.; Weatherly, R.A.; Dillon, J.E.; Hodges, E.K.; Marcus, C.L.; Guire, K.E. Sleep disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 2006, 117, e769–e778. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.L.; Mayo, M.S.; Smith, H.J.; Reese, M.; Weatherly, R.A. Improved behavior and sleep after adenotonsillectomy in children with Sleep-Disorders Breathing. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.D.; Nguyen, C.D.; Weedon, J.; Goldstein, N.A. Child behavior and quality of life in pediatric obstructive sleep apnea. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Huang, Y.S.; Chen, N.H.; Fang, T.J.; Lee, L.A. Impact of adenotonsillectomy on behavior in children with sleep-disordered breathing. Laryngoscope 2006, 116, 1142–1147. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Kelly, J. Long-term changes in behavior after adenotonsillectomy for obstructive sleep apnea syndrome in children. Otolaryngol. Head Neck Surg. 2006, 134, 374–378. [Google Scholar] [CrossRef]
- Stewart, M.G.; Glaze, D.G.; Friedman, E.M.; Smith, E.O.; Bautista, M. Quality of life and sleep study findings after adenotonsillectomy in children with obstructive sleep apnea. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 308–314. [Google Scholar] [CrossRef]
- de Serres, L.M.; Derkay, C.; Sie, K.; Biavati, M.; Jones, J.; Tunkel, D.; Manning, S.; Inglis, A.F.; Haddad, J., Jr.; Tampakopoulou, D.; et al. Impact of adenotonsillectomy on quality of life in children with obstructive sleep disorders. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 489–496. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Kelly, J.; Call, E.; Yao, N. Long-term changes in quality of life after surgery for pediatric obstructive sleep apnea. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 409–412. [Google Scholar] [CrossRef]
- Diez-Montiel, A.; de Diego, J.I.; Prim, M.P.; Martín-Martínez, M.A.; Pérez-Fernández, E.; Rabanal, I. Quality of life after surgical treatment of children with obstructive sleep apnea: Long-term results. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.B.; Kelly, J. Adenotonsillectomy for obstructive sleep apnea in obese children. Otolaryngol. Head Neck Surg. 2004, 131, 104–108. [Google Scholar] [CrossRef]
- Mitchell, R.B. Adenotonsillectomy for obstructive sleep in children: Outcome evaluated by pre- and postoperative polysomnography. Laryngoscope 2007, 117, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.B.; Kelly, J. Child behavior after adenotonsillectomy for obstructive sleep apnea syndrome. Laryngoscope 2005, 115, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, C.M.; Alam, L.; Vigilar, M.; Benke, J.; Martin, C.; Ishman, S. Correlation between REM AHI and quality-of-life scores in children with sleep-disordered breathing. Otolaryngol. Head Neck Surg. 2014, 151, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Ishman, S.L.; Yang, C.J.; Cohen, A.P.; Benke, J.R.; Meinzen-Derr, J.K.; Anderson, R.M.; Madden, M.E.; Tabangin, M.E. Is the OSA-18 predictive of obstructive sleep apnea: Comparison to polysomnography. Laryngoscope 2015, 125, 1491–1495. [Google Scholar] [CrossRef]
- Kobayashi, R.; Miyazaki, S.; Karaki, M.; Hoshikawa, H.; Nakata, S.; Hara, H.; Kodama, S.; Kikuchi, A.; Kitamura, T.; Mori, N. Evaluation of adenotonsillectomy and tonsillectomy for pediatric obstructive sleep apnea by rhinomanometry and the OSA-18 questionnaire. Acta Oto-Laryngologica 2014, 134, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Isaiah, A.; Hamdan, H.; Johnson, R.F.; Naqvi, K.; Mitchell, R.B. Very severe obstructive sleep apnea in children: Outcomes of adenotonsillectomy and risk factors for persistence. Otolaryngol. Head Neck Surg. 2017, 157, 128–134. [Google Scholar] [CrossRef]
- Imanguli, M.; Ulualp, S.O. Risk factors for residual obstructive sleep apnea after adenotonsillectomy in children: Risk factors for residual OSA after surgery. Laryngoscope 2016, 126, 2624–2629. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, K.; Cavallazzi, R.; Senthilvel, E. Outcomes of adenotonsillectomy in severe pediatric obstructive sleep apnea. Ear Nose Throat J. 2017, 96, E6–E9. [Google Scholar] [PubMed]

| Variables | Total Cohort (n = 65) | |
|---|---|---|
| Males, n (%) | 43 (66.2) | |
| Median (IQR) age at diagnosis, years | 4 (4–6) | |
| Median (IQR) weight, kg | 17 (15–20) | |
| Mean (SD) height, cm | 107 (11.7) | |
| Median (IQR) BMI z-score | −0.27 (−1.18; 0.32) | |
| Median (IQR) BMI, kg/m2 | 15.4 (14.2–16.6) | |
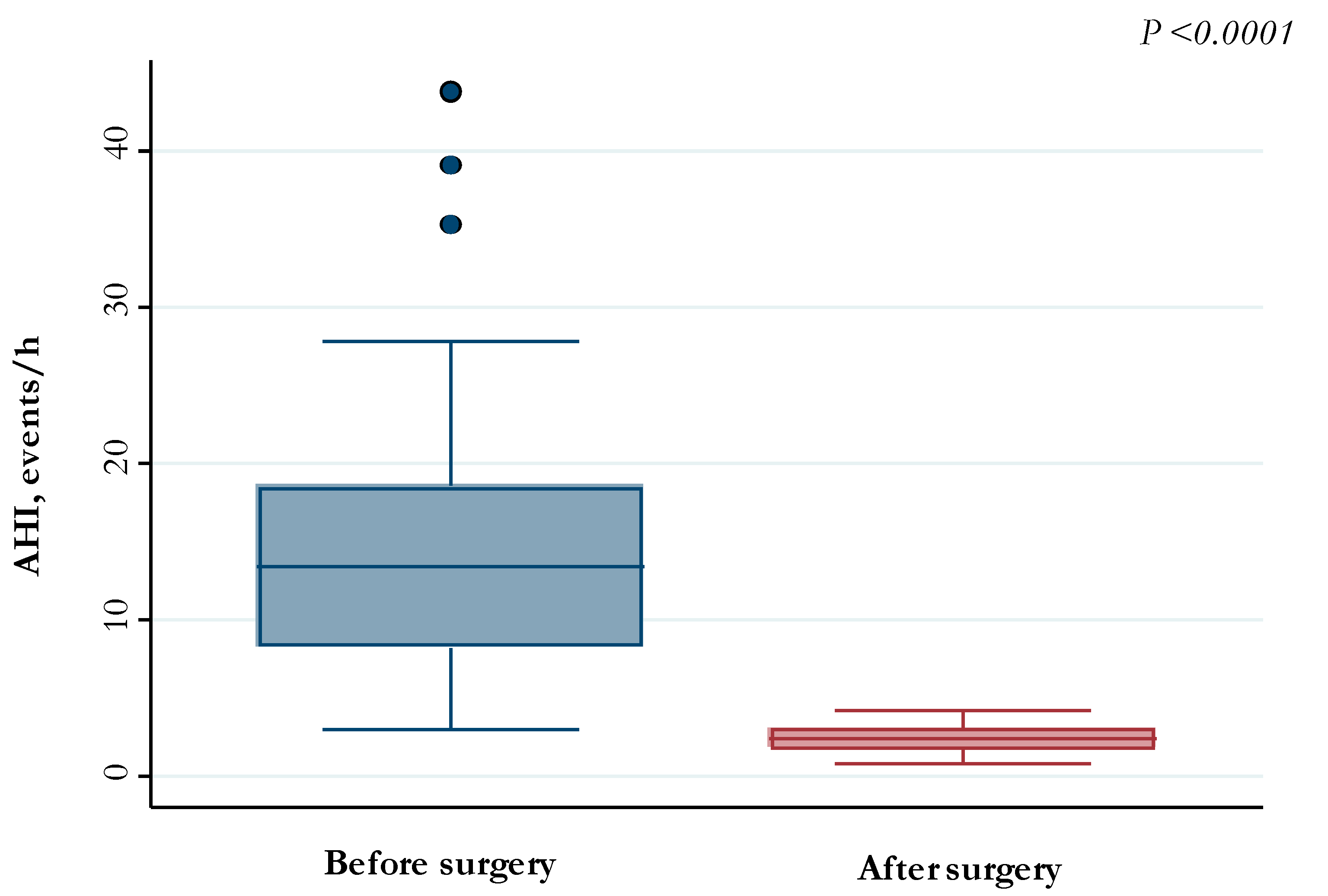
| Median (IQR) pre-operative AHI, events/h | 13.4 (8.3–18.5) | |
| Severity levels of OSAS | Mild OSAS (AHI < 5) | 4 (6.2) |
| Moderate OSAS (AHI 5–9.9) | 16 (24.6) | |
| Severe OSAS (AHI ≥ 10) | 45 (69.2) | |
| Mean (SD) post-operative AHI, events/h | 2.4 (0.9) | |
| Post-operative AHI ≥ 3, n (%) | 18 (27.7) | |
| Tonsil size grading III–IV, n (%) | 51 (78.5) | |
| Friedman palate position III–IV, n (%) | 26 (40.0) | |
| Median (IQR) pre-operative SpO2 nadir, % | 89 (84–92) | |
| Median (IQR) post-operative SpO2 nadir, % | 94 (93–95) | |
| Oral breathing, n (%) | 53 (81.5) | |
| Nasal airway patency, n (%) | 55 (84.6) | |
| Snoring, n (%) | 61 (93.9) | |
| Allergen sensitization, n (%) | 13 (20.0) | |
| Asthma, n (%) | 5 (7.7) | |
| Rhinitis, n (%) | 24 (36.9) | |
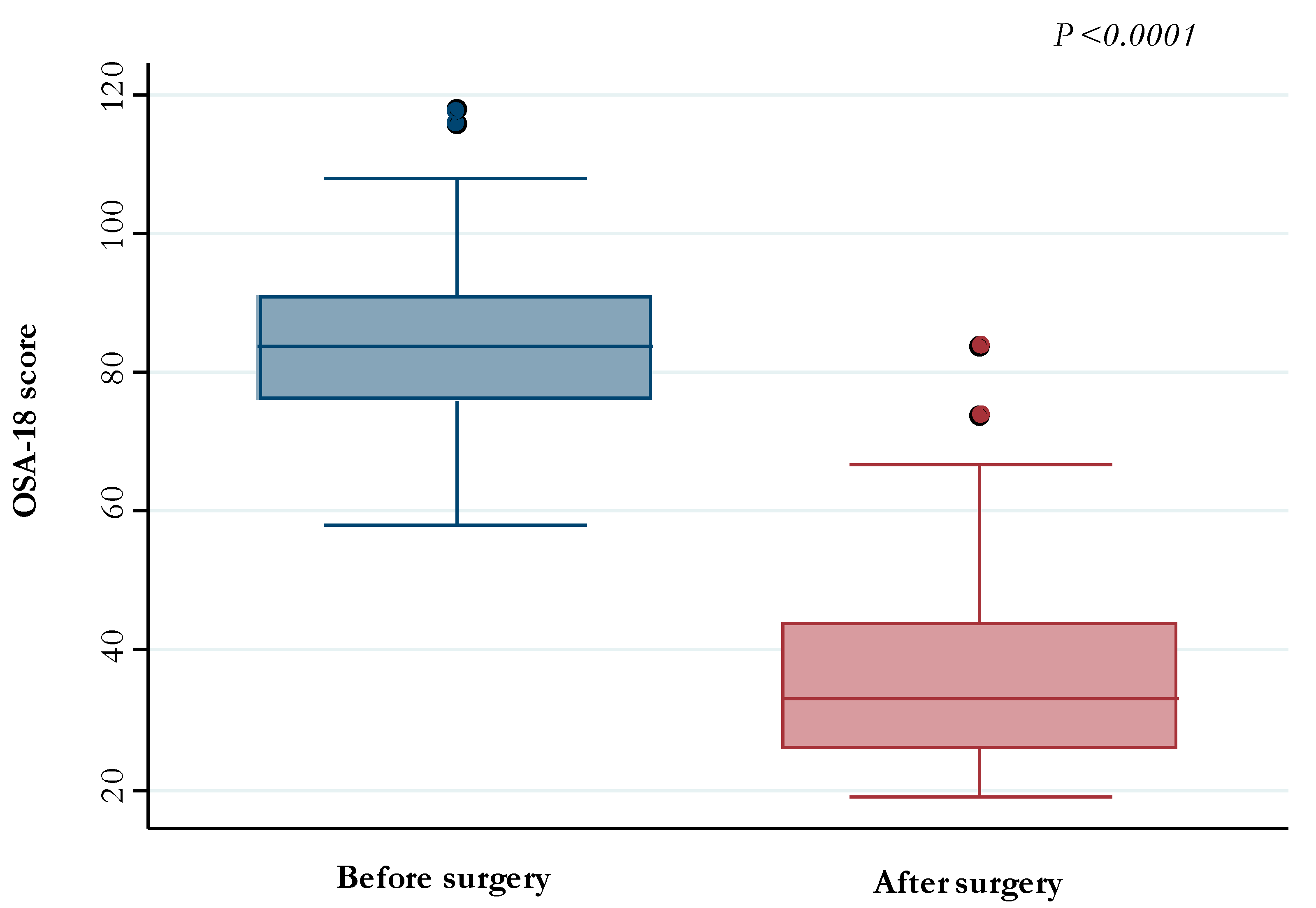
| Median (IQR) pre-operative OSA-18 | 84 (76–91) | |
| Median (IQR) post-operative OSA-18 | 33 (26–44) | |
| Variables | Age < 5 Years (n = 42) | Age ≥ 5 Years (n = 23) | p-Value | |
|---|---|---|---|---|
| Males, n (%) | 28 (66.7) | 15 (65.2) | 0.91 | |
| Median (IQR) BMI z-score | −0.48 (−1.32; 0.24) | −0.06 (−1.13; 1.12) | 0.19 | |
| Median (IQR) BMI, kg/m2 | 15.2 (14.1–16.2) | 16.0 (14.4–19.8) | 0.08 | |
| Median (IQR) pre-operative AHI, events/h | 13.6 (8.8–18.0) | 12.1 (8.1–19.6) | 0.58 | |
| Severity levels of OSAS, n (%) | Mild OSAS (AHI < 5) | 2 (4.8) | 2 (8.7) | 0.75 |
| Moderate OSAS (AHI 5–9.9) | 10 (23.8) | 6 (29.1) | ||
| Severe OSAS (AHI ≥ 10) | 30 (71.4) | 15 (65.2) | ||
| Median (IQR) post-operative AHI, events/h | 2.4 (1.6–2.9) | 2.6 (1.8–3.3) | 0.49 | |
| Post-operative AHI ≥ 3, n (%) | 10 (23.8) | 8 (34.8) | 0.34 | |
| Tonsil size grading III–IV, n (%) | 34 (81.0) | 17 (73.9) | 0.51 | |
| Friedman palate position III–IV, n (%) | 16 (38.1) | 10 (43.5) | 0.67 | |
| Median (IQR) pre-operative SpO2 nadir, % | 89 (86–92) | 89 (81–92) | 0.91 | |
| Median (IQR) post-operative SpO2 nadir, % | 94 (93–95) | 94 (93–95) | 0.83 | |
| Oral breathing, n (%) | 37 (88.1) | 16 (69.6) | 0.07 | |
| Nasal airway patency, n (%) | 37 (88.1) | 18 (79.3) | 0.31 | |
| Snoring, n (%) | 39 (92.9) | 22 (95.7) | 1.00 | |
| Allergen sensitization, n (%) | 6 (14.3) | 7 (30.4) | 0.12 | |
| Asthma, n (%) | 2 (4.8) | 3 (13.0) | 0.34 | |
| Rhinitis, n (%) | 13 (31.0) | 11 (47.8) | 0.18 | |
| Median (IQR) pre-operative OSA-18 | 85.5 (79–91) | 81 (69–93) | 0.15 | |
| Median (IQR) post-operative OSA-18 | 35 (27–45) | 31 (24–36) | 0.19 | |
| Variables | Mild OSAS AHI < 5 (n = 4) | Moderate OSAS AHI 5–9.9 (n = 16) | Severe OSAS AHI ≥ 10 (n = 45) | p-Value |
|---|---|---|---|---|
| Males, n (%) | 2 (50.0) | 11 (68.8) | 30 (66.7) | 0.82 |
| Median (IQR) age at diagnosis, years | 5 (3–6) | 4 (3.5–6.5) | 4 (4–5) | 0.95 |
| Median (IQR) weight, kg | 17.8 (14.5–19.3) | 17.5 (15–25) | 17 (15.0–19.7) | 0.74 |
| Median (IQR) height, cm | 108 (93.5–116.0) | 111 (98.0–117.5) | 105 (101–113) | 0.89 |
| Median (IQR) BMI z-score | −0.10 (−0.69; 0.53) | −0.08 (−1.01; 0.80) | −0.29 (−1.32; 0.24) | 0.54 |
| Median (IQR) BMI, kg/m2 | 16.0 (14.9–17.2) | 15.8 (14.4–18.2) | 15.4 (14.1–16.2) | 0.34 |
| Median (IQR) post-operative AHI, events/h | 1.9 (1.2–3.0) | 2.3 (1.7–2.7) | 2.5 (1.9–3.2) | 0.51 |
| Postoperative AHI ≥ 3, n (%) | 1 (25.0) | 3 (18.8) | 14 (31.1) | 0.79 |
| Tonsil size grading III–IV, n (%) | 3 (75.0) | 8 (50.0) | 40 (88.9) | 0.005 |
| Friedman palate position III–IV, n (%) | 1 (25.0) | 2 (12.5) | 23 (51.1) | 0.01 (1) |
| Median (IQR) pre-operative SpO2 nadir, % | 93 (89–95) | 92.5 (91–94) | 86 (79–90) | 0.0001 (2) |
| Median (IQR) post-operative SpO2 nadir, % | 95 (94.5–95.5) | 94.5 (93–95.5) | 94 (93–95) | 0.38 (3) |
| Oral breathing, n (%) | 1 (25.0) | 10 (62.5) | 42 (93.3) | <0.0001 (4) |
| Nasal airway patency, n (%) | 1 (25.0) | 13 (81.3) | 41 (91.1) | 0.002 (5) |
| Snoring, n (%) | 2 (50.0) | 14 (87.5) | 45 (100.0) | 0.001 (6) |
| Allergen sensitization, n (%) | 1 (25.0) | 4 (25.0) | 8 (17.8) | 0.67 |
| Asthma, n (%) | 0 (0.0) | 2 (12.5) | 3 (6.7) | 0.71 |
| Rhinitis, n (%) | 1 (25.0) | 7 (43.8) | 16 (35.6) | 0.76 |
| Median (IQR) pre-operative OSA-18 | 84.5 (70.5–88.5) | 78 (73–93) | 84 (79–91) | 0.69 |
| Median (IQR) post-operative OSA-18 | 50 (29–68) | 37 (24.5–48.5) | 31 (26–41) | 0.33 |
| Variables | Pre-Operative AHI | Post-Operative AHI |
|---|---|---|
| Pre-operative OSA-18, rho (p-value) | 0.19 (0.13) | - |
| Post-operative OSA-18, rho (p-value) | - | 0.31 (0.01) |
| Variables | Univariate Analysis | ||
|---|---|---|---|
| OR (95% CI) | p-Value | ||
| Males | 13.7 (1.69–111.8) | 0.01 | |
| Age at diagnosis, years | 1.09 (0.77–1.53) | 0.63 | |
| Weight, kg | 1.04 (0.96–1.13) | 0.36 | |
| Height, cm | 1.02 (0.97–1.07) | 0.47 | |
| BMI z-score | 1.42 (0.88–2.29) | 0.16 | |
| BMI, kg/m2 | 1.17 (091–1.50) | 0.21 | |
| Pre-operative AHI, events/h | 1.06 (0.99–1.13) | 0.09 | |
| Severity levels of OSAS | Mild OSAS (AHI < 5) | Ref. | Ref. |
| Moderate OSAS (AHI 5–9.9) | 0.69 (0.05–9.21) | 0.78 | |
| Severe OSAS (AHI ≥ 10) | 1.36 (0.13–14.2) | 0.80 | |
| Tonsil size grading III–IV | 0.95 (0.26–3.51) | 0.94 | |
| Friedman palate position III–IV | 3.35 (1.08–10.4) | 0.04 | |
| Pre-operative SpO2 nadir, % | 0.97 (0.91–1.03) | 0.24 | |
| Oral breathing | 0.72 (0.19–2.76) | 0.63 | |
| Nasal airway patency | 1.64 (0.31–8.59) | 0.56 | |
| Snoring | 1.16 (0.11–11.9) | 0.90 | |
| Allergen sensitization | 2.86 (0.81–10.1) | 0.10 | |
| Asthma | 0.63 (0.07–6.07) | 0.69 | |
| Rhinitis | 1.12 (0.37–3.44) | 0.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locci, C.; Cenere, C.; Sotgiu, G.; Puci, M.V.; Saderi, L.; Rizzo, D.; Bussu, F.; Antonucci, R. Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes. J. Clin. Med. 2023, 12, 5826. https://doi.org/10.3390/jcm12185826
Locci C, Cenere C, Sotgiu G, Puci MV, Saderi L, Rizzo D, Bussu F, Antonucci R. Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes. Journal of Clinical Medicine. 2023; 12(18):5826. https://doi.org/10.3390/jcm12185826
Chicago/Turabian StyleLocci, Cristian, Caterina Cenere, Giovanni Sotgiu, Mariangela Valentina Puci, Laura Saderi, Davide Rizzo, Francesco Bussu, and Roberto Antonucci. 2023. "Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes" Journal of Clinical Medicine 12, no. 18: 5826. https://doi.org/10.3390/jcm12185826
APA StyleLocci, C., Cenere, C., Sotgiu, G., Puci, M. V., Saderi, L., Rizzo, D., Bussu, F., & Antonucci, R. (2023). Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes. Journal of Clinical Medicine, 12(18), 5826. https://doi.org/10.3390/jcm12185826








